A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes
Abstract
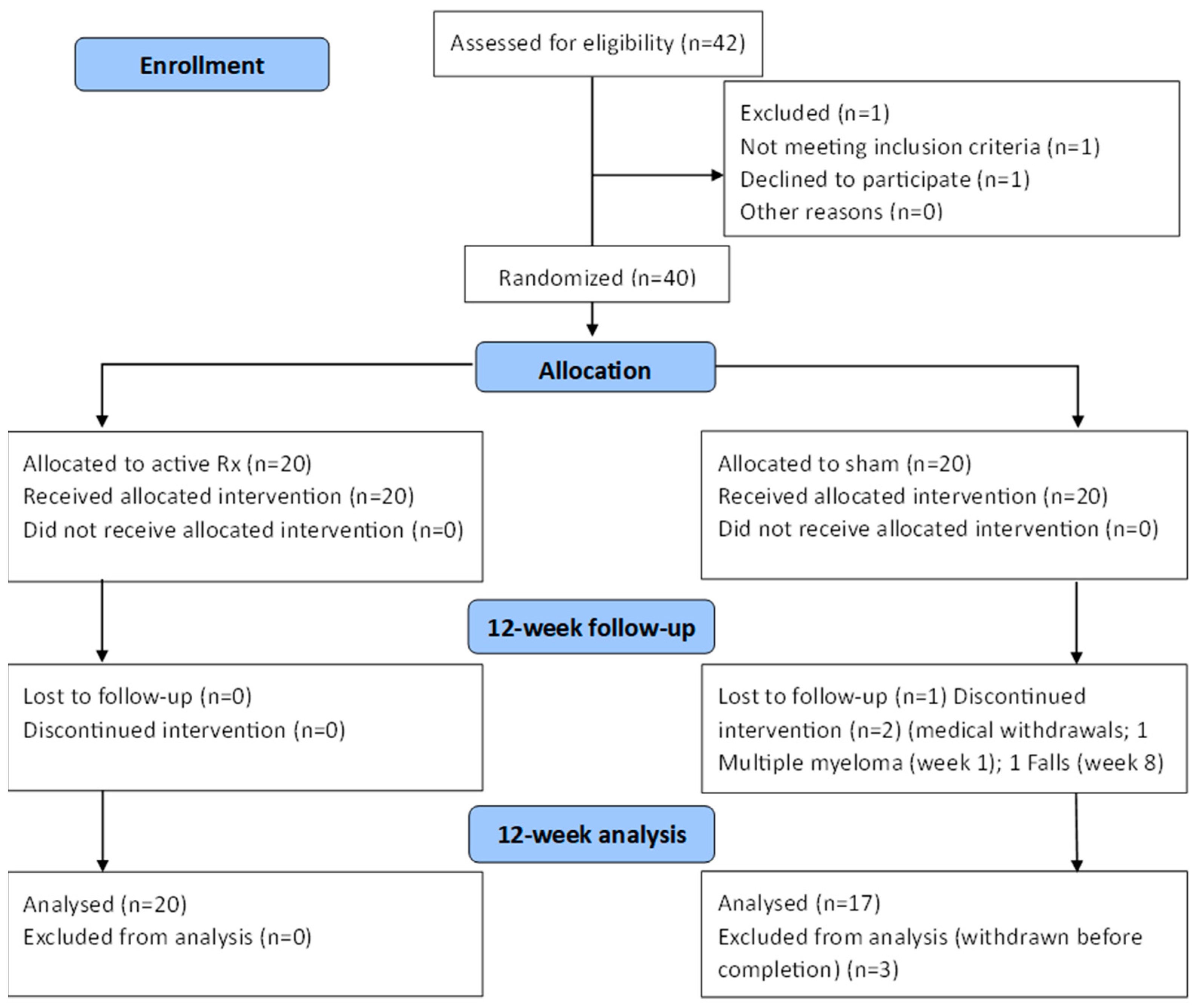
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zeng, X.S.; Geng, W.S.; Jia, J.J.; Chen, L.; Zhang, P.P. Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front. Aging Neurosci. 2018, 10, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocca, W.A. The future burden of Parkinson’s disease. Mov. Dis. 2018, 33, 8–9. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Twelves, D.; Perkins, K.S.M.; Counsell, C. Systematic review of incidence studies of Parkinson’s disease. Mov. Dis. 2003, 18, 19–31. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, X.; Lin, F.; Wu, D.; Chen, L.; Weng, H.; Yu, J.; Wang, Y.; Chen, Y.; Chen, X.; Ye, Q.; et al. Comparative Efficacy and Safety of Dopamine Agonists in Advanced Parkinson’s Disease with Motor Fluctuations: A Systematic Review and Network Meta-Analysis of Double-Blind Randomized Controlled Trials. Front. Neurosci. 2021, 15, PMC8586709. [Google Scholar] [CrossRef]
- Limousin, P.; Foltynie, T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol. 2019, 15, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological Treatment of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Singapore, 2018; pp. 129–144. [Google Scholar] [CrossRef]
- Hill, E.J.; Mangleburg, C.G.; Alfradique-Dunham, I.; Ripperger, B.; Stillwell, A.; Saade, H.; Rao, S.; Fagbongbe, O.; von Coelln, R.; Tarakad, A.; et al. Quantitative mobility measures complement the MDS-UPDRS for characterization of Parkinson’s disease heterogeneity. Park. Relat. Disord. 2021, 84, 105–111. [Google Scholar] [CrossRef]
- Campbell, M.C.; Myers, P.S.; Weigand, A.J.; Foster, E.R.; Cairns, N.J.; Jackson, J.J.; Lessov-Schlaggar, C.N.; Perlmutter, J.S. Parkinson disease clinical subtypes: Key features & clinical milestones. Ann. Clin. Transl. Neurol. 2020, 7, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Salehpour, F. Photobiomodulation of the Brain: Shining Light on Alzheimer’s and Other Neuropathological Diseases. J. Alzheimer’s Dis. 2021, 83, 1395–1397. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers: Vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R.; Liebert, A. Photobiomodulation Therapy Mechanisms Beyond Cytochrome c Oxidase. Photobiomodulation Photomed. Laser Surg. 2022, 40, 75–77. [Google Scholar] [CrossRef]
- Salehpour, F.; Hamblin, M. Photobiomodulation for Parkinson’s Disease in Animal Models: A Systematic Review. Biomolecules 2020, 10, 610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zomorrodi, R.; Loheswaran, G.; Pushparaj, A.; Lim, L. Pulsed Near Infrared Transcranial and Intranasal Photobiomodulation Significantly Modulates Neural Oscillations: A pilot exploratory study. Sci. Rep. 2019, 9, 6309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nizamutdinov, D.; Qi, X.; Berman, M.H.; Dougal, G.; Dayawansa, S.; Wu, E.; Yi, S.S.; Stevens, A.B.; Huang, J.H. Transcranial near infrared light stimulations improve cognition in patients with dementia. Aging Dis. 2021, 12, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Naeser, M.A.; Martin, P.I.; Ho, M.D.; Krengel, M.H.; Bogdanova, Y.; Knight, J.A.; Hamblin, M.R.; Fedoruk, A.E.; Poole, L.G.; Cheng, C.; et al. Transcranial Photobiomodulation Treatment: Significant Improvements in Four Ex-Football Players with Possible Chronic Traumatic Encephalopathy. J. Alzheimer’s Dis. Rep. 2023, 7, 77–105. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B.; Laakso, E.L.; Heller, G.; Jalilitabaei, P.; Tilley, S.; Mitrofanis, J.; Kiat, H. Improvements in clinical signs of Parkinson’s disease using photobiomodulation: A prospective proof-of-concept study. BMC Neurol. 2021, 21, 256. [Google Scholar] [CrossRef] [PubMed]
- McGee, C.; Liebert, A.; Herkes, G.; Bicknell, B.; Pang, V.; McLachlan, C.S.; Kiat, H. Protocol for randomized controlled trial to evaluate the safety and feasibility of a novel helmet to deliver transcranial light emitting diodes photobiomodulation therapy to patients with Parkinson’s disease. Front. Neurosci. 2022, 16, PMCID:PMC9428720. [Google Scholar] [CrossRef]
- Karanicolas, P.J.; Farrokhyar, F.; Bhandari, M. Practical tips for surgical research: Blinding: Who, what, when, why, how? Can. J. Surg. 2010, 53, 345–348. [Google Scholar]
- Tarolli, C.G.; Andrzejewski, K.; Zimmerman, G.A.; Bull, M.; Goldenthal, S.; Auinger, P.; O’Brien, M.; Dorsey, E.; Biglan, K.; Simuni, T. Feasibility, Reliability, and Value of Remote Video-Based Trial Visits in Parkinson’s Disease. J. Parkinsons. Dis. 2020, 10, 1779–1786. [Google Scholar] [CrossRef]
- Stillerova, T.; Liddle, J.; Gustafsson, L.; Lamont, R.; Silburn, P. Remotely Assessing Symptoms of Parkinson’s Disease Using Videoconferencing: A Feasibility Study. Neurol. Res. Int. 2016, 2016, 4802570. [Google Scholar] [CrossRef]
- Abdolahi, A.; Scoglio, N.; Killoran, A.; Dorsey, E.R.; Biglan, K.M. Potential reliability and validity of a modified version of the Unified Parkinson’s Disease Rating Scale that could be administered remotely. Park. Relat. Disord. 2013, 19, 218–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulman, L.M.; Gruber-Baldini, A.L.; Anderson, K.E.; Fishman, P.S.; Reich, S.G.; Weiner, W.J. The Clinically Important Difference on the Unified Parkinson’s Disease Rating Scale. Arch. Neurol. 2010, 67, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, C.G.; Wuu, J.; McDermott, M.P.; Adler, C.H.; Fahn, S.; Freed, C.R.; Hauser, R.A.; Olanow, W.C.; Shoulson, I.; Tandon, P.K.; et al. Placebo response in Parkinson’s disease: Comparisons among 11 trials covering medical and surgical interventions. Mov. Dis. 2008, 23, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Lidstone, S.C.; Schulzer, M.; Dinelle, K.; Mak, E.; Sossi, V.; Ruth, T.J.; de la Fuente-Fernández, R.; Phillips, A.G.; Stoessl, A.J. Effects of Expectation on Placebo-Induced Dopamine Release in Parkinson Disease. Arch. Gen. Psychiatry 2010, 67, 857. [Google Scholar] [CrossRef] [Green Version]
- Lidstone, S.C. Great Expectations: The Placebo Effect in Parkinson’s Disease. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 139–147. [Google Scholar]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Dis. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Athauda, D.; Maclagan, K.; Budnik, N.; Zampedri, L.; Hibbert, S.; Aviles-Olmos, I.; Chowdhury, K.; Skene, S.S.; Limousin, P.; Foltynie, T. Post hoc analysis of the Exenatide-PD trial-Factors that predict response. Eur. J. Neurosci. 2019, 49, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Paparella, G.; Fasano, A.; Hallett, M.; Berardelli, A. Evolving concepts on bradykinesia. Brain 2020, 143, 727–750. [Google Scholar] [CrossRef]
- Askari, A.; Zhu, B.J.; Lyu, X.; Chou, K.L.; Patil, P.G. Characterization and localization of upper and lower extremity motor improvements in STN DBS for Parkinson’s disease. Park. Rela Disord. 2022, 94, 84–88. [Google Scholar] [CrossRef]
- Regnault, A.; Boroojerdi, B.; Meunier, J.; Bani, M.; Morel, T.; Cano, S. Does the MDS-UPDRS provide the precision to assess progression in early Parkinson’s disease? Learnings from the Parkinson’s progression marker initiative cohort. J. Neurol. 2019, 266, 1927–1936. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, R.; Tiwari, P.; Pahuja, M.; Dada, R.; Tripathi, M. Anti-seizure medications and quality of life in person with epilepsy. Heliyon 2022, 8, e11073. [Google Scholar] [CrossRef] [PubMed]
- Stubendorff, K.; Larsson, V.; Ballard, C.; Minthon, L.; Aarsland, D.; Londos, E. Treatment effect of memantine on survival in dementia with Lewy bodies and Parkinson’s disease with dementia: A prospective study. BMJ Open 2014, 4, e005158. [Google Scholar] [CrossRef] [PubMed]
- Standaert, D.G.; Boyd, J.T.; Odin, P.; Robieson, W.Z.; Zamudio, J.; Chatamra, K. Systematic evaluation of levodopa-carbidopa intestinal gel patient-responder characteristics. NPJ Park. Dis. 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Group | Baseline Mean (SD) | 12-Week Mean (SD) | Mean Difference | Paired t-Test | |||
|---|---|---|---|---|---|---|---|
| Mean % Improvement | T Score | p Value | |||||
| UPDRS scores for all participants (df: active = 19; sham = 17) | |||||||
| Total score | Active | 21.35 (9.43) | 16.45 (9.48) | −4.90 (7.67) | 23% | 2.84 | 0.010 * |
| Sham | 26.00 (13.81) | 20.47 (12.83) | −5.52 (7.93) | 21% | 2.85 | 0.011 * | |
| Facial | Active | 2.26 (1.44) | 1.73 (1.66) | −0.53 (0.77) | 23% | 2.92 | 0.008 * |
| Sham | 2.24 (1.44) | 1.88 (1.49) | −0.36 (0.93) | 16% | 1.56 | 0.138 | |
| Upper limb | Active | 6.63 (3.53) | 4.84 (3.82) | −1.79 (3.88) | 27% | 1.84 | 0.060 |
| Sham | 7.24 (4.68) | 6.59 (4.87) | −0.64 (3.37) | 9% | 0.79 | 0.440 | |
| Lower limb | Active | 4.26 (2.51) | 2.47 (2.38) | −2.26 (2.62) | 53% | 2.61 | 0.017 * |
| Sham | 6.24 (3.68) | 3.88 (2.29) | −2.36 (3.16) | 38% | 3.04 | 0.007 * | |
| Gait | Active | 3.37 (1.54) | 2.79 (1.87) | −0.58 (1.46) | 17% | 1.87 | 0.102 |
| Sham | 5.00 (2.80) | 3.65 (2.85) | −1.35 (2.57) | 27% | 2.16 | 0.046 * | |
| Tremor | Active | 4.84 (3.48) | 4.11 (2.96) | −0.74 (2.58) | 15% | 0.51 | 0.229 |
| Sham | 5.29 (5.59) | 4.47 (4.45) | −0.82 (3.6) | 16% | 0.93 | 0.361 | |
| UPDRS scores for responders (df: active = 13; sham = 9) | |||||||
| Total | Active | 22.86 (10.39) | 14.57 (8.87) | −8.29 (5.17) | 36% | 6.00 | <0.001 * |
| score | Sham | 29.80 10.39) | 18.80 (14.31) | −11.00 (2.98) | 37% | 11.67 | <0.001 * |
| Facial | Active | 2.07 (1.38) | 1.50 (1.51) | −0.57 (0.76) | 28% | 2.83 | 0.014 * |
| Sham | 2.10 (1.52) | 1.50 (1.43) | −0.60 (0.97) | 29% | 1.97 | 0.081 | |
| Upper Limb | Active | 7.07 (3.73) | 4.29 (3.58) | −2.79 (3.89) | 40% | 2.68 | 0.019 * |
| Sham | 8.30 (5.31) | 6.30 (5.25) | −2.00 (2.91) | 24% | 2.18 | 0.058 | |
| Lower Limb | Active | 4.29 (2.73) | 1.79 (2.12) | −2.50 (2.41) | 58% | 3.88 | 0.002 * |
| Sham | 7.60 (3.57) | 3.70 (2.41) | −3.90 (2.57) | 51% | 4.82 | 0.001 * | |
| Gait | Active | 3.57 (1.40) | 2.57 (1.79) | −1.00 (1.24) | 28% | 3.01 | 0.010 * |
| Sham | 5.60 (2.99) | 3.60 (2.91 | −2.00 (2.98) | 36% | 2.12 | 0.063 | |
| Tremor | Active | 5.86 (3.39) | 4.43 (3.03) | −1.43 (2.34) | 24% | 2.28 | 0.040 * |
| Sham | 6.20 (6.51) | 3.70 (4.53) | −2.50 (3.63) | 40% | 2.18 | 0.057 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGee, C.; Liebert, A.; Bicknell, B.; Pang, V.; Isaac, V.; McLachlan, C.S.; Kiat, H.; Herkes, G. A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes. J. Clin. Med. 2023, 12, 2846. https://doi.org/10.3390/jcm12082846
McGee C, Liebert A, Bicknell B, Pang V, Isaac V, McLachlan CS, Kiat H, Herkes G. A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes. Journal of Clinical Medicine. 2023; 12(8):2846. https://doi.org/10.3390/jcm12082846
Chicago/Turabian StyleMcGee, Claire, Ann Liebert, Brian Bicknell, Vincent Pang, Vivian Isaac, Craig S. McLachlan, Hosen Kiat, and Geoffrey Herkes. 2023. "A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes" Journal of Clinical Medicine 12, no. 8: 2846. https://doi.org/10.3390/jcm12082846
APA StyleMcGee, C., Liebert, A., Bicknell, B., Pang, V., Isaac, V., McLachlan, C. S., Kiat, H., & Herkes, G. (2023). A Randomized Placebo-Controlled Study of a Transcranial Photobiomodulation Helmet in Parkinson’s Disease: Post-Hoc Analysis of Motor Outcomes. Journal of Clinical Medicine, 12(8), 2846. https://doi.org/10.3390/jcm12082846








