Intrahepatic Cholangiocarcinoma and Acute Intermittent Porphyria: A Case Report
Abstract
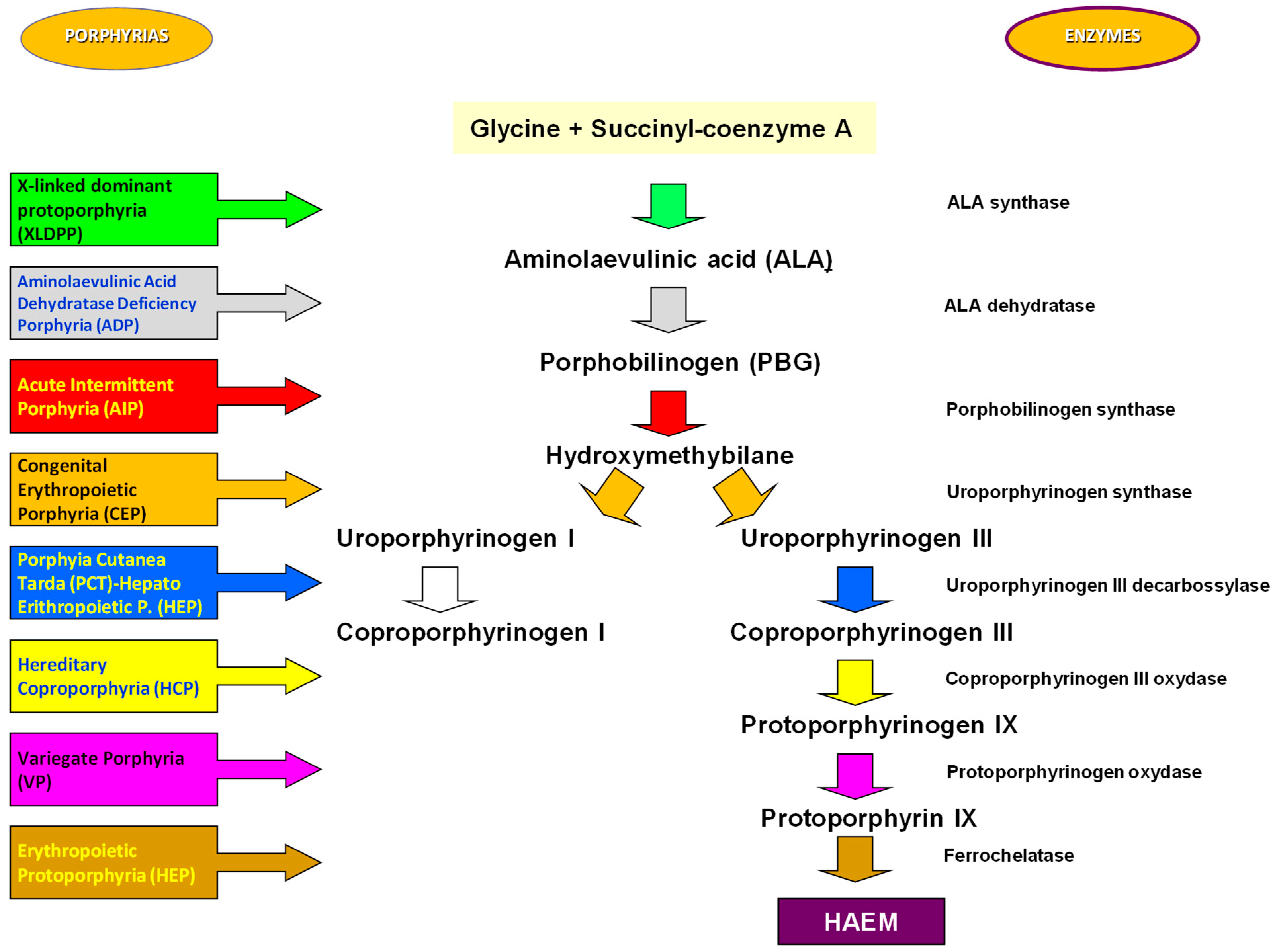
1. Introduction
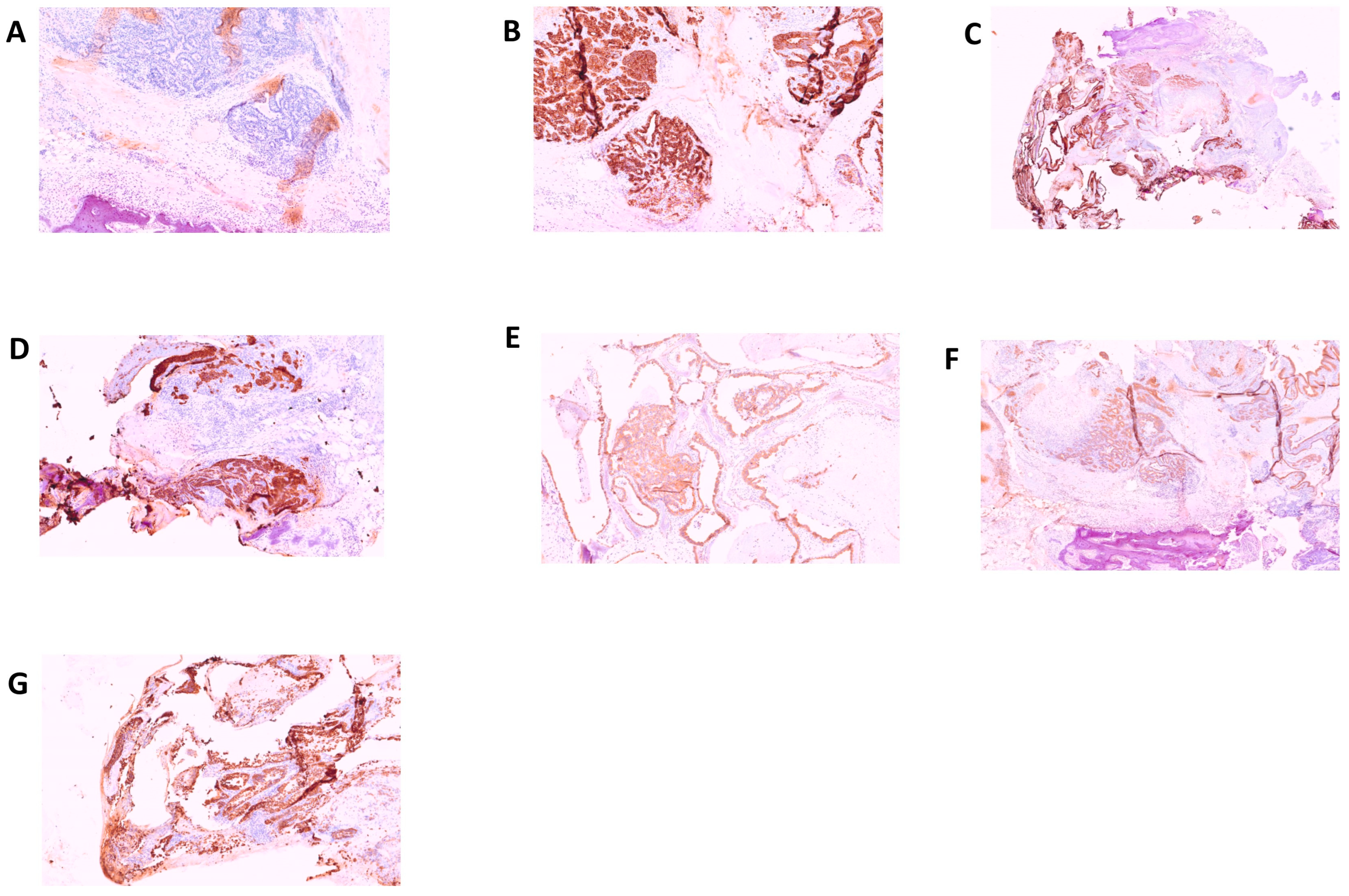
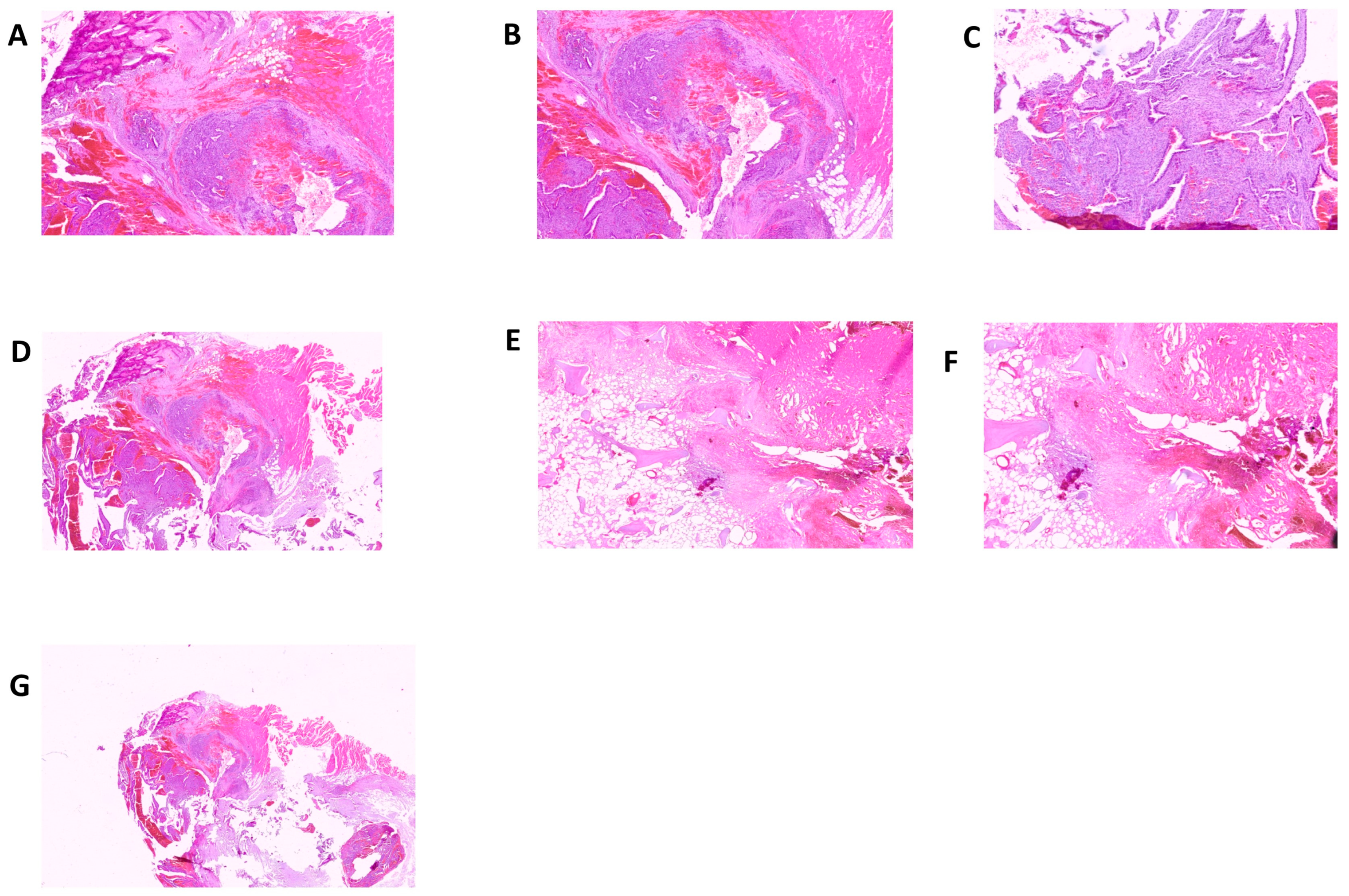
2. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szlendak, U.; Bykowska, K.; Lipniacka, A. Clinical, Biochemical and Molecular Characteristics of the Main Types of Porphyria. Adv. Clin. Exp. Med. 2016, 25, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Bissell, D.M.; Anderson, K.E.; Bonkovsky, H.L. Porphyria. N. Engl. J. Med. 2017, 377, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Bustad, H.; Kallio, J.; Vorland, M.; Fiorentino, V.; Sandberg, S.; Schmitt, C.; Aarsand, A.; Martinez, A. Acute Intermittent Porphyria: An Overview of Therapy Developments and Future Perspectives Focusing on Stabilisation of HMBS and Proteostasis Regulators. Int. J. Mol. Sci. 2021, 22, 675. [Google Scholar] [CrossRef]
- Yasuda, M.; Chen, B.; Desnick, R.J. Recent advances on porphyria genetics: Inheritance, penetrance & molecular heterogeneity, including new modifying/causative genes. Mol. Genet. Metab. 2018, 128, 320–331. [Google Scholar]
- Wylie, K.; Testai, F.D. Neurological Manifestations of Acute Porphyrias. Curr. Neurol. Neurosci. Rep. 2022, 22, 355–362. [Google Scholar] [CrossRef]
- Puy, H.; Gouya, L.; Deybach, J.-C. Porphyrias. Lancet 2010, 375, 924–937. [Google Scholar] [CrossRef]
- Stein, P.E.; Badminton, M.N.; Rees, D.C. Update review of the acute porphyrias. Br. J. Haematol. 2016, 176, 527–538. [Google Scholar] [CrossRef]
- Baravelli, C.M.; Sandberg, S.; Aarsand, A.K.; Nilsen, R.M.; Tollånes, M.C. Acute hepatic porphyria and cancer risk: A nationwide cohort study. J. Intern. Med. 2017, 282, 229–240. [Google Scholar] [CrossRef]
- Saberi, B.; Naik, H.; Overbey, J.R.; Erwin, A.L.; Anderson, K.E.; Bissell, D.M.; Bonkovsky, H.L.; Phillips, J.D.; Wang, B.; Singal, A.K. Hepatocellular Carcinoma in Acute Hepatic Porphyrias: Results from the Longitudinal Study of the U.S. Porphyrias Consortium. Hepatology 2021, 73, 1736–1746. [Google Scholar] [CrossRef]
- Ogun, A.S.; Joy, N.V.; Valentine, M. Biochemistry. Heme Synthesis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Valle, G.; Guida, C.C.; Nasuto, M.; Totaro, M.; Aucella, F.; Frusciante, V.; Di Mauro, L.; Potenza, A.; Savino, M.; Stanislao, M.; et al. Cerebral Hypoperfusion in Hereditary Coproporphyria (HCP): A Single Photon Emission Computed Tomography (SPECT) Study. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Guida, C.C.; Manzini, P.; Cuoghi, C.; Ventura, P. Kidney Involvement in Acute Hepatic Porphyrias: Pathophysiology and Diagnostic Implications. Diagnostics 2021, 11, 2324. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Cappellini, M.D.; Biolcati, G.; Guida, C.C.; Rocchi, E.; Gruppo Italiano Porfiria (GrIP). A challenging diagnosis for potential fatal diseases: Recommendations for diagnosing acute porphyrias. Eur. J. Intern. Med. 2014, 25, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Savino, M.; Guida, C.C.; Nardella, M.; Murgo, E.; Augello, B.; Merla, G.; De Cosmo, S.; Savino, A.F.; Tarquini, R.; Cei, F.; et al. Circadian Genes Expression Patterns in Disorders Due to Enzyme Deficiencies in the Heme Biosynthetic Pathway. Biomedicines 2022, 10, 3198. [Google Scholar] [CrossRef]
- Ramai, D.; Deliwala, S.S.; Chandan, S.; Lester, J.; Singh, J.; Samanta, J.; di Nunzio, S.; Perversi, F.; Cappellini, F.; Shah, A.; et al. Risk of Hepatocellular Carcinoma in Patients with Porphyria: A Systematic Review. Cancers 2022, 14, 2947. [Google Scholar] [CrossRef]
- Balwani, M.; Wang, B.; Anderson, K.E.; Bloomer, J.R.; Bissell, D.M.; Bonkovsky, H.L.; Phillips, J.D.; Desnick, R.J.; Porphyrias Consortium of the Rare Diseases Clinical Research Network. Acute hepatic porphyrias: Recommendations for evaluation and long-term management. Hepatology (Baltim. Md.) 2017, 66, 1314–1322. [Google Scholar] [CrossRef]
- Innala, E.; Andersson, C. Screening for hepatocellular carcinoma in acute intermittent porphyria: A 15-year follow-up in northern Sweden. J. Intern. Med. 2011, 269, 538–545. [Google Scholar] [CrossRef]
- Neeleman, R.A.; Wagenmakers, M.A.E.M.; Koole-Lesuis, R.H.; Mijnhout, G.S.; Wilson, J.H.P.; Friesema, E.C.H.; Langendonk, J.G. Medical and financial burden of acute intermittent porphyria. J. Inherit. Metab. Dis. 2018, 41, 809–817. [Google Scholar] [CrossRef]
- Lissing, M.; Vassiliou, D.; Floderus, Y.; Harper, P.; Bottai, M.; Kotopouli, M.; Hagström, H.; Sardh, E.; Wahlin, S. Risk of primary liver cancer in acute hepatic porphyria patients: A matched cohort study of 1244 individuals. J. Intern. Med. 2022, 291, 824–836. [Google Scholar] [CrossRef]
- Lissing, M.; Nowak, G.; Adam, R.; Karam, V.; Boyd, A.; Gouya, L.; Meersseman, W.; Melum, E.; Ołdakowska-Jedynak, U.; Reiter, F.P.; et al. Liver Transplantation for Acute Intermittent Porphyria. Liver Transplant. 2021, 27, 491–501. [Google Scholar] [CrossRef]
- Haverkamp, T.; Bronisch, O.; Knösel, T.; Mogler, C.; Weichert, W.; Stauch, T.; Schmid, C.; Rummeny, C.; Beykirch, M.K.; Petrides, P.E. Heterogeneous molecular behavior in liver tumors (HCC and CCA) of two patients with acute intermittent porphyria. J. Cancer Res. Clin. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Peoc’h, K.; Manceau, H.; Karim, Z.; Wahlin, S.; Gouya, L.; Puy, H.; Deybach, J.C. Hepatocellular carcinoma in acute hepatic porphyrias: A Damocles Sword. Mol. Genet. Metab. 2019, 128, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Findley, H.; Philips, A.; Cole, D.; Nair, A. Porphyrias: Implications for anaesthesia, critical care, and pain medicine. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 128–133. [Google Scholar] [CrossRef]
- James, M.F.; Hift, R.J. Porphyrias. Br. J. Anaesth. 2000, 85, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Baig, N.; Badminton, M.; Schulenburg-Brand, D. Acute hepatic porphyria and anaesthesia: A practical approach to the prevention and management of acute neurovisceral attacks. BJA Educ. 2021, 21, 66–74. [Google Scholar] [CrossRef]

| Normal Value | 10/12 | 12/12 | 13/12 | 14/12 | 20/12 | 22/12 | 26/12 | |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 14–18 | 7.30 | 9.90 | 10.20 | 10.20 | 9.90 | ||
| White blood cells count (103/µL) | 4.0–9.0 | 17.70 | 12.20 | 12.40 | 14.01 | 13.57 | ||
| Red blood cells count (106/µL) | 4.7–6.1 | 2.85 | 3.65 | 3.67 | 3.70 | 3.43 | ||
| Platelets count (103/µL) | 150–400 | 174 | 193 | 180 | 175 | 147 | ||
| PT-INR (%) (Prothrombin time test) | 70–130 | 59 | 66 | |||||
| PTT (seconds) (Partial Thromboplastin Time Test) | 25–32 | 21.8 | 22.5 | |||||
| D-dimer (ng/mL) | 0–500 | 3576 | ||||||
| Glucose (mg/dL) | 70–100 | 102 | 56 | 133 | 141 | |||
| Urea (mg/dL) | 10–50 | 65 | 65 | 31 | 59 | |||
| Creatinine (mg/dL) | 0.55–1.02 | 1.30 | 1.20 | 1.10 | 0.80 | 1.00 | ||
| CKD-EPI | 60–162 | 41 | 45 | 50 | 74 | 56 | ||
| Total serum proteins (g/dL) | 6.4–8.2 | 4.50 | 4.90 | |||||
| Uric acid (mg/dL) | 3.5–7.2 | 12 | 12.1 | |||||
| Sodium (mEq/L) | 136–145 | 139 | 136 | 134 | 132 | |||
| Potassium (mmol/L) | 3.5–5.0 | 2.8 | 2.8 | 3.5 | 4.9 | |||
| Aspartate aminotransferase (ALT. IU/L) | 8–30 | 115 | 119 | |||||
| Alanine aminotransferase (AST. IU/L) | 13–57 | 36 | 19 | |||||
| Gamma-glutamyl transferase (GGT) (IU/L) | 5–85 | 431 | ||||||
| Alkaline phosphatase (IU/L) | 45–117 | 277 | ||||||
| Ammonia (µmol/L) | 19–54 | 20 | ||||||
| Carcinoembryonic Antigen (CEA) (µg/L) | <5.00 | 2.75 | ||||||
| α-Fetoprotein (AFP) (ng/mL) | <12.00 | 232 | ||||||
| Cancer antigen (CA) 15-3 (U/mL) | <30.00 | 63.10 | ||||||
| Cancer antigen (CA) 125 (U/mL) | <35.00 | 544.30 | ||||||
| Cancer antigen (CA) 19-9 (U/mL) | <37.00 | 186.51 | ||||||
| C reactive protein CRP (mg/dL) | <0.30 | 13.10 | 11.50 | 15.10 | ||||
| Total porphyrins (µg/mL/24 h) | <0.50 | 15.81 | 2.36 | 2.23 | 7.83 | |||
| δ-aminolevulinic acid (ALA, mg/dL) | 0.00–5.00 | 12.20 | ||||||
| Porphobilinogen (PBG, µg/mL/24 h) | 0.00–2.00 | 2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guida, C.C.; Nardella, M.; Fiorentino, L.; Latiano, T.; Napolitano, F.; Ferrara, G.; Crisetti, A.; Mazzoccoli, G.; Aucella, F.; Aucella, F. Intrahepatic Cholangiocarcinoma and Acute Intermittent Porphyria: A Case Report. J. Clin. Med. 2023, 12, 3091. https://doi.org/10.3390/jcm12093091
Guida CC, Nardella M, Fiorentino L, Latiano T, Napolitano F, Ferrara G, Crisetti A, Mazzoccoli G, Aucella F, Aucella F. Intrahepatic Cholangiocarcinoma and Acute Intermittent Porphyria: A Case Report. Journal of Clinical Medicine. 2023; 12(9):3091. https://doi.org/10.3390/jcm12093091
Chicago/Turabian StyleGuida, Claudio Carmine, Maria Nardella, Leonardo Fiorentino, Tiziana Latiano, Francesco Napolitano, Gaetano Ferrara, Annalisa Crisetti, Gianluigi Mazzoccoli, Francesco Aucella, and Filippo Aucella. 2023. "Intrahepatic Cholangiocarcinoma and Acute Intermittent Porphyria: A Case Report" Journal of Clinical Medicine 12, no. 9: 3091. https://doi.org/10.3390/jcm12093091
APA StyleGuida, C. C., Nardella, M., Fiorentino, L., Latiano, T., Napolitano, F., Ferrara, G., Crisetti, A., Mazzoccoli, G., Aucella, F., & Aucella, F. (2023). Intrahepatic Cholangiocarcinoma and Acute Intermittent Porphyria: A Case Report. Journal of Clinical Medicine, 12(9), 3091. https://doi.org/10.3390/jcm12093091








