A Comparison of Tissue Adhesive Material and Suture as Wound-Closure Techniques following Carpal Tunnel Decompression: A Single-Center Randomized Control Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Flow
2.3. Intervention Protocols
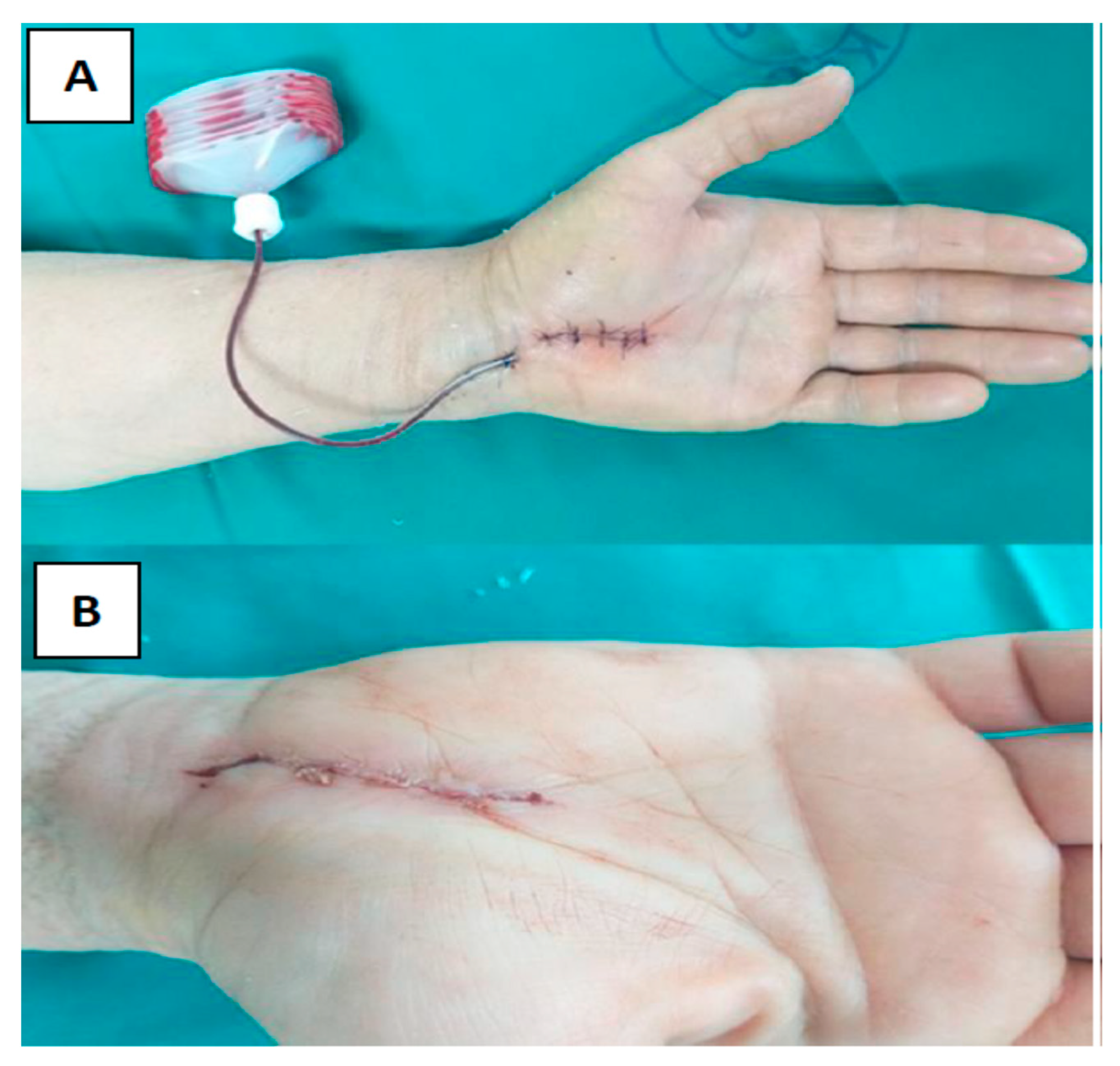
- The skin is stitched with transcutaneous nylon sutures (polypropylene-polyethylene monofilament, non-absorbable surgical suture) 4-0. (Optilene® DSMP 19, 3/8 needle, thread size 4/0, B. Braun Surgical, S.A. Carretera de Terrassa, Spain) (Figure 2A).
- After subcutaneous buried running continuous stitch with 4-0 Coated VicrylTM Plus PS-2, 3/8 (Ethicon Inc., Cincinnati, OH, USA), a two-component skin adhesive, Glubran Tiss 2® (GEM S.r.l., Viareggio, Italy), was applied. Glubran Tiss 2® is composed of NBCA (n-butyl 2 cyanoacrylate) and OCA (2-octyl cyanoacrylate) as a synthetic surgical glue with hemostatic, adhesive, sealing, and bacteriostatic properties [10]. When applied to wet tissue, it immediately polymerizes into a thin, elastic film with a great tensile strength that clings securely to the architecture of the tissue. Polymerized glue is a bioinert material. Each subject received 0.35 mL of Glubran Tiss® on the open wound, and before bandaging, subjects rested for 20 s for a polymerization process (Figure 2B).
2.4. Estimation of Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiménez Del Barrio, S.; Bueno Gracia, E.; Hidalgo García, C.; Estébanez de Miguel, E.; Tricás Moreno, J.M.; Rodríguez Marco, S.; Laita, L.C. Conservative treatment in patients with mild to moderate carpal tunnel syndrome: A systematic review. Neurologia 2018, 33, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Pace, V.; Marzano, F.; Placella, G. Update on surgical procedures for carpal tunnel syndrome: What is the current evidence and practice? What are the future research directions? World J. Orthop. 2023, 14, 6–12. [Google Scholar] [CrossRef]
- Wipperman, J.; Goerl, K. Carpal Tunnel Syndrome: Diagnosis and Management. Am. Fam. Physician 2016, 15, 993–999. [Google Scholar]
- Kluge, W.; Simpson, R.G.; Nicol, A.C. Late complications after open carpal tunnel decompression. J. Hand Surg. Br. 1996, 21, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Bolster, M.; Schipper, C.; Van Sterkenburg, S.; Ruettermann, M.; Reijnen, M. Single interrupted sutures compared with Donati sutures after open carpal tunnel release: A prospective randomised trial. J. Plast. Surg. Hand Surg. 2013, 47, 289–291. [Google Scholar] [CrossRef]
- Dietz, U.A.; Kuhfuss, I.; Debus, E.-S.; Thiede, A. Mario Donati and the vertical mattress suture of the skin. World J. Surg. 2006, 30, 141–148. [Google Scholar] [CrossRef]
- Coulthard, P.; Esposito, M.; Worthington, H.V.; van der Elst, M.; van Waes, O.J.; Darcey, J. Tissue adhesives for closure of surgical incisions. Cochrane Database Syst. Rev. 2010, 12, CD004287. [Google Scholar] [CrossRef]
- Dumville, J.C.; Coulthard, P.; Worthington, H.V.; Riley, P.; Patel, N.; Darcey, J.; Esposito, M.; van der Elst, M.; van Waes, O.J.F. Tissue adhesives for closure of surgical incisions. Cochrane Database Syst. Rev. 2014, 28, CD004287. [Google Scholar] [CrossRef]
- Steinberg, D.R. Surgical release of the carpal tunnel. Hand Clin. 2002, 18, 291–298. [Google Scholar] [CrossRef]
- Kakaei, F.; Seyyed Sadeghi, M.S.; Sanei, B.; Hashemzadeh, S.; Habibzadeh, A. A randomized clinical trial comparing the effect of different haemostatic agents for haemostasis of the liver after hepatic resection. HPB Surg. 2013, 2013, 587608. [Google Scholar] [CrossRef]
- Stavrou, D.; Haik, J.; Weissman, O.; Goldan, O.; Tessone, A.; Winkler, E. Patient and observer scar assessment scale: How good is it? J. Wound Care. 2009, 18, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.K.; Abdul Ghafar, M.A.; Shamsuddin, N.S.; Roslan, N.A.; Kaharuddin, H.; Nik Muhamad, N.A. The Assessment of Acute Pain in Pre-Hospital Care Using Verbal Numerical Rating and Visual Analogue Scales. J. Emerg. Med. 2015, 49, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.A.; Joshi, M.A.; Gadhire, M.; Shotriya, R.; Phad, B.; Singh, J. Prospective randomized comparative study of skin adhesive glue (2- methyl -2- cyanopropionate or cyanoacrylate) versus conventional skin suturing by suture material/skin stapler in clean surgical cases. Int. J. Surg. 2017, 5, 168. [Google Scholar] [CrossRef]
- Boya, H.; Özcan, Ö.; Özteki, N.H.H. Long-term complications of open carpal tunnel release. Muscle Nerve 2008, 38, 1443–1446. [Google Scholar] [CrossRef]
- Ogawa, R.; Akaishi, S. Endothelial dysfunction may play a key role in keloid and hypertrophic scar pathogenesis—Keloids and hypertrophic scars may be vascular disorders. Med. Hypotheses. 2016, 96, 51–60. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Jeschke, M.G.; Branski, L.K.; Barret, J.P.; Dziewulski, P.; Herndon, D.N. Hypertrophic scarring: The greatest unmet challenge after burn injury. Lancet. 2016, 388, 1427–1436. [Google Scholar] [CrossRef]
- Ruiz, R.; Hersant, B.; La Padula, S.; Meningaud, J.P. Facelifts: Improving the long-term outcomes of lower face and neck rejuvenation surgery: The lower face and neck rejuvenation combined method. J. Cranio-Maxillofacial Surg. 2018, 46, 697–704. [Google Scholar] [CrossRef]
- La Padula, S.; Hersant, B.; Bompy, L.; Meningaud, J.P. In search of a universal and objective method to assess facial aging: The new face objective photo-numerical assessment scale. J. Cranio-Maxillofacial Surg. 2019, 47, 1209–1215. [Google Scholar] [CrossRef]
- Suwannaphisit, S.; Aonsong, W.; Suwanno, P.; Yuenyongviwat, V. Comparing the running subcuticular technique versus the Donati technique in open carpal tunnel release: A randomized controlled trial. J. Orthop. Surg. Res. 2021, 16, 565. [Google Scholar] [CrossRef]
- Siegmeth, A.W.; Hopkinson-Woolley, J.A. Standard open decompression in carpal tunnel syndrome compared with a modified open technique preserving the superficial skin nerves: A prospective randomized study. J. Hand Surg. Am. 2006, 31, 1483–1489. [Google Scholar] [CrossRef]
- Ahcan, U.; Arnez, Z.M.; Bajrović, F.; Zorman, P. Surgical technique to reduce scar discomfort after carpal tunnel surgery. J. Hand Surg. Am. 2002, 27, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.G.; Wormald, J.C.; Figus, A. Absorbable versus non-absorbable sutures for skin closure after carpal tunnel decompression surgery. Cochrane Database Syst. Rev. 2018, 2, CD011757. [Google Scholar] [CrossRef] [PubMed]
- Theopold, C.; Potter, S.; Dempsey, M.; O’Shaughnessy, M. A randomised controlled trial of absorbable versus non-absorbable sutures for skin closure after open carpal tunnel release. J. Hand Surg. Eur. Vol. 2012, 37, 350–353. [Google Scholar] [CrossRef]
- Grimaldi, L.; Cuomo, R.; Brandi, C.; Botteri, G.; Nisi, G.; D’Aniello, C. Octyl-2-cyanoacrylate adhesive for skin closure: Eight years experience. In Vivo 2015, 29, 145–148. [Google Scholar] [PubMed]
- Martin, J.G.; Hollenbeck, S.T.; Janas, G.; Makar, R.A.; Pabon-Ramos, W.M.; Suhocki, P.V.; Miller, M.J.; Sopko, D.R.; Smith, T.P.; Kim, C.Y. Randomized Controlled Trial of Octyl Cyanoacrylate Skin Adhesive versus Subcuticular Suture for Skin Closure after Implantable Venous Port Placement. J. Vasc. Interv. Radiol. 2017, 28, 111–116. [Google Scholar] [CrossRef]
- Rushbrook, J.L.; White, G.; Kidger, L.; Marsh, P.; Taggart, T.F. The antibacterial effect of 2-octyl cyanoacrylate (Dermabond®) skin adhesive. J. Infect. Prev. 2014, 15, 236–239. [Google Scholar] [CrossRef]
- Lee, C.S.; Han, S.R.; Kye, B.H.; Bae, J.H.; Koh, W.; Lee, I.K.; Lee, D.-S.; Lee, Y.S. Surgical skin adhesive bond is safe and feasible wound closure method to reduce surgical site infection following minimally invasive colorectal cancer surgery. Ann. Surg. Treat. Res. 2020, 99, 146–152. [Google Scholar] [CrossRef]
- Farion, K.J.; Russell, K.F.; Osmond, M.H.; Hartling, L.; Klassen, T.P.; Durec, T.; Vandermeer, B. Tissue adhesives for traumatic lacerations in children and adults. Cochrane Database Syst. Rev. 2002, 2002, CD003326. [Google Scholar] [CrossRef]
- Lewis, T.L.; Goff, T.A.J.; Ray, R.; Varrall, C.R.; Robinson, P.W.; Fogarty, K.; Chang, A.; Dhaliwal, J.; Dearden, P.M.C.; Wines, A. Randomized Controlled Trial of Topical Skin Adhesive vs Nylon Sutures for Incision Closure in Forefoot Surgery. Foot Ankle Int. 2021, 42, 1106–1114. [Google Scholar] [CrossRef]
- McQuillan, T.J.; Vora, M.; Hawkins, J.; Kenney, D.; Diaz, R.; Ladd, A.L. Adhesive Taping Shows Better Cosmetic Outcomes Than Tissue Adhesives for Sutured Upper Extremity Incisions: A Single-Blind Prospective Randomized Controlled Trial. Orthopedics 2022, 45, e42–e46. [Google Scholar] [CrossRef]
- Sinha, S.; Naik, M.; Wright, V.; Timmons, J.; Campbell, A.C. A single blind, prospective, randomized trial comparing n-butyl 2-cyanoacrylate tissue adhesive (Indermil) and sutures for skin closure in hand surgery. J. Hand Surg. Br. 2001, 26, 264–265. [Google Scholar] [CrossRef] [PubMed]


| All (n = 100) | Males (n = 30) | Females (n = 70) | p | ||||
|---|---|---|---|---|---|---|---|
| Age (years) | 61.56 ± 12.03 | 66.30 ± 10.88 | 59.53 ± 12.00 | 0.005 | |||
| BMI (kg/m2) | 24.92 ± 2.74 | 26.44 ± 2.12 | 24.26 ± 2.73 | <0.001 | |||
| Time_stitching (min) | 3.25 ± 0.31 | 3.29 ± 0.27 | 3.23 ± 0.32 | 0.198 | |||
| Time_bandage (min) | 12.87 ± 1.01 | 12.70 ± 0.99 | 12.94 ± 1.02 | 0.131 | |||
| Sugical_decompression (min) | 8.71 ± 0.57 | 8.60 ± 0.53 | 8.75 ± 0.58 | 0.115 | |||
| cosmetic-VAS 2 weeks | 95.00 | (90.00–95.00) | 90.00 | (90.00–95.00) | 95.00 | (90.00–95.00) | 0.270 |
| cosmetic-VAS 6 weeks | 100.00 | (95.00–100.00) | 100.00 | (95.00–100.00) | 100.00 | (95.00–100.00) | 0.159 |
| cosmetic-VAS 12 weeks | 100.00 | (100.00–100.00) | 100.00 | (100.00–100.00) | 100.00 | (100.00–100.00) | 0.092 |
| POSAS 2 weeks pt. | 17.00 | (16.00–17.00) | 17.00 | (16.00–17.00) | 17.00 | (16.00–17.25) | 0.117 |
| POSAS 2 weeks observer | 17.00 | (16.00–17.00) | 16.00 | (16.00–17.00) | 17.00 | (16.00–17.00) | 0.006 |
| POSAS 6 weeks pt. | 15.00 | (14.00–15.00) | 14.00 | (13.00–15.00) | 15.00 | (14.00–15.00) | 0.143 |
| POSAS 6 weeks observer | 14.00 | (13.00–15.00) | 14.00 | (13.00–14.00) | 14.00 | (13.75–15.00) | 0.060 |
| POSAS 12 weeks pt. | 11.00 | (10.00–12.00) | 11.00 | (11.00–12.00) | 11.00 | (10.00–12.00) | 0.145 |
| POSAS 12 weeks observer | 11.00 | (10.00–12.00) | 11.00 | (10.00–12.00) | 11.00 | (10.00–12.00) | 0.180 |
| VNRS prior surgery | 5.00 | (4.00–6.00) | 5.00 | (4.00–6.00) | 5.00 | (4.00–5.25) | 0.403 |
| VNRS on surgery day | 5.00 | (4.00–6.00) | 4.50 | (4.00–6.00) | 5.00 | (4.00–6.00) | 0.084 |
| VNRS 2 weeks post surgery | 3.00 | (3.00–4.00) | 3.00 | (2.00–4.00) | 3.00 | (3.00–4.00) | 0.023 |
| VNRS 6 weeks post surgery | 2.00 | (1.00–2.00) | 2.00 | (1.00–2.00) | 2.00 | (1.75–2.00) | 0.087 |
| VNRS 12 weeks post surgery | 0.00 | (0.00–0.00) | 0.00 | (0.00–0.00) | 0.00 | (0.00–0.00) | 0.374 |
| wound length (mm) | 17.00 | (17.00–18.00) | 17.00 | (17.00–18.00) | 18.00 | (17.00–18.00) | 0.045 |
| Glue-Based Technique (n = 50) | Suture-Based Technique (n = 50) | p | |||
|---|---|---|---|---|---|
| Age (years) | 63.02 ± 12.97 | 60.10 ± 10.95 | 0.113 | ||
| BMI (kg/m2) | 24.79 ± 3.17 | 25.04 ± 2.25 | 0.325 | ||
| Time_stitching (min) | 3.19 ± 0.27 | 3.31 ± 0.33 | 0.021 | ||
| Time_bandage (min) | 12.93 ± 1.00 | 12.81 ± 1.03 | 0.291 | ||
| Sugical_decompression (min) | 8.74 ± 0.57 | 8.67 ± 0.57 | 0.264 | ||
| cosmetic-VAS 2 weeks | 95.00 | (90.00–95.00) | 90.00 | (90.00–95.00) | 0.014 |
| cosmetic-VAS 6 weeks | 100.00 | (100.00–100.00) | 100.00 | (95.00–100.00) | 0.003 |
| cosmetic-VAS 12 weeks | 100.00 | (100.00–100.00) | 100.00 | (100.00–100.00) | 0.153 |
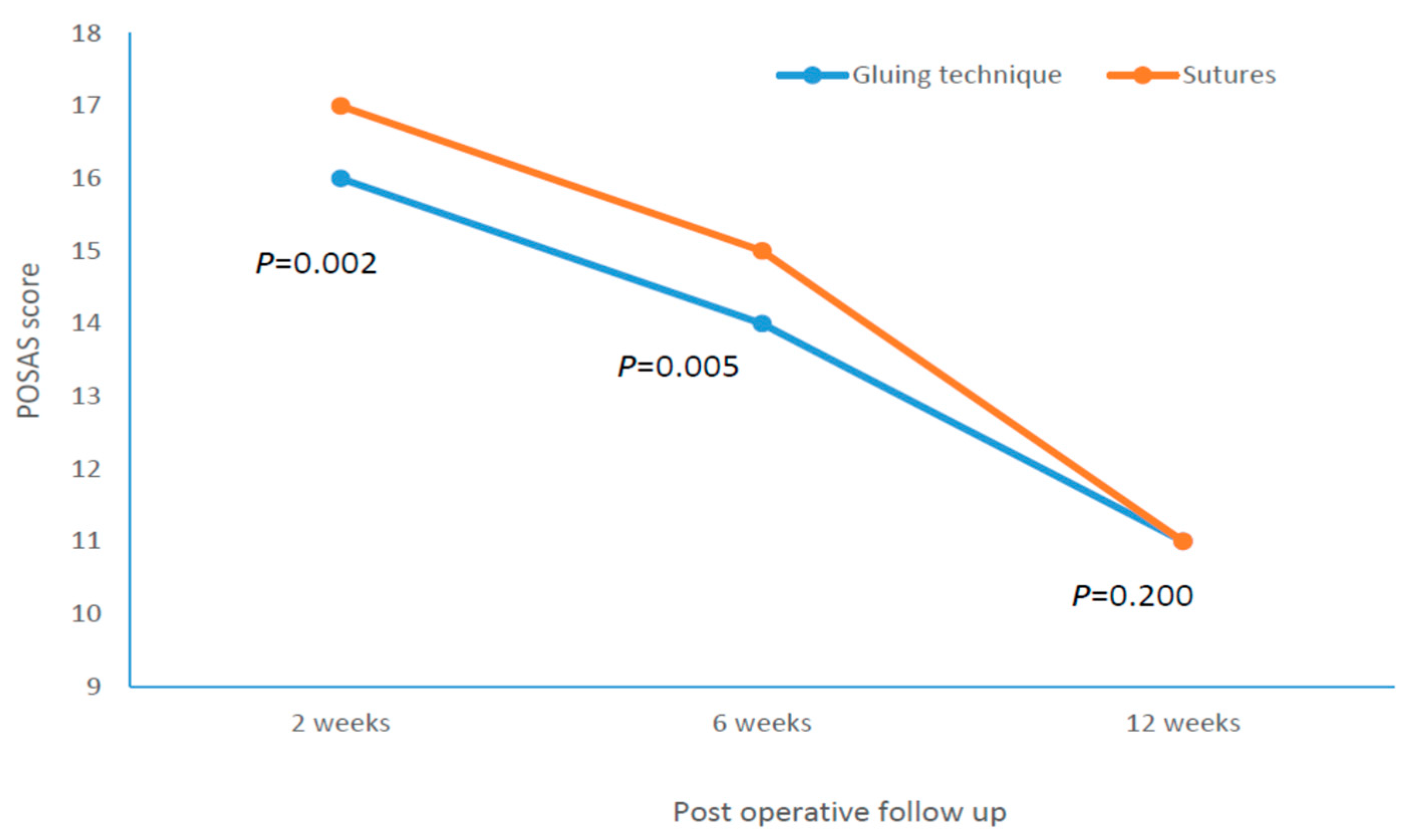
| POSAS 2 weeks pt. | 16.00 | (16.00–17.00) | 17.00 | (17.00–18.00) | 0.002 |
| POSAS 2 weeks observer | 16.00 | (16.00–17.00) | 17.00 | (16.00–18.00) | <0.001 |
| POSAS 6 weeks pt. | 14.00 | (14.00–15.00) | 15.00 | (14.00–15.00) | 0.005 |
| POSAS 6 weeks observer | 14.00 | (13.00–14.00) | 14.00 | (13.75–15.00) | 0.038 |
| POSAS 12 weeks pt. | 11.00 | (10.00–12.00) | 11.00 | (10.00–12.00) | 0.200 |
| POSAS 12 weeks observer | 11.00 | (10.00–11.25) | 11.00 | (10.00–12.00) | 0.064 |
| VNRS prior surgery | 5.00 | (4.00–6.00) | 5.00 | (4.00–5.25) | 0.387 |
| VNRS on surgery day | 5.00 | (4.00–6.00) | 5.00 | (4.00–6.00) | 0.134 |
| VNRS 2 weeks post surgery | 3.00 | (3.00–4.00) | 3.00 | (3.00–4.00) | 0.027 |
| VNRS 6 weeks post surgery | 2.00 | (1.00–2.00) | 2.00 | (2.00–3.00) | 0.001 |
| VNRS 12 weeks post surgery | 0.00 | (0.00–0.00) | 0.00 | (0.00–0.00) | 0.232 |
| wound length (mm) | 17.00 | (17.00–18.00) | 17.00 | (17.00–18.00) | 0.355 |
| Spearman’s Rho | p | |
|---|---|---|
| wound length | −0.088 | 0.273 |
| Time_stitching | 0.085 | 0.279 |
| Time_bandage | 0.016 | 0.456 |
| Sugical_decompression | 0.239 | 0.048 |
| cosmetic-VAS 2 weeks | −0.183 | 0.101 |
| cosmetic-VAS 6 weeks | −0.290 | 0.021 |
| cosmetic-VAS 12 weeks | −0.064 | 0.329 |
| POSAS 2 weeks pt. | −0.252 | 0.039 |
| POSAS 2 weeks observer | −0.294 | 0.019 |
| POSAS 6 weeks pt. | −0.460 | <0.001 |
| POSAS 6 weeks observer | −0.407 | 0.002 |
| POSAS 12 weeks pt. | −0.005 | 0.487 |
| POSAS 12 weeks observer | 0.104 | 0.237 |
| VNRS prior surgery | 0.062 | 0.335 |
| VNRS on surgery day | −0.230 | 0.054 |
| VNRS 2 weeks post surgery | −0.355 | 0.006 |
| VNRS 6 weeks post surgery | −0.208 | 0.074 |
| VNRS 12 weeks post surgery | 0.219 | 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunjic Roguljic, V.; Roguljic, L.; Kovacic, V.; Jukic, I. A Comparison of Tissue Adhesive Material and Suture as Wound-Closure Techniques following Carpal Tunnel Decompression: A Single-Center Randomized Control Trial. J. Clin. Med. 2023, 12, 2864. https://doi.org/10.3390/jcm12082864
Sunjic Roguljic V, Roguljic L, Kovacic V, Jukic I. A Comparison of Tissue Adhesive Material and Suture as Wound-Closure Techniques following Carpal Tunnel Decompression: A Single-Center Randomized Control Trial. Journal of Clinical Medicine. 2023; 12(8):2864. https://doi.org/10.3390/jcm12082864
Chicago/Turabian StyleSunjic Roguljic, Veridijana, Luka Roguljic, Vedran Kovacic, and Ivana Jukic. 2023. "A Comparison of Tissue Adhesive Material and Suture as Wound-Closure Techniques following Carpal Tunnel Decompression: A Single-Center Randomized Control Trial" Journal of Clinical Medicine 12, no. 8: 2864. https://doi.org/10.3390/jcm12082864
APA StyleSunjic Roguljic, V., Roguljic, L., Kovacic, V., & Jukic, I. (2023). A Comparison of Tissue Adhesive Material and Suture as Wound-Closure Techniques following Carpal Tunnel Decompression: A Single-Center Randomized Control Trial. Journal of Clinical Medicine, 12(8), 2864. https://doi.org/10.3390/jcm12082864








