Early Retinal Microvascular Changes Assessed with Swept-Source OCT Angiography in Type 1 Diabetes Patients without Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
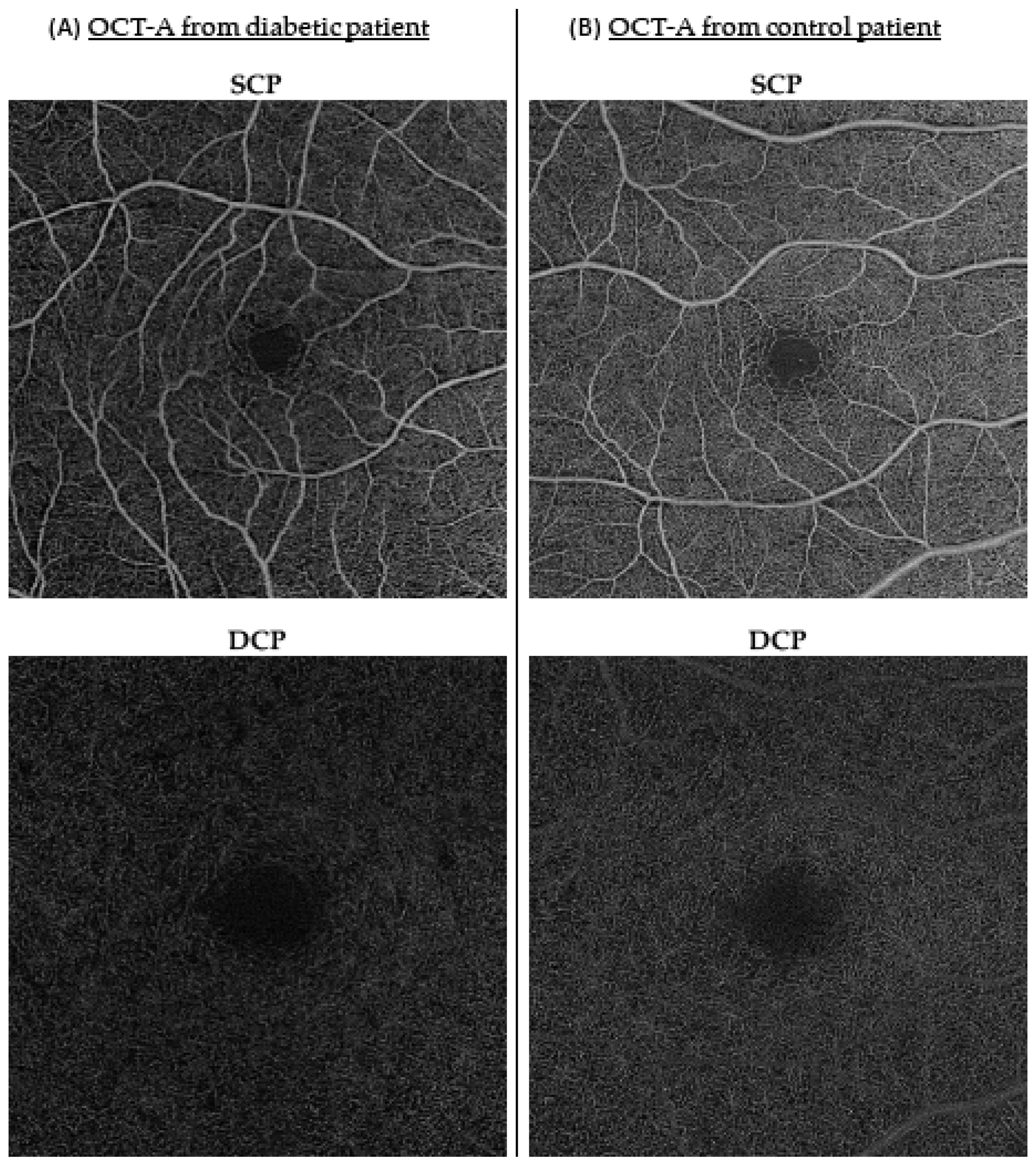
2.2. Imaging
2.3. Mean Amplitude of Glycemic Excursions (MAGE)
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Population
3.2. Retinal Microvascularization Comparison between the Diabetes and Control Groups
3.3. Retinal Microvascularization and Diabetic Characteristics in the Diabetes Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gale, E.A. Type 1 diabetes in the young: The harvest of sorrow goes on. Diabetologia 2005, 48, 1435–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs–An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology 2020, 127, S99–S119. [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Horton, W.B.; Barrett, E.J. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr. Rev. 2021, 42, 29–55. [Google Scholar] [CrossRef]
- Hainsworth, D.P.; Bebu, I.; Aiello, L.P.; Sivitz, W.; Gubitosi-Klug, R.; Malone, J.; White, N.H.; Danis, R.; Wallia, A.; Gao, X.; et al. Risk Factors for Retinopathy in Type 1 Diabetes: The DCCT/EDIC Study. Diabetes Care 2019, 42, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, I.B.; Brownlee, M. Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. JAMA 2010, 303, 2291–2292. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.R.L.; Leite, N.C.; Dib, E.; Salles, G.F. Predictors of Development and Progression of Retinopathy in Patients with Type 2 Diabetes: Importance of Blood Pressure Parameters. Sci. Rep. 2017, 7, 4867. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chou, Y.; Zhao, X.; Yang, J.; Chen, Y. Early Detection of Microvascular Impairments With Optical Coherence Tomography Angiography in Diabetic Patients Without Clinical Retinopathy: A Meta-analysis. Am. J. Ophthalmol. 2021, 222, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Inanc, M.; Tekin, K.; Kiziltoprak, H.; Ozalkak, S.; Doguizi, S.; Aycan, Z. Changes in Retinal Microcirculation Precede the Clinical Onset of Diabetic Retinopathy in Children With Type 1 Diabetes Mellitus. Am. J. Ophthalmol. 2019, 207, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Khouri, A.S.; Zarbin, M.A.; Szirth, B.C. Early retinal microvascular abnormalities in young adults with type 1 diabetes mellitus without clinically evident diabetic retinopathy. Retina 2021, 41, 1478–1486. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [Green Version]
- Yapanis, M.; James, S.; Craig, M.E.; O’Neal, D.; Ekinci, E.I. Complications of Diabetes and Metrics of Glycemic Management Derived From Continuous Glucose Monitoring. J. Clin. Endocrinol. Metab. 2022, 107, e2221–e2236. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.K.; Menghini, M.; Hansen, A.; Mackey, D.A.; Constable, I.J.; Sampson, D.M. Intrasession Repeatability and Interocular Symmetry of Foveal Avascular Zone and Retinal Vessel Density in OCT Angiography. Transl. Vis. Sci. Technol. 2018, 7, 6. [Google Scholar] [CrossRef]
- Querques, G.; Borrelli, E.; Battista, M.; Sacconi, R.; Bandello, F. Optical coherence tomography angiography in diabetes: Focus on microaneurysms. Eye 2021, 35, 142–148. [Google Scholar] [CrossRef]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. Am. J. Ophthalmol. 2015, 160, 35–44.e31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilotto, E.; Torresin, T.; Leonardi, F.; Gutierrez De Rubalcava Doblas, J.; Midena, G.; Moretti, C.; Midena, E. Retinal Microvascular and Neuronal Changes Are Also Present, Even If Differently, in Adolescents with Type 1 Diabetes without Clinical Diabetic Retinopathy. J. Clin. Med. 2022, 11, 3982. [Google Scholar] [CrossRef]
- Simonett, J.M.; Scarinci, F.; Picconi, F.; Giorno, P.; De Geronimo, D.; Di Renzo, A.; Varano, M.; Frontoni, S.; Parravano, M. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017, 95, e751–e755. [Google Scholar] [CrossRef] [Green Version]
- Carnevali, A.; Sacconi, R.; Corbelli, E.; Tomasso, L.; Querques, L.; Zerbini, G.; Scorcia, V.; Bandello, F.; Querques, G. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017, 54, 695–702. [Google Scholar] [CrossRef]
- Campbell, J.P.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, 42201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gołębiewska, J.; Olechowski, A.; Wysocka-Mincewicz, M.; Odrobina, D.; Baszyńska-Wilk, M.; Groszek, A.; Szalecki, M.; Hautz, W. Optical coherence tomography angiography vessel density in children with type 1 diabetes. PLoS ONE 2017, 12, e0186479. [Google Scholar] [CrossRef]
- Conrath, J.; Giorgi, R.; Raccah, D.; Ridings, B. Foveal avascular zone in diabetic retinopathy: Quantitative vs. qualitative assessment. Eye 2005, 19, 322–326. [Google Scholar] [CrossRef] [Green Version]
- Durbin, M.K.; An, L.; Shemonski, N.D.; Soares, M.; Santos, T.; Lopes, M.; Neves, C.; Cunha-Vaz, J. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmol. 2017, 135, 370–376. [Google Scholar] [CrossRef] [PubMed]
- El-Fayoumi, D.; Badr Eldine, N.M.; Esmael, A.F.; Ghalwash, D.; Soliman, H.M. Retinal Nerve Fiber Layer and Ganglion Cell Complex Thicknesses Are Reduced in Children With Type 1 Diabetes With No Evidence of Vascular Retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5355–5360. [Google Scholar] [CrossRef]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care 2016, 39, 686–693. [CrossRef] [PubMed] [Green Version]
- van den Boom, L.; Kostev, K. Patterns of insulin therapy and insulin daily doses in children and adolescents with type 1 diabetes in Germany. Diabetes Obes. Metab. 2022, 24, 296–301. [Google Scholar] [CrossRef]
- Martínez-Ortega, A.J.; Muñoz-Gómez, C.; Gros-Herguido, N.; Remón-Ruiz, P.J.; Acosta-Delgado, D.; Losada-Viñau, F.; Pumar-López, A.; Mangas-Cruz, M.; González-Navarro, I.; López-Gallardo, G.; et al. Description of a Cohort of Type 1 Diabetes Patients: Analysis of Comorbidities, Prevalence of Complications and Risk of Hypoglycemia. J. Clin. Med. 2022, 11, 1039. [Google Scholar] [CrossRef]
- Pauley, M.E.; Tommerdahl, K.L.; Snell-Bergeon, J.K.; Forlenza, G.P. Continuous Glucose Monitor, Insulin Pump, and Automated Insulin Delivery Therapies for Type 1 Diabetes: An Update on Potential for Cardiovascular Benefits. Curr. Cardiol. Rep. 2022, 24, 2043–2056. [Google Scholar] [CrossRef]
- Eid, P.; Arnould, L.; Gabrielle, P.H.; Aho, L.S.; Farnier, M.; Creuzot-Garcher, C.; Cottin, Y. Retinal Microvascular Changes in Familial Hypercholesterolemia: Analysis with Swept-Source Optical Coherence Tomography Angiography. J. Pers. Med. 2022, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Arnould, L.; Guenancia, C.; Azemar, A.; Alan, G.; Pitois, S.; Bichat, F.; Zeller, M.; Gabrielle, P.H.; Bron, A.M.; Creuzot-Garcher, C.; et al. The EYE-MI Pilot Study: A Prospective Acute Coronary Syndrome Cohort Evaluated With Retinal Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4299–4306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustin, A.J.; Atorf, J. The Value of Optical Coherence Tomography Angiography (OCT-A) in Neurological Diseases. Diagnostics 2022, 12, 468. [Google Scholar] [CrossRef] [PubMed]
| Diabetes Group (n = 98) | Control Group (n = 71) | p-Value | |||
|---|---|---|---|---|---|
| n | n | ||||
| Laterality (right) | 53 (54%) | 98 | 43 (61%) | 71 | 0.40 |
| Age (years) | 31.8 (25.3–42.6) | 98 | 29.4 (27.0–35.5) | 71 | 0.36 |
| Sex (male) | 42 (43%) | 28 (39%) | 0.66 | ||
| Duration of diabetes (years) | 18.0 (8.75–24.0) | 96 | |||
| BMI (kg/m2) | 24.1 (21.2–27.3) | 95 | |||
| Creatinine (μmol/L) | 64.5 (56.0–71.6) | 90 | |||
| HbA1c (%) | 7.7 (7.2–8.8) | 94 | |||
| MAGE (mg/dL) | 79.5 (63.9–101.0) | 46 | |||
| Diabetic Group | Control Group | Multivariate Analysis (n = 169) | ||
|---|---|---|---|---|
| Median (Q25–75) | Median (Q25–75) | β (95% CI) | p-Value | |
| FAZ | ||||
| Size (mm2) | 0.223 (0.167–0.310) | 0.221 (0.173–0.294) | −0.115 (−0.818; 0.0353) | 0.62 |
| Circularity index | 0.730 (0.657–0.769) | 0.762 (0.680–0.801) | 0.0231 (−0.00559; 0.0572) | 0.16 |
| SCP | ||||
| Central density (mm−1) | 11.4 (8.82–13.0) | 12.3 (10.3–13.8) | 0.677 (−0.298; 1.65) | 0.17 |
| Inner circle density (mm−1) | 17.6 (16.1–18.8) | 18.4 (17.2–19.2) | 1.00 (0.468; 1.79) | <0.01 * |
| Inner superior density (mm−1) | 18.5 (16.9–19.6) | 19.4 (18.3–20.1) | 0.981 (0.475; 1.61) | <0.01 * |
| Inner nasal density (mm−1) | 18.6 (17.2–19.8) | 19.3 (18.3–20.0) | 1.08 (0.502; 1.86) | <0.01 |
| Inner inferior density (mm−1) | 18.4 (17.1–19.5) | 19.3 (17.8–20.1) | 1.06 (0.469; 1.83) | <0.01 * |
| Inner temporal density (mm−1) | 18.3 (17.1–19.6) | 18.6 (17.9–19.9) | 0.912 (0.136; 1.69) | 0.021 |
| Outer circle density (mm−1) | 18.6 (17.4–19.4) | 19.0 (18.1–19.6) | 0.509 (0.107; 1.08) | 0.086 |
| DCP | ||||
| Central density (mm−1) | 0.633 (0.264–1.34) | 0.704 (0.342–1.57) | 0.187 (−0.197; 0.761) | 0.41 |
| Inner circle density (mm−1) | 12.3 (9.60–14.0) | 12.6 (10.4–14.1) | 0.558 (−0.350; 1.53) | 0.27 |
| Outer circle density (mm−1) | 13.4 (10.9–15.1) | 13.5 (10.8–15.4) | 0.343 (−0.703; 1.39) | 0.52 * |
| Diabetes Duration (n = 96) | HbA1c (n = 94) | MAGE (n = 46) | Total Daily Insulin (n = 64) | |||||
|---|---|---|---|---|---|---|---|---|
| r (95% CI) | p-Value | r (95% CI) | p-Value | r (95% CI) | p-Value | r (95% CI) | p-Value | |
| FAZ | ||||||||
| Size (mm2) | 0.000875 (−0.208; 0.209) | 0.99 | 0.0252 (−0.187; 0.235) | 0.81 | −0.0487 (−0.342; 0.253) | 0.75 | −0.164 (−0.404; 0.0966) 0.1145 | 0.20 |
| Circularity index | −0.176 (−0.370; 0.0340) | 0.090 | −0.0710 (−0.278; 0.142) | 0.50 | −0.106 (−0.392; 0.199) | 0.49 | (−0.147; 0.361) | 0.38 |
| SCP | ||||||||
| Central density (mm−1) | −0.0490 (−0.253; 0.159) | 0.64 | −0.114 (−0.315; 0.0967) | 0.27 | −0.0998 (−0.387; 0.205) | 0.51 | 0.173 (−0.0833; 0.408) | 0.17 |
| Inner circle density (mm−1) | −0.0549 (−0.258; 0.153) | 0.59 | −0.0326 (−0.239; 0.177) | 0.76 | −0.0371 (−0.332; 0.264) | 0.81 | 0.2063 (−0.049; 0.436) | 0.10 |
| Outer circle density (mm−1) | −0.0372 (−0.242; 0.170) | 0.72 | −0.0443 (−0.250; 0.166) | 0.67 | −0.0139 (−0.311; 0.286) | 0.93 | 0.162 (−0.095; 0.398) | 0.20 |
| DCP | ||||||||
| Central density (mm−1) | −0.176 (−0.394; 0.0596) | 0.13 | −0.028 (−0.263; 0.210) | 0.82 | −0.106 (−0.424; 0.235) | 0.53 | 0.294 (0.00223; 0.539) | 0.042 |
| Inner circle density (mm−1) | −0.0699 (−0.272; 0.138) | 0.49 | 0.0218 (−0.187; 0.229) | 0.84 | −0.173 (−0.448; 0.132) | 0.25 | 0.08217 (−0.174; 0.328) | 0.52 |
| Outer circle density (mm−1) | −0.0126 (−0.218; 0.194) | 0.90 | −0.0596 (−0.265; 0.151) | 0.57 | −0.132 (−0.414; 0.173) | 0.39 | 0.009891 (−0.244; 0.262) | 0.94 |
| Equipped with FSL Device (n = 46) | No FSL Device (n = 52) | Multivariate Analysis | ||
|---|---|---|---|---|
| Median (Q25–75) | Median (Q25–75) | β (95% CI) | p-Value | |
| FAZ | ||||
| Size (mm2) | 0.210 (0.165–0.299) | 0.250 (0.176–0.310) | −0.240 (−1.36; 0.0189) | 0.31 |
| Circularity index | 0.728 (0.672–0.777) | 0.730 (0.650–0.765) | 0.00353 (−0.0369; 0.0467) | 0.87 |
| SCP | ||||
| Central density (mm−1) | 12.3 (9.21–13.3) | 11.0 (8.59–12.5) | 0.805 (−0.498; 2.11) | 0.22 * |
| Inner circle density (mm−1) | 18.4 (16.1–19.1) | 17.4 (16.2–18.2) | 0.871 (−0.0489; 1.93) | 0.11 * |
| Outer circle density (mm−1) | 19.1 (17.9–19.8) | 18.2 (17.2–18.9) | 0.922 (0.226; 1.87) | 0.034 |
| DCP | ||||
| Central density (mm−1) | 0.812 (0.358–1.82) | 0.432 (0.209–1.14) | 0.595 (0.103; 1.45) | 0.052 |
| Inner circle density (mm−1) | 13.8 (11.7–14.8) | 11.5 (9.06–12.9) | 1.92 (0.520; 3.24) | <0.01 * |
| Outer circle density (mm−1) | 14.3 (12.4–15.7) | 12.9 (9.95–14.0) | 1.80 (0.427; 3.24) | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eid, P.; Creuzot-Garcher, C.; Aho, L.S.; Gabrielle, P.-H.; Charpin, E.; Haddad, D.; Steinberg, L.-A.; Bron, A.; Verges, B.; Arnould, L. Early Retinal Microvascular Changes Assessed with Swept-Source OCT Angiography in Type 1 Diabetes Patients without Retinopathy. J. Clin. Med. 2023, 12, 2687. https://doi.org/10.3390/jcm12072687
Eid P, Creuzot-Garcher C, Aho LS, Gabrielle P-H, Charpin E, Haddad D, Steinberg L-A, Bron A, Verges B, Arnould L. Early Retinal Microvascular Changes Assessed with Swept-Source OCT Angiography in Type 1 Diabetes Patients without Retinopathy. Journal of Clinical Medicine. 2023; 12(7):2687. https://doi.org/10.3390/jcm12072687
Chicago/Turabian StyleEid, Pétra, Catherine Creuzot-Garcher, Ludwig Serge Aho, Pierre-Henry Gabrielle, Estelle Charpin, Déa Haddad, Laure-Anne Steinberg, Alain Bron, Bruno Verges, and Louis Arnould. 2023. "Early Retinal Microvascular Changes Assessed with Swept-Source OCT Angiography in Type 1 Diabetes Patients without Retinopathy" Journal of Clinical Medicine 12, no. 7: 2687. https://doi.org/10.3390/jcm12072687
APA StyleEid, P., Creuzot-Garcher, C., Aho, L. S., Gabrielle, P.-H., Charpin, E., Haddad, D., Steinberg, L.-A., Bron, A., Verges, B., & Arnould, L. (2023). Early Retinal Microvascular Changes Assessed with Swept-Source OCT Angiography in Type 1 Diabetes Patients without Retinopathy. Journal of Clinical Medicine, 12(7), 2687. https://doi.org/10.3390/jcm12072687







