Emerging Role of Alarmins in Food Allergy: An Update on Pathophysiological Insights, Potential Use as Disease Biomarkers, and Therapeutic Implications
Abstract
:1. Introduction
2. Search Strategy
3. Results
3.1. IgE-Mediated FA
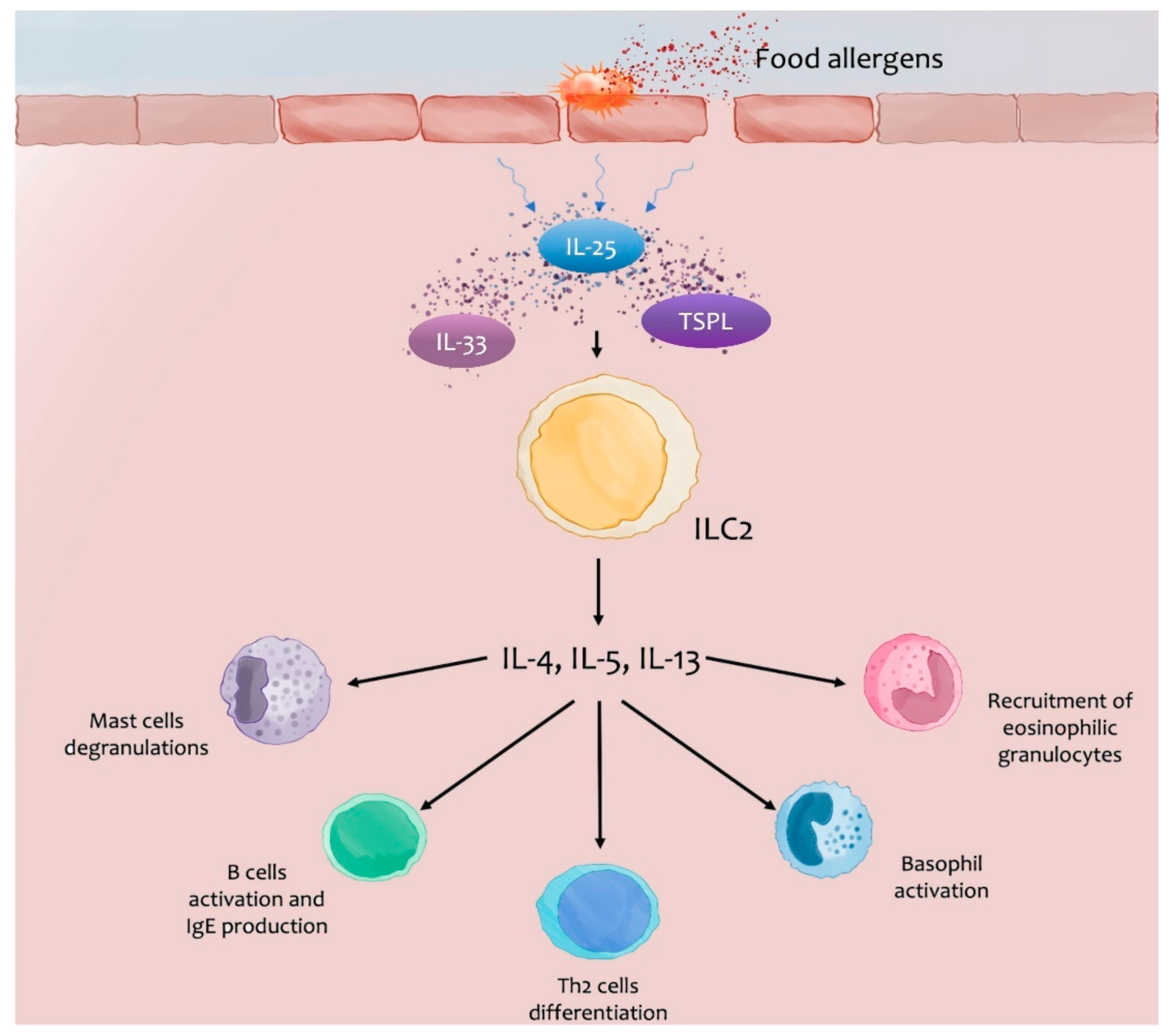
3.1.1. Nuclear Alarmins: Thymic Stromal Lymphopoietin (TSLP), IL-33, IL-25
Studies on Animal Models
Studies on Humans
3.1.2. Granule Derived Alarmins: Eosinophil-Derived Neurotoxin (EDN)
Studies on Humans
3.1.3. Cytoplasmic Alarmins: S100, Calprotectin, Adenosine 5′-Triphosphate (ATP), Heat-Shock Protein (HSP), Uric Acid
Studies on Animal Models
Studies on Humans
3.2. Non-IgE-Mediated FA
3.2.1. Non-IgE Cow’s Milk Protein Allergy (NICMPA)
Granule Derived Alarmins: Defensins (α, β), EDN
- Studies on humans
Cytoplasmic Alarmins: S100, Calprotectin
- Studies on humans
3.2.2. Food Protein-Induced Allergic Proctocolitis (FPIAP)
Granule Derived Alarmins: EDN
- Studies on humans
Cytoplasmic Alarmins: S100, Calprotectin
- Studies on humans
3.2.3. Food Protein-Induced Enterocolitis Syndrome (FPIES)
Granule Derived Alarmins: EDN
- Studies on humans
Cytoplasmic Alarmins: S100, Calprotectin
- Studies on humans
3.3. Mixed FA
3.3.1. Eosinophilic Esophagitis
Nuclear Alarmins: TSLP and IL-33
- Studies on animal models
- Studies on humans
Granule Derived Alarmins: Defensins (α, β)
- Studies on humans
| Alarmins | FA Type | Food | Study Design | Age (Year/Months) | F/M | N° of Patients | Country | Year [Ref.] |
|---|---|---|---|---|---|---|---|---|
| TSLP | IgE | Shrimp | Case-Control | 42ys | 19/18 | 37 | Poland | 2021 [48] |
| IgE | Peanut | Multi-omic | 10ys | 17/39 | 56 | USA | 2021 [50] | |
| Mixed (EE) | Cross-sectional | Unknown | 18 | Switzerland | 2015 [103] | |||
| Mixed (EE) | Retrospective | Unknown | 56/253 | 309 | USA | 2018 [104] | ||
| Mixed (EE) | Cohort | 10ys | 96/255 | 351 | USA | 2010 [105] | ||
| Mixed (EE) | Cohort | 9ys | 54/118 | 172 | USA | 2010 [106] | ||
| IL-33 | IgE | Shrimp | Case-Control | 42ys | 19/18 | 37 | Poland | 2021 [48] |
| IgE | Peanut | Case-Control | 10ys | 17/39 | 56 | USA | 2021 [50] | |
| Mixed (EE) | Cross-sectional | Unknown | 18 | Switzerland | 2015 [103] | |||
| Mixed (EE) | Cross-sectional | 39ys | 35/40 | 75 | USA | 2022 [107] | ||
| Mixed (EE) | Cross-sectional | 48ys | 10/19 | 29 | Japan | 2017 [108] | ||
| IL-25 | IgE | Shrimp | Case-Control | 42ys | 19/18 | 37 | Poland | 2021 [48] |
| IgE | Peanut | Cross-sectional | 8ys | 8/22 | 30 | Netherlands | 2013 [51] | |
| Mixed (EE) | Cross-sectional | Unknown | 18 | Switzerland | 2015 [103] | |||
| IL-1α | Not evaluated | |||||||
| HMGB1 | Not evaluated | |||||||
| HMGN1 | Not evaluated | |||||||
| Alarmins | FA Type | Food | Study Design | Age (Year/Months) | F/M | N° of Patients | Country | Year [Ref.] |
|---|---|---|---|---|---|---|---|---|
| Cathelicidin (LL37/CRAMP) | Not evaluated | |||||||
| Defensins (α, β) | Non-IgE | Cow’s milk | Cross-sectional | 9ms | Unknown | 57 | Finland | 2014 [79] |
| Mixed (EE) | Cross-sectional | 41ys | 2/16 | 18 | Switzerland | 2015 [103] | ||
| Mixed (EE) | Cross-sectional | 10ys | 11/11 | 22 | USA | 2013 [109] | ||
| EDN | IgE | Cow’s milk, egg, cod | Cohort | Unknown | 6/2 | 8 | Sweden | 2016 [55] |
| IgE | Cow’s milk | Longitudinal | 17ys | 14/10 | 24 | Finland | 2018 [56] | |
| Non-IgE (NICMPA) | Cow’s milk | Cross-sectional | 1m | 14/16 | 30 | Spain | 2021 [82] | |
| Non-IgE (NICMPA) | Cow’s milk | Case report | Newborn | 1/0 | 1 | Japan | 2007 [80] | |
| Non-IgE (FPIAP) | Cow’s milk | Cross-sectional | 4ms | 9/18 | 27 | Poland | 2020 [91] | |
| Non-IgE (FPIAP) | Prospective, open-label pilot | 7ms | 6/5 | 11 | USA | 2020 [92] | ||
| Non-IgE (FPIES) | Fish, cow’s milk, egg, wheat, rice | Cross-sectional | 3ms | 5/3 | 8 | Japan | 2014 [94] | |
| Non-IgE (FPIES) | Fish, egg, wheat, rice | Cross-sectional | 3ms | 3/2 | 5 | Japan | 2016 [95] | |
| Granulysin | Not evaluated | |||||||
| Alarmins | FA Type | Food | Study Design | Age (Year/Months) | F/M | N° of Patients | Country | Year [Ref.] |
|---|---|---|---|---|---|---|---|---|
| Cytoplasmic | ||||||||
| S100 proteins (calprotectin) | IgE | Cow’s milk | Case–Control | 0–9ms | 49/41 | 90 | China | 2021 [65] |
| IgE | Cow’s milk | Longitudinal | 33ys | 127/33 | 160 | Italy | 2011 [66] | |
| IgE | Cow’s milk, Egg, Cod | Cohort | Unknown | 6/2 | 8 | Sweden | 2016 [55] | |
| Non-IgE (NICMPA) | Cow’s milk | Cross-sectional | 9ms | 3/5 | 8 | Turkey | 2014 [81] | |
| Non-IgE (NICMPA) | Cow’s milk | Cross-sectional | 8ms | Unknown | 18 | Finland | 2014 [79] | |
| Non-IgE (NICMPA) | Cow’s milk | Cross-sectional | 1m | 14/16 | 30 | Spain | 2021 [82] | |
| Non-IgE (NICMPA) | Cow’s milk | Prospective cohort | 12–24ms | 8/9 | 17 | Spain, Italy | 2018 [83] | |
| Non-IgE (NICMPA) | Cow’s milk | Cross-sectional | 4ms | 15/25 | 40 | Spain | 2016 [84] | |
| Non-IgE (FPIAP) | Cow’s milk | Cross-sectional | 4ms | 9/18 | 27 | Poland | 2020 [91] | |
| Non-IgE (FPIES) | Fish, cow’s milk, egg, wheat, rice | Cross-sectional | 3ms | 5/3 | 8 | Japan | 2014 [94] | |
| HSP | IgE | Milk | Comparative | 53ys | 13/13 | 26 | USA | 2002 [70] |
| IgE | Peanut, soy, tree nuts, crab, clam, lobster, egg. | Cross-sectional, proteomic analysis | 8ys | 5/6 | 11 | USA | 2011 [71] | |
| ATP | Not evaluated | |||||||
| Uric acid | IgE | Cow’s milk | Survey | Unknown | 64/87 | 151 | Republic of Korea, USA * | 2016 [77] |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sicherer, S.H.; Sampson, H.A. Food Allergy: A Review and Update on Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Johansson, S.G.O.; Hourihane, J.B.; Bousquet, J.; Bruijnzeel-Koomen, C.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; Van Cauwenberge, P.; et al. A Revised Nomenclature for Allergy: An EAACI Position Statement from the EAACI Nomenclature Task Force. Allergy 2001, 56, 813–824. [Google Scholar] [CrossRef]
- Sampath, V.; Abrams, E.M.; Adlou, B.; Akdis, C.; Akdis, M.; Brough, H.A.; Chan, S.; Chatchatee, P.; Chinthrajah, R.S.; Cocco, R.R.; et al. Food Allergy across the Globe. J. Allergy Clin. Immunol. 2021, 148, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Grabenhenrich, L.; Trendelenburg, V.; Bellach, J.; Yürek, S.; Reich, A.; Fiandor, A.; Rivero, D.; Sigurdardottir, S.; Clausen, M.; Papadopoulos, N.G.; et al. Frequency of Food Allergy in School-Aged Children in Eight European Countries-The EuroPrevall-IFAAM Birth Cohort. Allergy 2020, 75, 2294–2308. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, S.T.; Jonasson, K.; Clausen, M.; Lilja Bjornsdottir, K.; Sigurdardottir, S.E.; Roberts, G.; Grimshaw, K.; Papadopoulos, N.G.; Xepapadaki, P.; Fiandor, A.; et al. Prevalence and Early-Life Risk Factors of School-Age Allergic Multimorbidity: The EuroPrevall-IFAAM Birth Cohort. Allergy 2021, 76, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Arasi, S.; Ballmer-Weber, B.; Baseggio Conrado, A.; Deschildre, A.; Gerdts, J.; Halken, S.; Muraro, A.; Patel, N.; Van Ree, R.; et al. Risk Factors for Severe Reactions in Food Allergy: Rapid Evidence Review with Meta-Analysis. Allergy 2022, 77, 2634–2652. [Google Scholar] [CrossRef]
- Okubo, Y.; Nochioka, K.; Testa, M.A. Nationwide Survey of Hospitalization Due to Pediatric Food-Induced Anaphylaxis in the United States. Pediatr. Emerg. Care 2019, 35, 769–773. [Google Scholar] [CrossRef]
- Patriarca, G.; Schiavino, D.; Pecora, V.; Lombardo, C.; Pollastrini, E.; Aruanno, A.; Sabato, V.; Colagiovanni, A.; Rizzi, A.; De Pasquale, T.; et al. Food Allergy and Food Intolerance: Diagnosis and Treatment. Intern. Emerg. Med. 2009, 4, 11–24. [Google Scholar] [CrossRef]
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [Green Version]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI Guideline: Preventing the Development of Food Allergy in Infants and Young Children (2020 Update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef]
- Seth, D.; Poowutikul, P.; Pansare, M.; Kamat, D. Food Allergy: A Review. Pediatr. Ann. 2020, 49, e50–e58. [Google Scholar] [CrossRef] [PubMed]
- Bruton, K.; Koenig, J.F.E.; Phelps, A.; Jordana, M. Perturbations to Homeostasis in Experimental Models Revealed Innate Pathways Driving Food Allergy. Front. Immunol. 2020, 11, 603272. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, U.M.; Layhadi, J.A.; Golebski, K.; István Komlósi, Z.; Peng, Y.; Sekerel, B.; Durham, S.R.; Brough, H.; Morita, H.; Akdis, M.; et al. Innate Lymphoid Cells: The Missing Part of a Puzzle in Food Allergy. Allergy 2021, 76, 2002–2016. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Borcard, L.; Walsh, K.P.; Pena Rodriguez, M.; Mueller, C.; Kim, B.S.; Kubo, M.; Artis, D.; Noti, M. Basophil-Derived IL-4 Promotes Epicutaneous Antigen Sensitization Concomitant with the Development of Food Allergy. J. Allergy Clin. Immunol. 2018, 141, 223–234.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, J.F.E.; Bruton, K.; Phelps, A.; Grydziuszko, E.; Jiménez-Saiz, R.; Jordana, M. Memory Generation and Re-Activation in Food Allergy. ImmunoTargets Ther. 2021, 10, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Wambre, E.; James, E.A.; Kwok, W.W. Characterization of CD4+ T Cell Subsets in Allergy. Curr. Opin. Immunol. 2012, 24, 700–706. [Google Scholar] [CrossRef] [Green Version]
- Bangert, C.; Rindler, K.; Krausgruber, T.; Alkon, N.; Thaler, F.M.; Kurz, H.; Ayub, T.; Demirtas, D.; Fortelny, N.; Vorstandlechner, V.; et al. Persistence of Mature Dendritic Cells, TH2A, and Tc2 Cells Characterize Clinically Resolved Atopic Dermatitis under IL-4Rα Blockade. Sci. Immunol. 2021, 6, eabe2749. [Google Scholar] [CrossRef]
- Jiménez-Saiz, R.; Bruton, K.; Jordana, M. Follicular T Cells: From Stability to Failure. Allergy 2020, 75, 1006–1007. [Google Scholar] [CrossRef]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T Follicular Helper Cell Subset That Drives Anaphylactic IgE. Science 2019, 365, eaaw6433. [Google Scholar] [CrossRef]
- Olivera, A.; Laky, K.; Hogan, S.P.; Frischmeyer-Guerrerio, P. Editorial: Innate Cells in the Pathogenesis of Food Allergy. Front. Immunol. 2021, 12, 709991. [Google Scholar] [CrossRef]
- Neeland, M.R.; Koplin, J.J.; Dang, T.D.; Dharmage, S.C.; Tang, M.L.; Prescott, S.L.; Saffery, R.; Martino, D.J.; Allen, K.J. Early Life Innate Immune Signatures of Persistent Food Allergy. J. Allergy Clin. Immunol. 2018, 142, 857–864.e3. [Google Scholar] [CrossRef] [Green Version]
- Oppenheim, J.J.; Tewary, P.; de la Rosa, G.; Yang, D. Alarmins Initiate Host Defense. Adv. Exp. Med. Biol. 2007, 601, 185–194. [Google Scholar] [CrossRef]
- Yang, D.; Han, Z.; Oppenheim, J.J. Alarmins and Immunity. Immunol. Rev. 2017, 280, 41–56. [Google Scholar] [CrossRef]
- Seong, S.-Y.; Matzinger, P. Hydrophobicity: An Ancient Damage-Associated Molecular Pattern That Initiates Innate Immune Responses. Nat. Rev. Immunol. 2004, 4, 469–478. [Google Scholar] [CrossRef]
- Taverna, S.; Tonacci, A.; Ferraro, M.; Cammarata, G.; Cuttitta, G.; Bucchieri, S.; Pace, E.; Gangemi, S. High Mobility Group Box 1: Biological Functions and Relevance in Oxidative Stress Related Chronic Diseases. Cells 2022, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. Tolerance, Danger, and the Extended Family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Malinczak, C.-A.; Parolia, A.; Fonseca, W.; Morris, S.; Rasky, A.J.; Bawa, P.; Zhang, Y.; Mire, M.M.; Ziegler, S.F.; Ptaschinski, C.; et al. TSLP-Driven Chromatin Remodeling and Trained Systemic Immunity after Neonatal Respiratory Viral Infection. J. Immunol. 2021, 206, 1315–1328. [Google Scholar] [CrossRef] [PubMed]
- Brough, H.A.; Nadeau, K.C.; Sindher, S.B.; Alkotob, S.S.; Chan, S.; Bahnson, H.T.; Leung, D.Y.; Lack, G. Epicutaneous Sensitization in the Development of Food Allergy: What Is the Evidence and How Can This Be Prevented? Allergy 2020, 75, 2185–2205. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, Y.; Jiménez-Saiz, R.; Spill, P.; Chu, D.K.; Waserman, S.; Jordana, M. The Initiation of Th2 Immunity Towards Food Allergens. Int. J. Mol. Sci. 2018, 19, 1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiter, B.; Shreffler, W.G. Innate Immunostimulatory Properties of Allergens and Their Relevance to Food Allergy. Semin. Immunopathol. 2012, 34, 617–632. [Google Scholar] [CrossRef] [Green Version]
- Ruiter, B.; Shreffler, W.G. The Role of Dendritic Cells in Food Allergy. J. Allergy Clin. Immunol. 2012, 129, 921–928. [Google Scholar] [CrossRef]
- Gasper, D.J.; Tejera, M.M.; Suresh, M. CD4 T-Cell Memory Generation and Maintenance. Crit. Rev. Immunol. 2014, 34, 121–146. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Liu, E.; Gertie, J.A.; Joseph, J.; Xu, L.; Pinker, E.Y.; Waizman, D.A.; Catanzaro, J.; Hamza, K.H.; Lahl, K.; et al. Divergent T Follicular Helper Cell Requirement for IgA and IgE Production to Peanut during Allergic Sensitization. Sci. Immunol. 2020, 5, eaay2754. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Charbonnier, L.-M.; Georgiev, P.; Oettgen, H.C.; Rachid, R.; Chatila, T.A. Regulatory T Cell Reprogramming toward a Th2-Cell-like Lineage Impairs Oral Tolerance and Promotes Food Allergy. Immunity 2015, 42, 512–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shik, D.; Tomar, S.; Lee, J.-B.; Chen, C.-Y.; Smith, A.; Wang, Y.-H. IL-9–Producing Cells in the Development of IgE-Mediated Food Allergy. Semin. Immunopathol. 2017, 39, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brough, H.A.; Cousins, D.J.; Munteanu, A.; Wong, Y.F.; Sudra, A.; Makinson, K.; Stephens, A.C.; Arno, M.; Ciortuz, L.; Lack, G.; et al. IL-9 Is a Key Component of Memory TH Cell Peanut-Specific Responses from Children with Peanut Allergy. J. Allergy Clin. Immunol. 2014, 134, 1329–1338.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blázquez, A.B.; Mayer, L.; Berin, M.C. Thymic Stromal Lymphopoietin Is Required for Gastrointestinal Allergy but Not Oral Tolerance. Gastroenterology 2010, 139, 1301–1309.e4. [Google Scholar] [CrossRef]
- Noti, M.; Kim, B.S.; Siracusa, M.C.; Rak, G.D.; Kubo, M.; Moghaddam, A.E.; Sattentau, Q.A.; Comeau, M.R.; Spergel, J.M.; Artis, D. Exposure to Food Allergens through Inflamed Skin Promotes Intestinal Food Allergy through the Thymic Stromal Lymphopoietin–Basophil Axis. J. Allergy Clin. Immunol. 2014, 133, 1390–1399.e6. [Google Scholar] [CrossRef] [Green Version]
- Frossard, C.P.; Zimmerli, S.C.; Rincon Garriz, J.M.; Eigenmann, P.A. Food Allergy in Mice Is Modulated through the Thymic Stromal Lymphopoietin Pathway. Clin. Transl. Allergy 2015, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Bonanno, A.; Gangemi, S.; La Grutta, S.; Malizia, V.; Riccobono, L.; Colombo, P.; Cibella, F.; Profita, M. 25-Hydroxyvitamin D, IL-31, and IL-33 in Children with Allergic Disease of the Airways. Mediat. Inflamm. 2014, 2014, 520241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegra, A.; Murdaca, G.; Gammeri, L.; Ettari, R.; Gangemi, S. Alarmins and MicroRNAs, a New Axis in the Genesis of Respiratory Diseases: Possible Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 1783. [Google Scholar] [CrossRef]
- Galand, C.; Leyva-Castillo, J.M.; Yoon, J.; Han, A.; Lee, M.S.; McKenzie, A.N.J.; Stassen, M.; Oyoshi, M.K.; Finkelman, F.D.; Geha, R.S. IL-33 Promotes Food Anaphylaxis in Epicutaneously Sensitized Mice by Targeting Mast Cells. J. Allergy Clin. Immunol. 2016, 138, 1356–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodoun, M.V.; Tomar, S.; Tocker, J.E.; Wang, Y.H.; Finkelman, F.D. Prevention of Food Allergy Development and Suppression of Established Food Allergy by Neutralization of Thymic Stromal Lymphopoietin, IL-25, and IL-33. J. Allergy Clin. Immunol. 2018, 141, 171–179.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tordesillas, L.; Goswami, R.; Benedé, S.; Grishina, G.; Dunkin, D.; Järvinen, K.M.; Maleki, S.J.; Sampson, H.A.; Berin, M.C. Skin Exposure Promotes a Th2-Dependent Sensitization to Peanut Allergens. J. Clin. Investig. 2014, 124, 4965–4975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Rodríguez, L.; Martínez-Blanco, M.; Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Egg Yolk Augments Type 2 Immunity by Activating Innate Cells. Eur. J. Nutr. 2020, 59, 3245–3256. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Llop-Guevara, A.; Walker, T.D.; Flader, K.; Goncharova, S.; Boudreau, J.E.; Moore, C.L.; In, T.S.; Waserman, S.; Coyle, A.J.; et al. IL-33, but Not Thymic Stromal Lymphopoietin or IL-25, Is Central to Mite and Peanut Allergic Sensitization. J. Allergy Clin. Immunol. 2013, 131, 187–200.e8. [Google Scholar] [CrossRef]
- Han, H.; Thelen, T.D.; Comeau, M.R.; Ziegler, S.F. Thymic Stromal Lymphopoietin–Mediated Epicutaneous Inflammation Promotes Acute Diarrhea and Anaphylaxis. J. Clin. Investig. 2014, 124, 5442–5452. [Google Scholar] [CrossRef] [Green Version]
- Ukleja-Sokołowska, N.; Żbikowska-Gotz, M.; Lis, K.; Adamczak, R.; Bartuzi, Z. Assessment of TSLP, IL 25 and IL 33 in Patients with Shrimp Allergy. Allergy Asthma. Clin. Immunol. 2021, 17, 76. [Google Scholar] [CrossRef]
- Paparo, L.; Picariello, G.; Bruno, C.; Pisapia, L.; Canale, V.; Sarracino, A.; Nocerino, R.; Carucci, L.; Cosenza, L.; Cozzolino, T.; et al. Tolerogenic Effect Elicited by Protein Fraction Derived From Different Formulas for Dietary Treatment of Cow’s Milk Allergy in Human Cells. Front. Immunol. 2020, 11, 604075. [Google Scholar] [CrossRef]
- Ho, H.-E.; Chun, Y.; Jeong, S.; Jumreornvong, O.; Sicherer, S.H.; Bunyavanich, S. Multidimensional Study of the Oral Microbiome, Metabolite, and Immunologic Environment in Peanut Allergy. J. Allergy Clin. Immunol. 2021, 148, 627–632.e3. [Google Scholar] [CrossRef]
- Aalberse, J.A.; van Thuijl, A.O.; Meijer, Y.; de Jager, W.; van der Palen-Merkus, T.; Sprikkelman, A.B.; Hoekstra, M.O.; Prakken, B.J.; van Wijk, F. Plasma IL-25 Is Elevated in a Subgroup of Patients with Clinical Reactivity to Peanut. Clin. Transl. Allergy 2013, 3, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majamaa, H.; Laine, S.; Miettinen, A. Eosinophil Protein X and Eosinophil Cationic Protein as Indicators of Intestinal Inflammation in Infants with Atopic Eczema and Food Allergy. Clin. Exp. Allergy 1999, 29, 1502–1506. [Google Scholar] [CrossRef]
- van Odijk, J.; Peterson, C.G.B.; Ahlstedt, S.; Bengtsson, U.; Borres, M.P.; Hulthén, L.; Magnusson, J.; Hansson, T. Measurements of Eosinophil Activation before and after Food Challenges in Adults with Food Hypersensitivity. Int. Arch. Allergy Immunol. 2006, 140, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Kalach, N.; Kapel, N.; Waligora-Dupriet, A.-J.; Castelain, M.-C.; Cousin, M.O.; Sauvage, C.; Ba, F.; Nicolis, I.; Campeotto, F.; Butel, M.J.; et al. Intestinal Permeability and Fecal Eosinophil-Derived Neurotoxin Are the Best Diagnosis Tools for Digestive Non-IgE-Mediated Cow’s Milk Allergy in Toddlers. Clin. Chem. Lab. Med. 2013, 51, 351–361. [Google Scholar] [CrossRef]
- Winberg, A.; Nagaeva, O.; Nagaev, I.; Lundell, C.; Arencibia, I.; Mincheva-Nilsson, L.; Rönmark, E.; West, C.E. Dynamics of Cytokine MRNA Expression and Fecal Biomarkers in School-Children Undergoing a Double-Blind Placebo-Controlled Food Challenge Series. Cytokine 2016, 88, 259–266. [Google Scholar] [CrossRef]
- Salmivesi, S.; Paassilta, M.; Huhtala, H.; Nieminen, R.; Moilanen, E.; Korppi, M. Elevated Serum Adipsin May Predict Unsuccessful Treatment for Cows’ Milk Allergy but Other Biomarkers Do Not. Acta Paediatr. 2018, 107, 328–332. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, J.; Ma, J.; Sheng, X.; Li, F. Changes in Inflammatory Factors in the Brown Norway Rat Model of Food Allergy. BMC Immunol. 2021, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, F.; Wang, J.; Ma, J.; Sheng, X. Upregulation of Calprotectin in Mild IgE-Mediated Ovalbumin Hypersensitivity. Oncotarget 2017, 8, 37342–37354. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B.; Abbracchio, M.P.; Burnstock, G.; Daly, J.W.; Harden, T.K.; Jacobson, K.A.; Leff, P.; Williams, M. Nomenclature and Classification of Purinoceptors. Pharmacol. Rev. 1994, 46, 143–156. [Google Scholar]
- Burnstock, G.; Campbell, G.; Satchell, D.; Smythe, A. Evidence That Adenosine Triphosphate or a Related Nucleotide Is the Transmitter Substance Released by Non-Adrenergic Inhibitory Nerves in the Gut. Br. J. Pharmacol. 1970, 40, 668–688. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.G.; Mulè, F.; Serio, R. Evidence That ATP or a Related Purine Is an Excitatory Neurotransmitter in the Longitudinal Muscle of Mouse Distal Colon. Br. J. Pharmacol. 2007, 151, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, D.L.; Gruber, H.E.; Wasserman, S.I. Adenosine Release from Stimulated Mast Cells. Proc. Natl. Acad. Sci. USA 1984, 81, 6192–6196. [Google Scholar] [CrossRef] [Green Version]
- Schulman, E.S.; Glaum, M.C.; Post, T.; Wang, Y.; Raible, D.G.; Mohanty, J.; Butterfield, J.H.; Pelleg, A. ATP Modulates Anti-IgE-Induced Release of Histamine from Human Lung Mast Cells. Am. J. Respir. Cell Mol. Biol. 1999, 20, 530–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Y.; Yamamoto, T.; Kadowaki, M. Alteration of Cholinergic, Purinergic and Sensory Neurotransmission in the Mouse Colon of Food Allergy Model. Neurosci. Lett. 2008, 445, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, J.; Ren, F.; Shen, L.; Li, F. Can Fecal Calprotectin Levels Be Used to Monitor Infant Milk Protein Allergies? Allergy Asthma Clin. Immunol. 2021, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Brusca, I.; Mansueto, P.; Soresi, M.; D’Alcamo, A.; Ambrosiano, G.; Pepe, I.; Iacono, G.; Lospalluti, M.L.; La Chiusa, S.M.; et al. Fecal Assays Detect Hypersensitivity to Cow’s Milk Protein and Gluten in Adults with Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2011, 9, 965–971.e3. [Google Scholar] [CrossRef]
- Wang, L.-C.; Liao, L.-X.; Lv, H.-N.; Liu, D.; Dong, W.; Zhu, J.; Chen, J.-F.; Shi, M.-L.; Fu, G.; Song, X.-M.; et al. Highly Selective Activation of Heat Shock Protein 70 by Allosteric Regulation Provides an Insight into Efficient Neuroinflammation Inhibition. EBioMedicine 2017, 23, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-H.; Chou, P.-C.; Chung, F.-T.; Lin, H.-C.; Huang, K.-H.; Kuo, H.-P. Heat Shock Protein70 Is Implicated in Modulating NF-ΚB Activation in Alveolar Macrophages of Patients with Active Pulmonary Tuberculosis. Sci. Rep. 2017, 7, 1214. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Q.; Wawrzyniuk, M.; Rutten, V.P.M.G.; van Eden, W.; Sijts, A.J.A.M.; Broere, F. Hsp70 and NF-KB Mediated Control of Innate Inflammatory Responses in a Canine Macrophage Cell Line. Int. J. Mol. Sci. 2020, 21, 6464. [Google Scholar] [CrossRef]
- Mj, D. Prevalence of Heat Shock Protein in Patients with Meniere’s Disease and Allergy. Otolaryngol. Head Neck Surg. 2002, 126, 677–682. [Google Scholar] [CrossRef]
- Nair, B.; Wheeler, J.C.; Sykes, D.E.; Brown, P.; Reynolds, J.L.; Aalinkeel, R.; Mahajan, S.D.; Schwartz, S.A. Proteomic Approach to Evaluate Mechanisms That Contribute to Food Allergenicity: Comparative 2D-DIGE Analysis of Radioallergosorbent Test Positive and Negative Patients. Int. J. Proteom. 2011, 2011, 673618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Rock, K.L. How Dying Cells Alert the Immune System to Danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef]
- Matzinger, P. The Danger Model: A Renewed Sense of Self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Kouzaki, H.; Kita, H. Human Eosinophils Recognize Endogenous Danger Signal Crystalline Uric Acid and Produce Proinflammatory Cytokines Mediated by Autocrine ATP. J. Immunol. 2010, 184, 6350–6358. [Google Scholar] [CrossRef] [Green Version]
- Willart, M.a.M.; Lambrecht, B.N. The Danger within: Endogenous Danger Signals, Atopy and Asthma. Clin. Exp. Allergy 2009, 39, 12–19. [Google Scholar] [CrossRef]
- Min, K.-B.; Min, J.-Y. Increased Risk for Hyperuricemia in Adults Sensitized to Cow Milk Allergen. Clin. Rheumatol. 2017, 36, 1407–1412. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Szajewska, H.; Lack, G. Food Allergy and the Gut. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 241–257. [Google Scholar] [CrossRef]
- Merras-Salmio, L.; Kolho, K.-L.; Pelkonen, A.S.; Kuitunen, M.; Mäkelä, M.J.; Savilahti, E. Markers of Gut Mucosal Inflammation and Cow’s Milk Specific Immunoglobulins in Non-IgE Cow’s Milk Allergy. Clin. Transl. Allergy 2014, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Wada, H.; Horisawa, T.; Inoue, M.; Yoshida, T.; Toma, T.; Yachie, A. Sequential Measurement of Fecal Parameters in a Case of Non-Immunoglobulin E-Mediated Milk Allergy. Pediatr. Int. 2007, 49, 109–111. [Google Scholar] [CrossRef]
- Beşer, O.F.; Sancak, S.; Erkan, T.; Kutlu, T.; Cokuğraş, H.; Cokuğraş, F.Ç. Can Fecal Calprotectin Level Be Used as a Markers of Inflammation in the Diagnosis and Follow-Up of Cow’s Milk Protein Allergy? Allergy Asthma Immunol. Res. 2014, 6, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca, M.; Donat, E.; Rodriguez Varela, A.; Carvajal, E.; Cano, F.; Armisen, A.; Ekoff, H.; Cañada-Martínez, A.J.; Rydell, N.; Ribes-Koninckx, C. Fecal Calprotectin and Eosinophil-Derived Neurotoxin in Children with Non-IgE-Mediated Cow’s Milk Protein Allergy. J. Clin. Med. 2021, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Guadamuro, L.; Espinosa-Martos, I.; Mancabelli, L.; Jiménez, S.; Molinos-Norniella, C.; Pérez-Solis, D.; Milani, C.; Rodríguez, J.M.; Ventura, M.; et al. Microbiota and Derived Parameters in Fecal Samples of Infants with Non-IgE Cow’s Milk Protein Allergy under a Restricted Diet. Nutrients 2018, 10, 1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trillo Belizón, C.; Ortega Páez, E.; Medina Claros, A.F.; Rodríguez Sánchez, I.; Reina González, A.; Vera Medialdea, R.; Ramón Salguero, J.M. Faecal calprotectin as an aid to the diagnosis of non-IgE mediated cow’s milk protein allergy. An. Pediatría 2016, 84, 318–323. [Google Scholar] [CrossRef]
- Maloney, J.; Nowak-Wegrzyn, A. Educational Clinical Case Series for Pediatric Allergy and Immunology: Allergic Proctocolitis, Food Protein-Induced Enterocolitis Syndrome and Allergic Eosinophilic Gastroenteritis with Protein-Losing Gastroenteropathy as Manifestations of Non-IgE-Mediated Cow’s Milk Allergy. Pediatr. Allergy Immunol. 2007, 18, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Labrosse, R.; Graham, F.; Caubet, J.-C. Non-IgE-Mediated Gastrointestinal Food Allergies in Children: An Update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef]
- Leonard, S.A. Non-IgE-Mediated Adverse Food Reactions. Curr. Allergy Asthma Rep. 2017, 17, 84. [Google Scholar] [CrossRef]
- Meyer, R.; Chebar Lozinsky, A.; Fleischer, D.M.; Vieira, M.C.; Du Toit, G.; Vandenplas, Y.; Dupont, C.; Knibb, R.; Uysal, P.; Cavkaytar, O.; et al. Diagnosis and Management of Non-IgE Gastrointestinal Allergies in Breastfed Infants-An EAACI Position Paper. Allergy 2020, 75, 14–32. [Google Scholar] [CrossRef] [Green Version]
- Mennini, M.; Fiocchi, A.G.; Cafarotti, A.; Montesano, M.; Mauro, A.; Villa, M.P.; Nardo, G.D. Food Protein-Induced Allergic Proctocolitis in Infants: Literature Review and Proposal of a Management Protocol. World Allergy Organ. J. 2020, 13, 100471. [Google Scholar] [CrossRef]
- Phadke, N.A.; Virkud, Y.V.; Martin, V.; Seay, H.L.; Keet, C.; Yuan, Q.; Shreffler, W.G. Food-Protein Induced Allergic Proctocolitis Is Prospectively Associated with IgE-Mediated Milk and Egg Allergies by Age 3. J. Allergy Clin. Immunol. 2019, 143, AB201. [Google Scholar] [CrossRef] [Green Version]
- Rycyk, A.; Cudowska, B.; Lebensztejn, D.M. Eosinophil-Derived Neurotoxin, Tumor Necrosis Factor Alpha, and Calprotectin as Non-Invasive Biomarkers of Food Protein-Induced Allergic Proctocolitis in Infants. J. Clin. Med. 2020, 9, 3147. [Google Scholar] [CrossRef] [PubMed]
- de Boer, J.; Deb, C.; Bornstein, J.; Horvath, K.; Mehta, D.; Smadi, Y. Using Eosinophil Biomarkers From Rectal Epithelial Samples to Diagnose Food Protein-Induced Proctocolitis: A Pilot Study. J. Pediatr. Gastroenterol. Nutr. 2020, 71, e109–e112. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.M.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International Consensus Guidelines for the Diagnosis and Management of Food Protein–Induced Enterocolitis Syndrome: Executive Summary—Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2017, 139, 1111–1126.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, T.; Toma, T.; Muraoka, M.; Matsuda, Y.; Yachie, A. Elevation of Fecal Eosinophil-Derived Neurotoxin in Infants with Food Protein-Induced Enterocolitis Syndrome. Pediatr. Allergy Immunol. 2014, 25, 617–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, T.; Matsuda, Y.; Toma, T.; Koizumi, E.; Okamoto, H.; Yachie, A. Increased CD69 Expression on Peripheral Eosinophils from Patients with Food Protein-Induced Enterocolitis Syndrome. Int. Arch. Allergy Immunol. 2016, 170, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Rank, M.A.; Sharaf, R.N.; Furuta, G.T.; Aceves, S.S.; Greenhawt, M.; Spergel, J.M.; Falck-Ytter, Y.T.; Dellon, E.S.; Chachu, K.A.; Day, L.; et al. Technical Review on the Management of Eosinophilic Esophagitis: A Report from the AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters. Ann. Allergy Asthma Immunol. 2020, 124, 424–440.e17. [Google Scholar] [CrossRef]
- Hirano, I.; Chan, E.S.; Rank, M.A.; Sharaf, R.N.; Stollman, N.H.; Stukus, D.R.; Wang, K.; Greenhawt, M.; Falck-Ytter, Y.T.; AGA Institute Clinical Guidelines Committee; et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Ann. Allergy Asthma Immunol. 2020, 124, 416–423. [Google Scholar] [CrossRef]
- Dhar, A.; Haboubi, H.N.; Attwood, S.E.; Auth, M.K.H.; Dunn, J.M.; Sweis, R.; Morris, D.; Epstein, J.; Novelli, M.R.; Hunter, H.; et al. British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) Joint Consensus Guidelines on the Diagnosis and Management of Eosinophilic Oesophagitis in Children and Adults. Gut 2022, 71, 1459–1487. [Google Scholar] [CrossRef]
- Navarro, P.; Laserna-Mendieta, E.J.; Casabona, S.; Savarino, E.; Pérez-Fernández, M.T.; Ghisa, M.; Pérez-Martínez, I.; Guagnozzi, D.; Perelló, A.; Guardiola-Arévalo, A.; et al. Accurate and Timely Diagnosis of Eosinophilic Esophagitis Improves over Time in Europe. An Analysis of the EoE CONNECT Registry. United Eur. Gastroenterol. J. 2022, 10, 507–517. [Google Scholar] [CrossRef]
- Cianferoni, A.; Spergel, J. Eosinophilic Esophagitis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Venturelli, N.; Lexmond, W.S.; Ohsaki, A.; Nurko, S.; Karasuyama, H.; Fiebiger, E.; Oyoshi, M.K. Allergic Skin Sensitization Promotes Eosinophilic Esophagitis through the IL-33-Basophil Axis in Mice. J. Allergy Clin. Immunol. 2016, 138, 1367–1380.e5. [Google Scholar] [CrossRef] [Green Version]
- Judd, L.M.; Heine, R.G.; Menheniott, T.R.; Buzzelli, J.; O’Brien-Simpson, N.; Pavlic, D.; O’Connor, L.; Al Gazali, K.; Hamilton, O.; Scurr, M.; et al. Elevated IL-33 Expression Is Associated with Pediatric Eosinophilic Esophagitis, and Exogenous IL-33 Promotes Eosinophilic Esophagitis Development in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G13–G25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.; Radonjic-Hösli, S.; Straumann, A.; Yousefi, S.; Simon, H.-U. Active Eosinophilic Esophagitis Is Characterized by Epithelial Barrier Defects and Eosinophil Extracellular Trap Formation. Allergy 2015, 70, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Fahey, L.M.; Chandramouleeswaran, P.M.; Guan, S.; Benitez, A.J.; Furuta, G.T.; Aceves, S.S.; Wang, M.-L.; Liacouras, C.A.; Muir, A.B.; Sleiman, P.M.; et al. Food Allergen Triggers Are Increased in Children with the TSLP Risk Allele and Eosinophilic Esophagitis. Clin. Transl. Gastroenterol. 2018, 9, 139. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Spergel, J.M.; Sherrill, J.D.; Annaiah, K.; Martin, L.J.; Cianferoni, A.; Gober, L.; Kim, C.; Glessner, J.; Frackelton, E.; et al. Common Variants at 5q22 Associate with Pediatric Eosinophilic Esophagitis. Nat. Genet. 2010, 42, 289–291. [Google Scholar] [CrossRef] [Green Version]
- Sherrill, J.D.; Gao, P.-S.; Stucke, E.M.; Blanchard, C.; Collins, M.H.; Putnam, P.E.; Franciosi, J.P.; Kushner, J.P.; Abonia, J.P.; Assa’ad, A.H.; et al. Variants of Thymic Stromal Lymphopoietin and Its Receptor Associate with Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2010, 126, 160–165.e3. [Google Scholar] [CrossRef] [Green Version]
- Uchida, A.M.; Lenehan, P.J.; Vimalathas, P.; Miller, K.C.; Valencia-Yang, M.; Qiang, L.; Canha, L.A.; Ali, L.R.; Dougan, M.; Garber, J.J.; et al. Tissue Eosinophils Express the IL-33 Receptor ST2 and Type 2 Cytokines in Patients with Eosinophilic Esophagitis. Allergy 2022, 77, 656–660. [Google Scholar] [CrossRef]
- Ishihara, S.; Shoda, T.; Ishimura, N.; Ohta, S.; Ono, J.; Azuma, Y.; Okimoto, E.; Izuhara, K.; Nomura, I.; Matsumoto, K.; et al. Serum Biomarkers for the Diagnosis of Eosinophilic Esophagitis and Eosinophilic Gastroenteritis. Intern. Med. 2017, 56, 2819–2825. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, S.; Robinson, Z.D.; Masterson, J.C.; Hosford, L.; Moore, W.; Pan, Z.; Harris, R.; Souza, R.F.; Spechler, S.J.; Fillon, S.A.; et al. Esophageal Human β-Defensin Expression in Eosinophilic Esophagitis. Pediatr. Res. 2013, 73, 647–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzi, A.; Lo Presti, E.; Chini, R.; Gammeri, L.; Inchingolo, R.; Lohmeyer, F.M.; Nucera, E.; Gangemi, S. Emerging Role of Alarmins in Food Allergy: An Update on Pathophysiological Insights, Potential Use as Disease Biomarkers, and Therapeutic Implications. J. Clin. Med. 2023, 12, 2699. https://doi.org/10.3390/jcm12072699
Rizzi A, Lo Presti E, Chini R, Gammeri L, Inchingolo R, Lohmeyer FM, Nucera E, Gangemi S. Emerging Role of Alarmins in Food Allergy: An Update on Pathophysiological Insights, Potential Use as Disease Biomarkers, and Therapeutic Implications. Journal of Clinical Medicine. 2023; 12(7):2699. https://doi.org/10.3390/jcm12072699
Chicago/Turabian StyleRizzi, Angela, Elena Lo Presti, Raffaella Chini, Luca Gammeri, Riccardo Inchingolo, Franziska Michaela Lohmeyer, Eleonora Nucera, and Sebastiano Gangemi. 2023. "Emerging Role of Alarmins in Food Allergy: An Update on Pathophysiological Insights, Potential Use as Disease Biomarkers, and Therapeutic Implications" Journal of Clinical Medicine 12, no. 7: 2699. https://doi.org/10.3390/jcm12072699
APA StyleRizzi, A., Lo Presti, E., Chini, R., Gammeri, L., Inchingolo, R., Lohmeyer, F. M., Nucera, E., & Gangemi, S. (2023). Emerging Role of Alarmins in Food Allergy: An Update on Pathophysiological Insights, Potential Use as Disease Biomarkers, and Therapeutic Implications. Journal of Clinical Medicine, 12(7), 2699. https://doi.org/10.3390/jcm12072699








