Abstract
(1) Background: Allergic rhinitis (AR) is a common disease in otolaryngology and novel biological therapies are required for clinical needs. To assess the tolerability of monoclonal antibodies, justifying their clinical applications, we presented a comprehensive safety profile of biologics in AR; (2) Methods: A systematic literature search was conducted following PRISMA guidelines for randomized clinical trials comparing monoclonal antibodies and placebo in AR. PubMed, Web of Science, Medline, and Cochrane were searched up until 9 January 2023. Among 3590 records in total, 12 studies with more than 2600 patients were included. Quality was assessed for all studies using Cochrane risk-of-bias tool for randomized trials, and subgrouped meta-analysis was performed; (3) Results: We accomplished an up-to-date literature overview and analysis on adverse events of monoclonal antibodies in AR. Total, common, severe, discontinuation-causing, and serious adverse events failed to reach statistical significance. Country was an essential factor for heterogeneity, and urticaria was the adverse event at highest risk (RR 2.81, 95% CI 0.79–9.95); (4) Conclusions: Monoclonal antibodies are considered well tolerated and relatively safe in patients with AR. The regions of patients and hypersensitive adverse reactions such as urticaria require a special caution in biological treatments in AR.
1. Introduction
Allergic rhinitis (AR) is one of the most common diseases in otolaryngology, affecting about 10–40% population worldwide [1], and its prevalence rate has increased progressively, especially in developed countries [2]. Classic symptoms of AR include nasal itching, sneezing, rhinorrhea, and nasal congestion. Additionally, ocular symptoms such as allergic rhinoconjunctivitis are common, causing itching and redness of the eyes and tearing, and other symptoms are itching of the palate, postnasal drip, and cough [3]. AR was traditionally subdivided into seasonal, perennial, and occupational rhinitis. Seasonal allergic rhinitis (SAR) is frequently induced by outdoor allergens such as pollens or molds, while perennial allergic rhinitis (PAR) is most often induced by indoor allergens such as dust mites, animal dander, molds, and cockroaches. Current classification is based on the severity of symptoms, including sleep disturbance, impairment of daily activities, leisure and/or sport, impairment of school or work, and troublesome symptoms. “Mild” indicates no mentioned symptoms, and “moderate-severe” means at least one symptom is present [3]. There are various traditional treatments of AR, including education, allergen avoidance, pharmacotherapy such as antihistamines and corticosteroids, and allergen-specific immunotherapy (AIT) [4,5]. Given traditional treatment regimens are not always efficacious, there is certainly a role for newer therapies, such as monoclonal antibodies (mAbs) [6]. As the progress of researches on the immunopathogenic mechanisms of AR, mAbs blocking essential disease-causing factors are proven to have promising therapeutic effects [7,8,9,10,11,12]. However, the overall safety of mAbs is still in question, with a poorly understanding of the underlying mechanisms of many mAb-related adverse reactions [13]. According to the FDA label of omalizumab (XOLAIR), common side effects include injection site reaction (45%), viral infections (23%), upper respiratory tract infection (20%), sinusitis (16%), headache (15%), and pharyngitis (11%); anaphylaxis and malignancies are the most serious adverse reactions occurring in clinical trials [14]. Besides, injection site reactions are also major adverse events in many other biological products. For example, a meta-analysis demonstrated that dupilumab increased the risk of injection site reactions in patients with allergic diseases [15]. Safety assessment on novel biological therapies is a crucial aspect and one of the fundamental criteria, as much as efficacy, for the justification of their clinical applications [16]. Therefore, a comprehensive safety profile of biologics in AR is certainly needed before widespread use.
In this systematic review, we aimed to exhaustively search and summarize studies investigating monoclonal antibody treatments and their adverse events in AR patients. In the following step, meta-analysis was conducted to statistically analyze the risk of total, common, severe, withdrawal-causing, and serious adverse events and assess the safety of mAb treatments in patients with AR from different aspects. It allowed us to summarize the current knowledge of adverse events in mAb RCTs and perform a comprehensive safety assessment to specify tolerability information on monoclonal antibody therapies in AR for novel clinical drug selections in the future.
2. Methods
2.1. Search Strategy
We searched PubMed, Web of Science, Medline and the Cochrane Central Register of Controlled Trials databases from inception to 9 January 2023 without language restrictions. Combinations of AR-related terms (“allergic rhinitis”, “rhinitis”, “rhino conjunctivitis”, “nasal allergy”, or “hay fever”), mAb-related terms (“monoclonal antibodies”, “humanized”, “anti-IgE”, “omalizumab”, “anti-IL4”, “anti-IL13”, “anti-IL4Ra”, or “dupilumab”) and therapy-related terms (“therapy”, “treatment”, or “management”) were used when screening titles/abstracts/keywords of articles. We also manually searched reference lists and similar articles for additional relevant studies. Randomized controlled trials (RCTs) reporting the efficacy and safety of mAbs for the treatment of allergic rhinitis against placebo were retrieved for a full-text review and assessed for eligibility. For missing, unclear, or incomplete results, we contacted researchers for clarification before exclusion. Studies that met the inclusion criteria were included for further analysis.
2.2. Selection Criteria
To reduce selection bias, two reviewers independently assessed each study and disagreements were resolved by consensus with a third reviewer, if necessary. The selection criteria were set prior to the literature search process. Studies that met the following criteria were eligible for inclusion: (1) the study population comprised allergic rhinitis patients of any age groups, confirmed with a physician diagnosis and evidence of clinically relevant allergic sensitization; (2) RCTs comparing the use of monoclonal antibodies therapy with placebo; (3) safety assessment was accomplished by reporting adverse events.
Studies were excluded if: (1) allergic rhinitis was treated as a clinical manifestation or optional comorbid of other diseases; (2) the study design didn’t follow RCTs; (3) patients were treated with any mAbs in the past 12 months before studies started; (4) the control group received treatment other than placebo; (5) the treatment group received mAbs along with any other treatments instead of mAbs alone.
2.3. Data Extraction and Analysis
Two investigators independently read through the included studies and recorded basic information such as first author and year of publishment. We also collected information on characteristics of the included RCTs, including countries where trials were conducted, sample size, monoclonal antibody given to the treatment group, intervention scheme, and follow-up time. Moreover, patients’ baseline demographics in the included studies were collected, including age (range and mean), sex (male/female), race (White/Black/Asian/others), weight (range and mean), type of AR (SAR/PAR), serum IgE (mean), and history of asthma and atopic dermatitis. Most importantly, for all included RCTs, we collected safety information about the frequency and detailed clinical manifestations of adverse events.
Meta-analysis was conducted through RStudio using Mantel-Haenszel method. Heterogeneity was evaluated according to heterogeneity test and I2 statistic. A p-value < 0.1 was considered as a significant heterogeneity. Meanwhile, I2 of 25%, 50%, and 75% represented a low, medium, and high heterogeneity, respectively. For I2 < 50%, a fixed-effects model was used for meta-analysis. However, if I2 > 50%, indicative of a high heterogeneity, a random-effects model should be used to maintain conservative. Lastly, we used RStudio to conduct subgroup analysis and present forest plots.
2.4. Quality Assessment
Two investigators independently conducted the quality assessment for each included study using Cochrane risk-of-bias tool for randomized trials (RoB 2). We assessed 6 domains representing the selection, performance, detection, attrition, and reporting bias, and evaluated the risk as 3 levels: low, unclear, and high [17]. The study was identified to be at low risk only if all 6 domains were at low risk. If at least 1 domain was at high risk, the overall risk level was high. If none of the domain was at high risk, but at least 1 domain had unclear risk, we considered the study as unclear risk. Furthermore, publication bias was assessed using funnel plots.
3. Results
3.1. Study Selection, Characteristics, and Quality
The systematic search resulted in 3590 records in total from 4 sources. After 1373 duplicates were identified using Mendeley Desktop (version 1.19.8) and removed manually, 2217 records were roughly screened using title and abstract for clinal trials meeting the inclusion criteria. Out of these, 263 full-text records were assessed for eligibility, and 12 studies were included in the following systematic review. The process of literature search and study selection until the final decision on included studies was presented in the PRISMA flow chart in Figure 1.
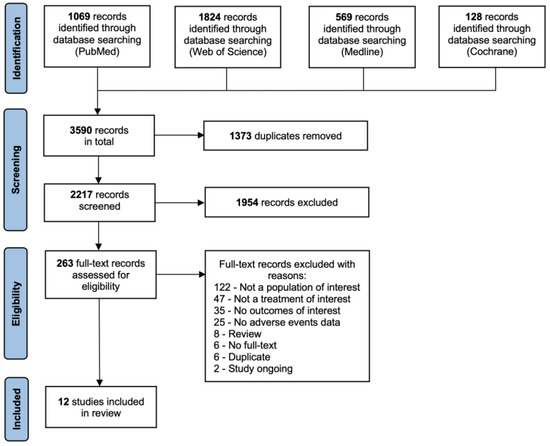
Figure 1.
PRISMA flow chart for the process of study selection and inclusion [18].
Among the included studies, 11 of them were published in academic journals from 1997 to 2022, and 1 presented data on governmental website for clinical trials (Identifier: NCT04709575) with no official publications. There were 6 trials conducted in only one country: 3 in the USA, 1 in Japan, 1 in Canada, and 1 in Belgium. The other 6 trials were multinational, including countries in North America, South America, Europe, Asia-Pacific, and Africa. The total sample size of included studies varied between 36 and 536 patients, while the experimental sample size varied from 24 to 400. For the included 12 trials, 7 of them investigated the safety of anti-IgE monoclonal antibodies, 2 investigated dupilumab, an anti-IL-4R mAb, 1 investigated REGN1908-1909, an anti-Fel d 1 mAb, and 2 investigated REGN5713/14/15, an anti-Bet v 1 mAb. For the anti-IgE mAbs, 2 used rhuMAb-E25, the former name of omalizumab, 4 used omalizumab, and 1 used quilizumab. Additionally, the follow-up time ranged from 8 to 28 weeks, and detailed intervention strategies were shown in Table 1. Moreover, Table 2 summarized the baseline demographics of 2630 included patients, of which 1598 belonged to the treatment group. All included studies recruited adults and patients’ mean ages were greater than 30, while 4 studies also recruited patients younger than 18 years old. All the studies recruited both male and female patients. 7 studies reported the detailed race composition of included patients and 6 studies provided information on weight, either range or mean. Among all 12 studies, 7 of them recruited SAR patients, 4 recruited PAR patients, and 1 recruited both. The SAR patients were induced by ragweed, birch pollen, Japanese cedar pollen, and grass pollen and 1 PAR study chose cat-induced PAR patients. 7 studies examined total serum IgE and reported the mean. There were 8 studies that recorded the asthma history of patients, of which 1 study excluded patients with asthma history, and 2 studies included patients with comorbid asthma history. Only 4 studies reported patients’ history of atopic dermatitis. Further information on other comorbid atopic diseases were not available.

Table 1.
Characteristics of included randomized controlled trials (RCTs).

Table 2.
Baseline demographics of included patients.
The quality assessment results were presented using a traffic light plot in Figure 2. According to the assessment, 2 studies were at low risk, 5 studies were at unclear risk, and 5 studies had a high risk of bias. Most of the unclear risk came from the random sequence generation process, leading to a selection bias. The highest risk of bias among the different studies was noted in the domain of incomplete outcome data, causing an attrition bias.
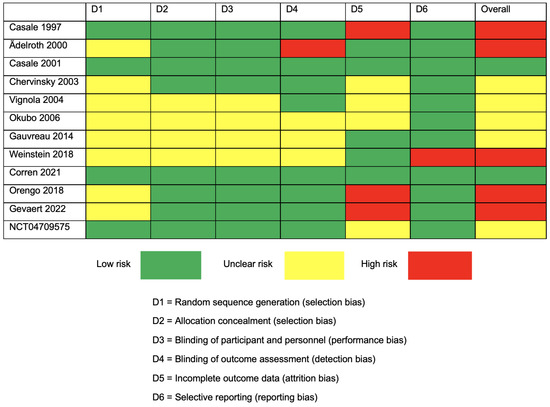
Figure 2.
Traffic light plot for Cochrane Collaboration’s proposal for the assessment of the risk of bias (RoB 2) for RCTs [19,20,21,22,23,24,25,26,27,28,29,30].
3.2. Evidence for Adverse Events
Adverse events reported in all 12 including RCTs were summarized in Table 3. Among them, 10 studies provided data about total subjects experiencing adverse events, in the treatment and control group, respectively. All studies documented the frequently reported adverse events and their frequencies. Researchers individually evaluated the severity of adverse events as 3 levels: mild, moderate, and severe. “Severe” adverse events were defined to cause severe discomfort and limited normal function, leading to a significant change from baseline and definite damage to health, or probably required a prolonged hospitalization [19]. We specifically noted adverse events leading to withdrawal of the study and serious adverse events (as opposed to severe).

Table 3.
Adverse events among the included studies.
3.2.1. Anti-IgE mAbs
Adverse events were reported in 6 omalizumab [19,20,21,22,23,24] and 1 quilizumab [25] trials. Except for 2 studies with no data reported, 373 out of 590 (63.2%) mAb-treated patients and 309 out of 489 (63.2%) placebo-treated patients experienced any adverse events during the trials. Frequently reported adverse events of omalizumab were infections, including upper respiratory infections, viral infections or influenza, nasopharyngitis, pharyngitis, and sinusitis; neurological symptoms, including headache, sinus headache, and special senses; respiratory symptoms, including coughing, worsen asthma, and sore throat; gastrointestinal symptoms, including nausea, colitis ulcerative, and diarrhea; musculoskeletal symptoms, including arthralgia, back pain, and sprains and strains; general and administration site reactions, including injection site reactions and urticaria; and systemic reactions, such as weight gain and skin and subcutaneous tissue disorders. The occurrence rates of severe, discontinuation-causing, and serious adverse events were 2.5%, 0.72%, and 8% for omalizumab-treated patients, and 5.4%, 0.49%, and 9.3% for placebo-treated patients, respectively. The study on quilizumab mainly reported infectious adverse events, including upper respiratory tract infections (29.2% in treatment group, and 16.7% in control group) and 1 severe gastroenteritis in the control group.
3.2.2. Anti-IL-4R mAb
For 2 included dupilumab trials, a total of 292 patients were included [26,27]. 77.6% dupilumab-treated patients and 74.3% placebo-treated patients experienced adverse events. Both studies reported injection site reactions, and 1 study also reported respiratory tract infections, neurological symptoms, and nasal congestions. There were no substantial differences between the 2 groups for severe and serious adverse events. However, patients in the treatment group had a higher possibility to discontinue the study due to adverse events than the control group (4.37% and 0.92%, respectively).
3.2.3. Anti-Fel d 1 mAb
There was only 1 trial reporting adverse events comparing the safety of REGN1908-1909 treatment and placebo [28]. The occurrence rates of adverse events were similar in 2 groups (63.9% REGN1908-1909-treated patients and 62.2% placebo-treated patients), and none of them withdrew the trial because of adverse events. Frequently reported adverse events included infections, neurological symptoms, gastrointestinal symptoms, and injection site reactions, and each group reported 1 serious adverse event.
3.2.4. Anti-Bet v 1 mAb
Two studies investigated the safety of REGN5713/14/15 [29,30], and a total of 413 patients were recruited. Overall, 118 out of 205 (57.6%) REGN5713/14/15-treated patients and 115 out of 208 (55.3%) placebo-treated patients suffered from at least 1 adverse event, showing no significant difference. Among them, 1 patient in the treatment group withdrew from the study, and 3 serious adverse events happened in the treatment group, with an occurrence rate of 0.73%. Frequently reported adverse events included headache, nasopharyngitis, and vaccination complication such as injection site reactions and hypersensitivity.
3.3. Meta-Analysis Results
Ten RCTs (n = 1857) informed the total subjects experiencing adverse events, and the test for heterogeneity indicated a medium heterogeneity (I2 = 49.7%, p = 0.0365). Subgroup analysis was conducted to detect the source of heterogeneity, and we found that country played a significant role (I2 = 0%, p = 0.5, Figure 3), but subgrouping based on type of AR, age group of patients, and type of mAb didn’t reduce heterogeneity (Figures S1–S3). However, neither the subgroup analysis nor the overall random effects model (RR 1.02, 95% CI 0.93–1.12, Figure 3) showed significant differences in experiencing adverse reactions between the mAb-treatment and placebo group. There was no strong evidence of publication bias.
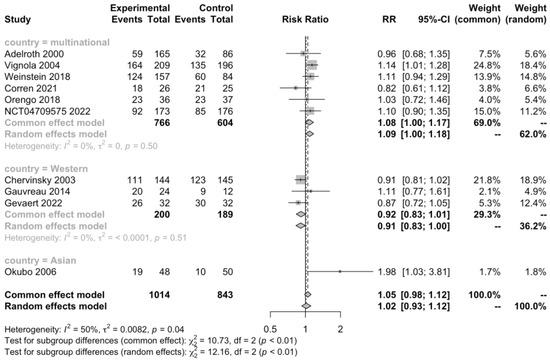
Figure 3.
Forest plot of RCTs comparing patients experiencing adverse events subgrouped by country [20,22,23,24,25,26,27,28,29,30].
Regarding common adverse events, the risk of experiencing headache (RR 0.88, 95% CI 0.73–1.06, Figure S4), upper respiratory infection (RR 1.05, 95% CI 0.76–1.47, Figure S5), viral infection (RR 0.9, 95% CI 0.59–1.36, Figure S6), pharyngitis (RR 1.12, 95% CI 0.69–1.81, Figure S7), nasopharyngitis (RR 0.92, 95% CI 0.72–1.17, Figure S8), injection site reactions (RR 1.21, 95% CI 0.85–1.73, Figure S9), and urticaria (RR 2.81, 95% CI 0.79–9.95, Figure 4) were not significantly different between patients treated with monoclonal antibodies and placebo. Among them, pharyngitis and urticaria occurred only in omalizumab trials, and it was reasonable to speculate that patients treated with omalizumab might have an increased risk to experience urticaria though it failed to reach statistically significant. There was no strong evidence of heterogeneity.
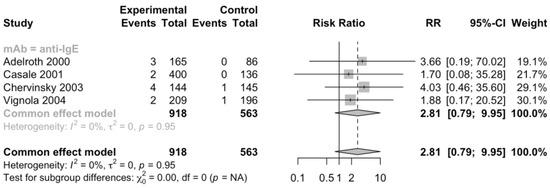
Figure 4.
Forest plot of RCTs comparing rates of patients with urticaria subgrouped by type of mAb [20,21,22,23].
Additionally, 5 RCTs (n = 1092) informed severe adverse events, 11 RCTs (n = 2393) informed adverse events leading to discontinuation, and 11 RCTs (n = 2097) informed serious adverse events (Figure 5, Figure 6 and Figure 7). No heterogeneity was detected. The risk ratio of severe adverse events was 0.68, with a 95% CI of 0.36–1.27, indicating that treatment with mAbs reduced 38% risk of severe adverse events. In contrast, the risk ratio of adverse events leading to discontinuation was 2.32 and the 95% CI was 0.87–6.18, which showed that patients treated with mAbs might have a higher possibility to discontinue due to adverse events. However, both the severe and withdrawal-causing adverse events failed to reach statistically significant, and there was also no significant difference in the risk of serious adverse events (RR 1.13, 95% CI 0.57–2.21).
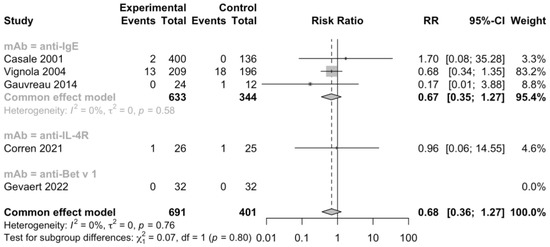
Figure 5.
Forest plot of RCTs comparing severe adverse events subgrouped by type of mAb [21,23,25,27,29].
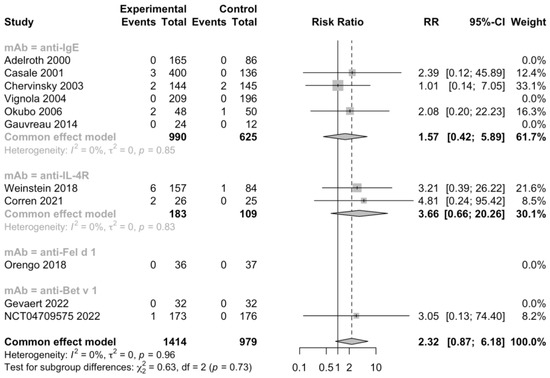
Figure 6.
Forest plot of RCTs comparing withdrawal-causing adverse events subgrouped by type of mAb [20,21,22,23,24,25,26,27,28,29,30].
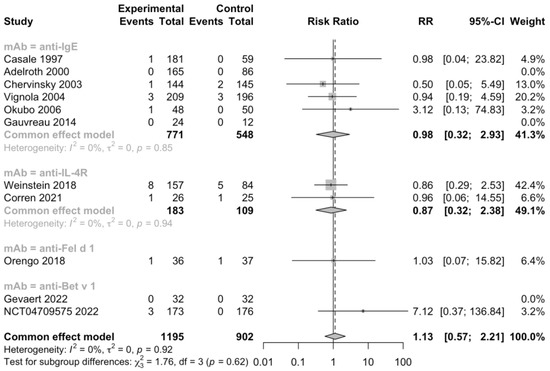
Figure 7.
Forest plot of RCTs comparing serious adverse events subgrouped by type of mAb [19,20,22,23,24,25,26,27,28,29,30].
4. Discussion
This systematic review and meta-analysis of more than 2600 patients with AR in 12 RCTs assessed the safety of 5 monoclonal antibodies, including 2 anti-IgE mAbs omalizumab and quilizumab, 1 Anti-IL-4R mAb dupilumab, 1 Anti-Fel d 1 mAb REGN1908-1909, and 1 Anti-Bet v 1 mAb REGN5713/14/15, and provided comprehensive evidence on comparing the incidence of adverse events of treatment with mAbs and placebo. According to the pathogenesis of AR, type 2 immunity plays an important role [4]. Thus, type 2 cytokines, such as IL-4, IL-5, and IL-13, are promising therapeutical targets for AR and also other allergic diseases, such as asthma and atopic dermatitis [7,9]. Unfortunately, according to our literature review, there were currently no RCTs investigating the safety of anti-IL-5/5Ra mAbs including mepolizumab, reslizumab and benralizumab, or anti-IL-13 mAbs including lebrikizumab and tralokinumab in AR. Further research on the tolerability of other types of mAbs should be conducted before more biological therapies are introduced to AR patients.
Our results demonstrated that total, serious, and common adverse events such as headache, upper respiratory infection, viral infection, pharyngitis, nasopharyngitis, and injection site reactions occurred with a similar incidence rate in the mAb and placebo groups, indicating the acceptable safety and tolerability of biologics in patients with AR in general. Our result was similar to the conclusions of other meta-analysis researches on the safety of biological treatments in AR, especially omalizumab [8,31]. We also found that countries where studies were conducted brought up a medium heterogeneity, and subgrouping the studies into Western, Asian, and multinational could eliminate heterogeneity, which indicated that the region of research might influence the incidence of adverse events. Region could not only reflect the racial compositions of participants to some extent, but also give a hint of the living environments and lifestyles of patients, which was proved to be essential factors causing AR, and even many other allergic diseases [32,33,34,35]. As AR has become a global health issue, the promotion of biological treatments worldwide required special attention on adverse events among patients in different areas with different ethnicities. According to ICH E5, frameworks were needed to register and review the global trials and decide the acceptability of foreign clinical data across different races [36]. Some studies found that the efficacy and safety of biological treatments varied in different populations. For example, a cohort study showed different rates of response and adverse effects to biological therapies between South Asian IBD patients, mainly migrants, and Caucasian American [37]. However, there was currently no information comparing tolerability differences of AR patients across regions. Therefore, future studies must address this issue to guide the clinical use of monoclonal antibodies in AR patients from different countries.
Furthermore, we suspected urticaria to be an adverse event at high risk for treatment with omalizumab, although the data failed to reach statistical significance. Systemic hypersensitivity reactions was found to be associated with multiple biological treatments such as omalizumab, dupilumab, and benralizumab [38,39]. In spite of some evidence showing that hypersensitivity reactions including urticaria, skin rush, and anaphylaxis had a low incidence rate and no significant difference between omalizumab and placebo-treated patients [40], anaphylaxis has been proved to be related to omalizumab since its initial FDA approval for allergic asthma in 2003 and the product label even includes a black box warning [41,42]. In the future, more investigations should evaluate the hypersensitive adverse reactions of biological therapies on allergic patients, especially AR. For novel monoclonal antibodies targeting allergic proteins, such as REGN1908-1909 which targets Fel d 1, the dominant allergen in cat dander triggering AR and asthma symptoms [43], and REGN5713/14/15 which targets Bet v 1, the most abundant and immunodominant allergen in birch pollen triggering birch-related AR12, the overall safety was acceptable based on the analysis of the included studies. However, the number of trials and patients included were insufficient, and there was no past research indicating tolerability information in other diseases for reference. Thus, more studies are expected to draw a strong conclusion on the safety of innovatory biologics for the advancement of future clinical drugs.
The strengths of our study included a comprehensive and up-to-date search with restricted selection criteria following scientific guidelines. All the included studies were placebo controlled RCTs, and quality assessment demonstrated that most studies were at low or unclear risk of bias. Additionally, there was no evidence of statistical heterogeneity in all the meta-analysis outcomes (one eliminated heterogeneity after subgrouping), strengthening the consistency and reliability of our analysis. However, several limitations of the evidence should be considered. The number of studies in quilizumab, dupilumab, REGN1908-1909, and REGN5713/14/15 was small (≤2 studies for each biological product), providing limited safety information, especially for adverse events with extremely low incidence rates. Besides, all the studies had a relatively short follow-up time (the maximum follow-up time was 28 weeks), and this might lead to the ignorance of some adverse events in long-term practical use. Finally, some studies reported the safety data incompletely. For example, they only reported frequent adverse events with an incidence rate >10% and failed to summarize the severity of adverse events.
In conclusion, this systematic review with meta-analysis indicated that biological therapies were well tolerated in patients with AR, with no common or serious adverse events reaching statistical significance. Country was a significant factor for heterogeneity, and hypersensitivity such as urticaria required special caution. From a safety perspective, monoclonal antibodies are promising novel treatment options for different types of AR. We expect more investigations and future studies to confirm our findings and assess the long-term safety of biologics in the use of AR patients worldwide.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12082848/s1, Figure S1: Forest plot of RCTs comparing patients experiencing adverse events subgrouped by AR type [20,22,23,24,25,26,27,28,29,30]; Figure S2. Forest plot of RCTs comparing patients experiencing adverse events subgrouped by age group [20,22,23,24,25,26,27,28,29,30]; Figure S3. Forest plot of RCTs comparing patients experiencing adverse events subgrouped by type of mAb [20,22,23,24,25,26,27,28,29,30]; Figure S4. Forest plot of RCTs comparing rates of patients with headache subgrouped by type of mAb [19,21,22,23,24,27,28,29,30]; Figure S5. Forest plot of RCTs comparing rates of patients with upper respiratory infection subgrouped by type of mAb [21,22,23,25,27,28]; Figure S6. Forest plot of RCTs comparing rates of patients with viral infection subgrouped by type of mAb [21,22,23,27,28]; Figure S7. Forest plot of RCTs comparing rates of patients with pharyngitis subgrouped by type of mAb [19,21,23]; Figure S8. Forest plot of RCTs comparing rates of patients with nasopharyngitis subgrouped by type of mAb [22,23,27,28,29,30]; Figure S9. Forest plot of RCTs comparing rates of patients with injection site reactions subgrouped by type of mAb [20,22,23,26,27,28,29].
Author Contributions
Conceptualization, Y.L. (Yuxi Lin); methodology, Y.L. (Yuxi Lin) and W.W.; software, Y.L. (Yuxi Lin); validation, Y.L. (Yuxi Lin), Z.Z. and S.A.; formal analysis, Y.L. (Yuxi Lin), Z.Z. and S.A.; investigation, L.W., Y.Z. and X.W.; resources, Y.L. (Yuzhuo Liu) and J.L.; data curation, Y.L. (Yuxi Lin); writing—original draft preparation, Y.L. (Yuxi Lin); writing—review and editing, Y.L. (Yuxi Lin) and W.W.; visualization, Y.L. (Yuxi Lin); supervision, W.L.; project administration, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National High Level Hospital Clinical Research Funding (grant number 2022-PUMCH-B-096, 2022-PUMCH-A-030 and 2022-PUMCH-C-050); National Natural Science Foundation of China (grant number 82071027 and 82101200).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Small, P.; Keith, P.K.; Kim, H. Allergic rhinitis. Allergy Asthma Clin. Immunol. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y. Increasing prevalence of allergic rhinitis in China. Allergy Asthma Immunol. Res. 2019, 11, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Canonica, G.W.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. Dis. Prim. 2020, 6, 95. [Google Scholar] [CrossRef]
- Scadding, G.K.; Kariyawasam, H.H.; Scadding, G.; Mirakian, R.; Buckley, R.J.; Dixon, T.; Durham, S.R.; Farooque, S.; Jones, N.; Leech, S.; et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017, First edition 2007). Clin. Exp. Allergy 2017, 47, 856–889. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, C.; Zhang, L. Advances and novel developments in allergic rhinitis. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 3069–3076. [Google Scholar] [CrossRef]
- Nur Husna, S.M.; Md Shukri, N.; Mohd Ashari, N.S.; Wong, K.K. IL-4/IL-13 axis as therapeutic targets in allergic rhinitis and asthma. PeerJ 2022, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, K.; Cui, X.; Lu, L.; Dong, J.; Wang, M.; Gao, X. Clinical Efficacy and Safety of Omalizumab in the Treatment of Allergic Rhinitis: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Am. J. Rhinol. Allergy 2019, 34, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, W.; Yuan, H.; Li, Y.; Lv, Z.; Cui, Y.; Liu, J.; Ying, S. Current State of Monoclonal Antibody Therapy for Allergic Diseases. Engineering 2021, 7, 1552–1556. [Google Scholar] [CrossRef]
- Shamji, M.H.; Singh, I.; Layhadi, J.A.; Ito, C.; Karamani, A.; Kouser, L.; Sharif, H.; Tang, J.; Handijiev, S.; Parkin, R.V.; et al. Passive prophylactic administration with a single dose of Anti–Fel d 1 monoclonal antibodies REGN1908–1909 in cat allergen–induced allergic rhinitis: A randomized, double-blind, placebo-controlled clinical trial. Am. J. Respir. Crit. Care Med. 2021, 204, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Dingman, R.; Wang, C.Q.; Lai, C.; Rajadhyaksha, M.; DeVeaux, M.; Orengo, J.M.; Radin, A.; Davis, J.D. REGN1908-1909 monoclonal antibodies block Fel d 1 in cat allergic subjects: Translational pharmacokinetics and pharmacodynamics. Clin. Transl. Sci. 2021, 14, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Atanasio, A.; Franklin, M.C.; Kamat, V.; Hernandez, A.R.; Badithe, A.; Ben, L.-H.; Jones, J.; Bautista, J.; Yancopoulos, G.D.; Olson, W.; et al. Targeting immunodominant Bet v 1 epitopes with monoclonal antibodies prevents the birch allergic response. J. Allergy Clin. Immunol. 2022, 149, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- US Food Drug Adm. XOLAIR® Omalizumab for Subcutaneous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/103976s5102lbl.pdf (accessed on 3 March 2023).
- Chen, X.; Liu, M.; Wu, S.; Huang, Z.; Li, X.; Lai, X.; Bao, H.; Huang, J.; Chang, L.; Zhang, G. Treatment-emergent adverse events in dupilumab-treated patients with allergic diseases: A meta-analysis. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Nencini, F.; Pratesi, S.; Maggi, E.; Vultaggio, A. An overview on safety of monoclonal antibodies. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, S.J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Casale, T.B.; Bernstein, I.L.; Busse, W.W.; LaForce, C.F.; Tinkelman, D.G.; Stoltz, R.R.; Dockhorn, R.J.; Reimann, J.; Su, J.Q.; Fick, R.B., Jr.; et al. Use of an anti-lgE humanized monoclonal antibody in ragweed-induced allergic rhinitis. J. Allergy Clin. Immunol. 1997, 100, 110–121. [Google Scholar] [CrossRef]
- Adelroth, E.; Rak, S.; Haahtela, T.; Aasand, G.; Rosenhall, L.; Zetterstrom, O.; Byrne, A.; Champain, K.; Thirlwell, J.; Della Cioppa, G.; et al. Recombinant humanized mAb-E25, an anti-IgE mAb, in birch pollen-induced seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2000, 106, 253–259. [Google Scholar] [CrossRef]
- Casale, T.B.; Condemi, J.; LaForce, C.; Nayak, A.; Rowe, M.; Watrous, M.; McAlary, M.; Fowler-Taylor, A.; Racine, A.; Gupta, N.; et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: A randomized controlled trial. JAMA 2001, 286, 2956–2967. [Google Scholar] [CrossRef] [PubMed]
- Chervinsky, P.; Casale, T.; Townley, R.; Tripathy, I.; Hedgecock, S.; Fowler-Taylor, A.; Shen, H.; Fox, H. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Ann. Allergy Asthma Immunol. 2003, 91, 160–167. [Google Scholar] [CrossRef]
- Vignola, A.M.; Humbert, M.; Bousquet, J.; Boulet, L.-P.; Hedgecock, S.; Blogg, M.; Fox, H.; Surrey, K. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy Eur. J. Allergy Clin. Immunol. 2004, 59, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Ogino, S.; Nagakura, T.; Ishikawa, T. Omalizumab is effective and safe in the treatment of Japanese cedar pollen-induced seasonal allergic rhinitis. Allergol. Int. 2006, 55, 379–386. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Harris, J.M.; Boulet, L.P.; Scheerens, H.; Fitzgerald, J.M.; Putnam, W.S.; Cockcroft, D.W.; Davis, B.E.; Leigh, R.; Zheng, Y.; et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci. Transl. Med. 2014, 6, 243ra85. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.F.; Katial, R.; Jayawardena, S.; Pirozzi, G.; Staudinger, H.; Eckert, L.; Joish, V.N.; Amin, N.; Maroni, J.; Rowe, P.; et al. Efficacy and safety of dupilumab in perennial allergic rhinitis and comorbid asthma. J. Allergy Clin. Immunol. 2018, 142, 171–177.e1. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Saini, S.S.; Gagnon, R.; Moss, M.H.; Sussman, G.; Jacobs, J.; Laws, E.; Chung, E.S.; Constant, T.; Sun, Y.; et al. Short-Term Subcutaneous Allergy Immunotherapy and Dupilumab are Well Tolerated in Allergic Rhinitis: A Randomized Trial. J. Asthma Allergy 2021, 14, 1045–1063. [Google Scholar] [CrossRef] [PubMed]
- Orengo, J.M.; Radin, A.R.; Kamat, V.; Badithe, A.; Ben, L.H.; Bennett, B.L.; Zhong, S.; Birchard, D.; Limnander, A.; Rafique, A.; et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat. Commun. 2018, 9, 1421. [Google Scholar] [CrossRef]
- Gevaert, P.; De Craemer, J.; De Ruyck, N.; Rottey, S.; de Hoon, J.; Hellings, P.W.; Volckaert, B.; Lesneuck, K.; Orengo, J.M.; Atanasio, A.; et al. Novel antibody cocktail targeting Bet v 1 rapidly and sustainably treats birch allergy symptoms in a phase 1 study. J. Allergy Clin. Immunol. 2022, 149, 189–199. [Google Scholar] [CrossRef] [PubMed]
- NCT04709575. A Study to Investigate the Efficacy of Anti-Bet v 1 Monoclonal Antibodies in Reducing Symptoms of Seasonal Allergic Rhinitis. 2020. Available online: https://beta.clinicaltrials.gov/study/NCT04709575 (accessed on 9 January 2023).
- Qiu, X.; Wang, H. Safety and efficacy of omalizumab for the treatment of allergic rhinitis: Meta-analysis of randomized clinical trials. J. Clin. Otorhinolaryngol. Head Neck Surg. 2016, 30, 694–697. [Google Scholar]
- Graham-Rowe, D. When allergies go west. Nature 2011, 479, 6–8. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Hu, Y.; Zou, Z.; Shen, L.; Huang, C. Home environment, lifestyles behaviors, and rhinitis in childhood. Int. J. Hyg Environ. Health 2016, 219, 220–231. [Google Scholar] [CrossRef]
- Chong, S.N.; Chew, F.T. Epidemiology of allergic rhinitis and associated risk factors in Asia. World Allergy Organ. J. 2018, 11, 17. [Google Scholar] [CrossRef]
- Xing, Y.; Wong, G.W.K. Environmental Influences and Allergic Diseases in the Asia-Pacific Region: What Will Happen in Next 30 Years? Allergy Asthma Immunol. Res. 2022, 14, 21–39. [Google Scholar] [CrossRef]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH topic E 5 (R1) Ethnic Factors in the Acceptability of Foreign Clinical Data. Drug Inf. J. 1998, 32, 1283S–1292S. Available online: https://database.ich.org/sites/default/files/E5_R1__Guideline.pdf (accessed on 20 February 2023). [CrossRef]
- Bodiwala, V.; Marhsall, T.; Seril, D. Comparing Efficacy and Adverse Effects of Biological Agents in South Asian Inflammatory Bowel Disease Patients Living in the United States to Caucasian American Patients. Am. J. Gastroenterol. 2019, 114, s373. [Google Scholar] [CrossRef]
- Sitek, A.N.; Li, J.T.; Pongdee, T. Risks and safety of biologics: A practical guide for allergists. World Allergy Organ. J. 2023, 16, 100737. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Bahna, S.L. Hypersensitivity and Adverse Reactions to Biologics for Asthma and Allergic Diseases; Taylor & Francis: Abingdon, UK, 2020; Volume 16. [Google Scholar] [CrossRef]
- Rodrigo, G.J.; Neffen, H. Systematic review on the use of omalizumab for the treatment of asthmatic children and adolescents. Pediatr. Allergy Immunol. 2015, 26, 551–556. [Google Scholar] [CrossRef]
- Cox, L.; Platts-Mills, T.A.E.; Finegold, I.; Schwartz, L.B.; Simons, F.E.R.; Wallace, D.V. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J. Allergy Clin. Immunol. 2007, 120, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.; Lieberman, P.; Wallace, D.; Simons, F.E.R.; Finegold, I.; Platts-Mills, T.; Schwartz, L. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma & Immunology Omalizumab-Associated Anaphylaxis Joint Task Force follow-up report. J. Allergy Clin. Immunol. 2011, 128, 210–212. [Google Scholar] [CrossRef] [PubMed]
- de Blay, F.J.; Gherasim, A.; Domis, N.; Meier, P.; Shawki, F.; Wang, C.Q.; Orengo, J.M.; DeVeaux, M.; Ramesh, D.; Jalbert, J.J.; et al. REGN1908/1909 prevented cat allergen–induced early asthmatic responses in an environmental exposure unit. J. Allergy Clin. Immunol. 2022, 150, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).