Clinical Applications of Optical Coherence Tomography Angiography in Inherited Retinal Diseases: An Up-to-Date Review of the Literature
Abstract
1. Introduction
2. Optical Coherence Tomography Angiography Technical Aspects
3. Clinical Applications
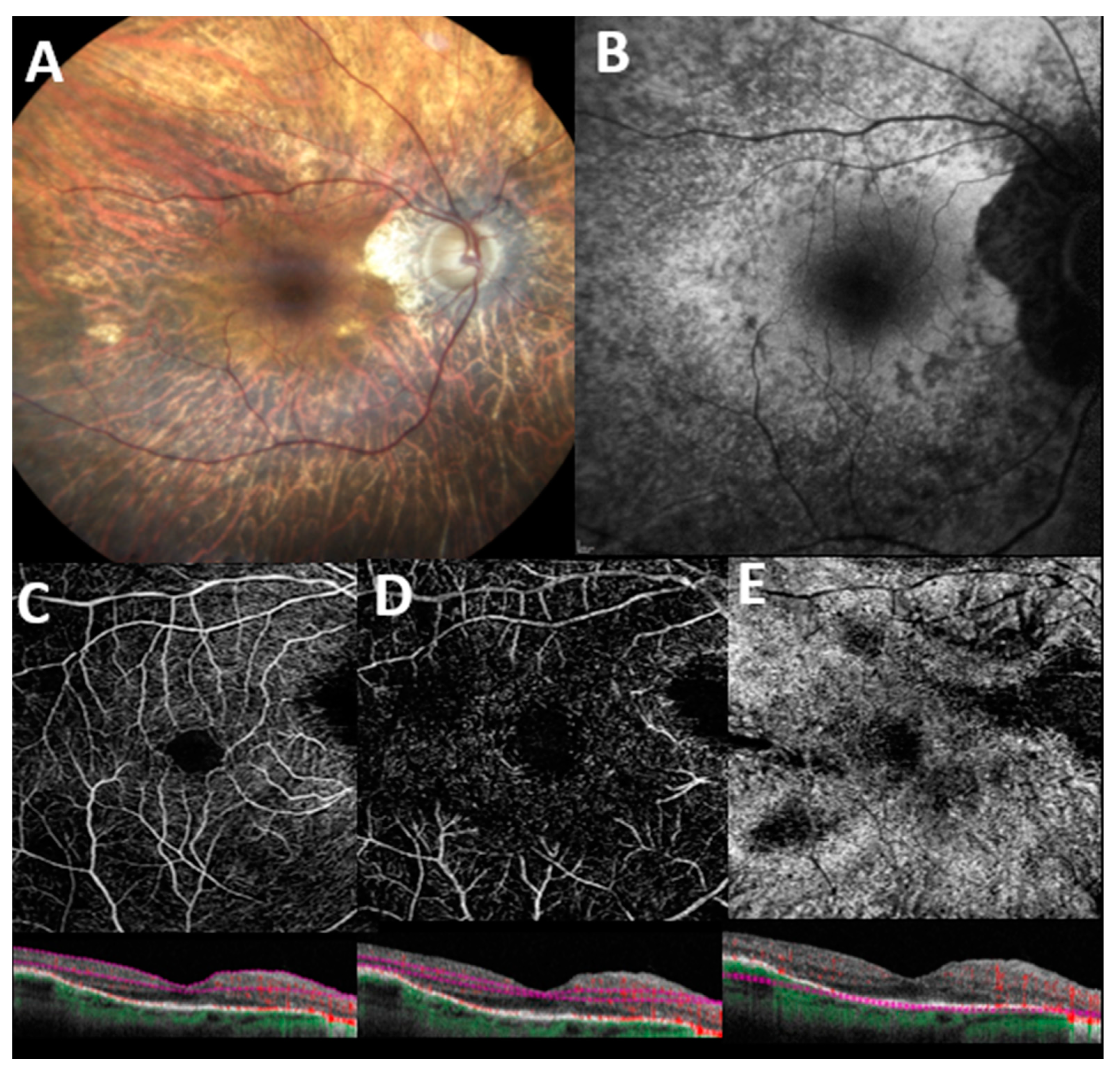
3.1. OCT-A in Retinitis Pigmentosa
3.2. OCT-A in Choroideremia
3.3. OCT-A in Best Disease
3.4. OCT-A in Stargardt Disease
3.5. OCT-A in Miscellaneous Diseases
3.5.1. OCT-A in Gyrate Atrophy
3.5.2. OCT-A in Bietti Dystrophy
3.5.3. OCT-A in Leber Hereditary Optic Neuropathy
3.5.4. OCT-A in X-Linked Retinoschisis
4. Limitations
5. Conclusions
6. Methods of Literature Search
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical Coherence Tomography Angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J. Retina and Vitreous; American Academy of Ophthalmology: San Francisco, CA, USA, 2017. [Google Scholar]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical Coherence Tomography Angiography: A Comprehensive Review of Current Methods and Clinical Applications HHS Public Access. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef] [PubMed]
- Novotny, H.R.; Alvis, D.L. A Method of Photographing Fluorescence in Circulating Blood in the Human Retina. Circulation 1961, 24, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Pellegrini, M.; Cornish, E.; Teo, K.Y.C.; Cereda, M.; Chabblani, J. Imaging the Choroid: From Indocyanine Green Angiography to Optical Coherence Tomography Angiography. Asia-Pac. J. Ophthalmol. 2020, 9, 335–348. [Google Scholar] [CrossRef]
- Chen, C.-L.; Wang, R.K. Optical Coherence Tomography Based Angiography [Invited]. Biomed. Opt. Express 2017, 8, 1056. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Q.; Chen, C.-L.; Wang, R.K. Methods and Algorithms for Optical Coherence Tomography-Based Angiography: A Review and Comparison. J. Biomed. Opt. 2015, 20, 100901. [Google Scholar] [CrossRef]
- Wang, R.K.; Jacques, S.L.; Ma, Z.; Hurst, S.; Hanson, S.R.; Gruber, A. Three Dimensional Optical Angiography. Opt. Express 2007, 15, 4083. [Google Scholar] [CrossRef]
- Barton, J.K.; Stromski, S. Flow Measurement without Phase Information in Optical Coherence Tomography Images. Opt. Express 2005, 13, 5234. [Google Scholar] [CrossRef]
- Wang, R.K.; An, L. Doppler Optical Micro-Angiography for Volumetric Imaging of Vascular Perfusion in Vivo. Opt. Express 2009, 17, 8926. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-Spectrum Amplitude-Decorrelation Angiography with Optical Coherence Tomography. Opt. Express 2012, 20, 4710. [Google Scholar] [CrossRef]
- Borrelli, E.; Sarraf, D.; Freund, K.B.; Sadda, S.R. OCT Angiography and Evaluation of the Choroid and Choroidal Vascular Disorders. Prog. Retin. Eye Res. 2018, 67, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Fineman, M.S.; Maguire, J.I.; Fineman, S.W.; Benson, W.E. Safety of Indocyanine Green Angiography during Pregnancy: A Survey of the Retina, Macula, and Vitreous Societies. Arch. Ophthalmol. 2001, 119, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Halperin, L.S.; Olk, R.J.; Soubrane, G.; Coscas, G. Safety of Fluorescein Angiography during Pregnancy. Am. J. Ophthalmol. 1990, 109, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Alnawaiseh, M.; Schubert, F.; Heiduschka, P.; Eter, N. Optical Coherence Tomography Angiography in Patients with Retinitis Pigmentosa. Retina 2019, 39, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Aragona, E.; Perra, C.; Bianco, L.; Antropoli, A.; Saladino, A.; Berni, A.; Basile, G.; Pina, A.; Bandello, F.; et al. Characterizing Macular Edema in Retinitis Pigmentosa through a Combined Structural and Microvascular Optical Coherence Tomography Investigation. Sci. Rep. 2023, 13, 800. [Google Scholar] [CrossRef]
- Ataş, F.; Kayabaşı, M.; Saatci, A.O. Vessel Density and Choroidal Vascularity Index in Patients with Bietti Crystalline Dystrophy and Retinitis Pigmentosa. Photodiagnosis Photodyn. Ther. 2022, 40, 103181. [Google Scholar] [CrossRef]
- Attaallah, H.R.; Mohamed, A.A.M.; Hamid, M.A. Quantification of Macular Microvascular Changes in Retinitis Pigmentosa Using Optical Coherence Tomography Angiography. Clin. Ophthalmol. 2020, 14, 1705–1713. [Google Scholar] [CrossRef]
- Deutsch, S.; Lommatzsch, A.; Weinitz, S.; Farmand, G.; Kellner, U. Optical Coherence Tomography Angiography (OCT-A) in Retinitis Pigmentosa and Macular Dystrophy Patients: A Retrospective Study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 1923–1931. [Google Scholar] [CrossRef]
- Giansanti, F.; Vicini, G.; Sodi, A.; Nicolosi, C.; Bellari, L.; Virgili, G.; Rizzo, S.; Bacherini, D. Optical Coherence Tomography Angiography for the Evaluation of Retinal and Choroidal Vasculature in Retinitis Pigmentosa: A Monocentric Experience. Diagnostics 2022, 12, 1020. [Google Scholar] [CrossRef]
- Hagag, A.M.; Wang, J.; Lu, K.; Harman, G.; Weleber, R.G.; Huang, D.; Yang, P.; Pennesi, M.E.; Jia, Y. Projection-Resolved Optical Coherence Tomographic Angiography of Retinal Plexuses in Retinitis Pigmentosa. Am. J. Ophthalmol. 2019, 204, 70–79. [Google Scholar] [CrossRef]
- Jauregui, R.; Park, K.S.; Duong, J.K.; Mahajan, V.B.; Tsang, S.H. Quantitative Progression of Retinitis Pigmentosa by Optical Coherence Tomography Angiography. Sci. Rep. 2018, 8, 13130. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, Y.; Murakami, Y.; Funatsu, J.; Akiyama, M.; Nakatake, S.; Fujiwara, K.; Tachibana, T.; Nakao, S.; Hisatomi, T.; Yoshida, S.; et al. Optical Coherence Tomography Angiography of the Macular Microvasculature Changes in Retinitis Pigmentosa. Acta Ophthalmol. 2018, 96, e59–e67. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lu, J.; Liu, Q.; Wang, Y.; Cao, D.; Wang, J.; Wang, X.; Pan, J.; Ma, L.; Jin, C.; et al. Effect of Choroidal Vessel Density on the Ellipsoid Zone and Visual Function in Retinitis Pigmentosa Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4328–4335. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, R.; D’Aloisio, R.; de Nicola, C.; Ferro, G.; Senatore, A.; Libertini, D.; di Marzio, G.; di Nicola, M.; di Martino, G.; di Antonio, L.; et al. Widefield Swept Source OCTA in Retinitis Pigmentosa. Diagnostics 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Oishi, A.; Hasegawa, T.; Oishi, M.; Numa, S.; Otsuka, Y.; Uji, A.; Kadomoto, S.; Hata, M.; Ikeda, H.O.; et al. Concentric Choriocapillaris Flow Deficits in Retinitis Pigmentosa Detected Using Wide-Angle Swept-Source Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Inoue, T.; Maruyama-Inoue, M.; Yanagi, Y.; Kadonosono, K.; Ogawa, A.; Hashimoto, Y.; Azuma, K.; Terao, R.; Asaoka, R.; et al. Relationship between the Vessel Density around the Optic Nerve Head and Visual Field Deterioration in Eyes with Retinitis Pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 1097–1103. [Google Scholar] [CrossRef]
- Nassisi, M.; Lavia, C.; Mohand-Said, S.; Smirnov, V.; Antonio, A.; Condroyer, C.; Sancho, S.; Varin, J.; Gaudric, A.; Zeitz, C.; et al. Near-Infrared Fundus Autofluorescence Alterations Correlate with Swept-Source Optical Coherence Tomography Angiography Findings in Patients with Retinitis Pigmentosa. Sci. Rep. 2021, 11, 3180. [Google Scholar] [CrossRef]
- Parodi, M.B.; Cicinelli, M.V.; Rabiolo, A.; Pierro, L.; Gagliardi, M.; Bolognesi, G.; Bandello, F. Vessel Density Analysis in Patients with Retinitis Pigmentosa by Means of Optical Coherence Tomography Angiography. Br. J. Ophthalmol. 2017, 101, 428–432. [Google Scholar] [CrossRef]
- Shen, C.; Li, Y.; Wang, Q.; Chen, Y.N.; Li, W.; Wei, W. Choroidal Vascular Changes in Retinitis Pigmentosa Patients Detected by Optical Coherence Tomography Angiography. BMC Ophthalmol. 2020, 20, 384. [Google Scholar] [CrossRef]
- Sugahara, M.; Miyata, M.; Ishihara, K.; Gotoh, N.; Morooka, S.; Ogino, K.; Hasegawa, T.; Hirashima, T.; Yoshikawa, M.; Hata, M.; et al. Optical Coherence Tomography Angiography to Estimate Retinal Blood Flow in Eyes with Retinitis Pigmentosa. Sci. Rep. 2017, 7, 46396. [Google Scholar] [CrossRef]
- Takagi, S.; Hirami, Y.; Takahashi, M.; Fujihara, M.; Mandai, M.; Miyakoshi, C.; Tomita, G.; Kurimoto, Y. Optical Coherence Tomography Angiography in Patients with Retinitis Pigmentosa Who Have Normal Visual Acuity. Acta Ophthalmol. 2018, 96, e636–e642. [Google Scholar] [CrossRef] [PubMed]
- Toto, L.; Borrelli, E.; Mastropasqua, R.; Senatore, A.; di Antonio, L.; di Nicola, M.; Carpineto, P.; Mastropasqua, L. Macular Features in Retinitis Pigmentosa: Correlations Among Ganglion Cell Complex Thickness, Capillary Density, and Macular Function. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6360–6366. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Zhao, Q.; Li, D.J.; Wang, Z.Y.; Chen, W.; Li, Y.F.; Cui, R.; Shen, L.; Wang, R.K.; Peng, X.Y.; et al. Quantitative Evaluation of Primary Retinitis Pigmentosa Patients Using Colour Doppler Flow Imaging and Optical Coherence Tomography Angiography. Acta Ophthalmol. 2019, 97, e993–e997. [Google Scholar] [CrossRef] [PubMed]
- Tolmachova, T.; Wavre-Shapton, S.T.; Barnard, A.R.; MacLaren, R.E.; Futter, C.E.; Seabra, M.C. Retinal Pigment Epithelium Defects Accelerate Photoreceptor Degeneration in Cell Type-Specific Knockout Mouse Models of Choroideremia. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4913–4920. [Google Scholar] [CrossRef] [PubMed]
- Tolmachova, T.; Anders, R.; Abrink, M.; Bugeon, L.; Dallman, M.J.; Futter, C.E.; Ramalho, J.S.; Tonagel, F.; Tanimoto, N.; Seeliger, M.W.; et al. Independent Degeneration of Photoreceptors and Retinal Pigment Epithelium in Conditional Knockout Mouse Models of Choroideremia. J. Clin. Investig. 2006, 116, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Jia, Y.; Gao, S.S.; Zhang, X.; Weleber, R.G.; Huang, D.; Pennesi, M.E. Optical Coherence Tomography Angiography in Choroideremia: Correlating Choriocapillaris Loss with Overlying Degeneration. JAMA Ophthalmol. 2016, 134, 697–702. [Google Scholar] [CrossRef]
- Schaal, K.B.; Freund, K.B.; Litts, K.M.; Zhang, Y.; Messinger, J.D.; Curcio, C.A. Outer Retinal Tubulation in Advanced Age-Related Macular Degeneration: Optical Coherence Tomographic Findings Correspond to Histology. Retina 2015, 35, 1339–1350. [Google Scholar] [CrossRef]
- Abbouda, A.; Dubis, A.M.; Webster, A.R.; Moosajee, M. Identifying Characteristic Features of the Retinal and Choroidal Vasculature in Choroideremia Using Optical Coherence Tomography Angiography. Eye 2018, 32, 563–571. [Google Scholar] [CrossRef]
- Battaglia Parodi, M.; Arrigo, A.; MacLaren, R.E.; Aragona, E.; Toto, L.; Mastropasqua, R.; Manitto, M.P.; Bandello, F. Vascular Alterations Revealed with Optical Coherence Tomography Angiography in Patients with Choroideremia. Retina 2019, 39, 1200–1205. [Google Scholar] [CrossRef]
- Murro, V.; Mucciolo, D.P.; Giorgio, D.; Sodi, A.; Passerini, I.; Virgili, G.; Rizzo, S. Optical Coherence Tomography Angiography (OCT-A) in Young Choroideremia (CHM) Patients. Ophthalmic Genet. 2019, 40, 201–206. [Google Scholar] [CrossRef]
- Murro, V.; Mucciolo, D.P.; Giorgio, D.; Sodi, A.; Passerini, I.; Virgili, G.; Rizzo, S. Optical Coherence Tomography Angiography (OCT-A) in Choroideremia (CHM) Carriers. Ophthalmic Genet. 2020, 41, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Romano, F.; Parodi, M.B.; Charbel Issa, P.; Birtel, J.; Bandello, F.; MacLaren, R.E. Reduced Vessel Density in Deep Capillary Plexus Correlates with Retinal Layer Thickness in Choroideremia. Br. J. Ophthalmol. 2021, 105, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.C.; Gao, S.S.; Zhang, M.; Alabduljalil, T.; Al-Qahtani, A.; Weleber, R.G.; Yang, P.; Jia, Y.; Huang, D.; Pennesi, M.E. Optical Coherence Tomography Angiography of Choroidal Neovascularization in Four Inherited Retinal Dystrophies. Retina 2016, 36, 2339–2347. [Google Scholar] [CrossRef]
- Ranjan, R.; Verghese, S.; Salian, R.; Manayath, G.J.; Saravanan, V.R.; Narendran, V. OCT Angiography for the Diagnosis and Management of Choroidal Neovascularization Secondary to Choroideremia. Am. J. Ophthalmol. Case Rep. 2021, 22, 101042. [Google Scholar] [CrossRef]
- Sodi, A.; Passerini, I.; Murro, V.; Caputo, R.; Bacci, G.M.; Bodoj, M.; Torricelli, F.; Menchini, U. BEST1 Sequence Variants in Italian Patients with Vitelliform Macular Dystrophy. Mol. Vis. 2012, 18, 2736–2748. [Google Scholar] [PubMed]
- Jauregui, R.; Parmann, R.; Nuzbrokh, Y.; Tsang, S.H.; Sparrow, J.R. Stage-Dependent Choriocapillaris Impairment in Best Vitelliform Macular Dystrophy Characterized by Optical Coherence Tomography Angiography. Sci. Rep. 2021, 11, 14300. [Google Scholar] [CrossRef]
- Wang, X.N.; You, Q.S.; Li, Q.; Li, Y.; Mao, Y.; Hu, F.; Zhao, H.Y.; Tsai, F.F.; Peng, X.Y. Findings of Optical Coherence Tomography Angiography in Best Vitelliform Macular Dystrophy. Ophthalmic Res. 2018, 60, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Battaglia Parodi, M.; Romano, F.; Cicinelli, M.V.; Rabiolo, A.; Arrigo, A.; Pierro, L.; Iacono, P.; Bandello, F. Retinal Vascular Impairment in Best Vitelliform Macular Dystrophy Assessed by Means of Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2018, 187, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Mirshahi, A.; Lashay, A.; Masoumi, A.; Abrishami, M. Optical Coherence Tomography Angiography in Best Vitelliform Macular Dystrophy. J. Curr. Ophthalmol. 2019, 31, 442–445. [Google Scholar] [CrossRef]
- Da, S.; Maurizio, P.; Parodi, B.; Toto, L.; Ravalico, G.; Parodi, M.B. Occult Choroidal Neovascularization in Adult-Onset Foveomacular Vitelliform Dystrophy. Ophthalmologica 2001, 215, 412–414. [Google Scholar]
- Parodi, M.B.; Arrigo, A.; Bandello, F. Optical Coherence Tomography Angiography Quantitative Assessment of Macular Neovascularization in Best Vitelliform Macular Dystrophy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 61. [Google Scholar] [CrossRef]
- Arrigo, A.; Bordato, A.; Aragona, E.; Amato, A.; Viganò, C.; Bandello, F.; Battaglia Parodi, M. Macular Neovascularization in AMD, CSC and Best Vitelliform Macular Dystrophy: Quantitative OCTA Detects Distinct Clinical Entities. Eye 2021, 35, 3266–3276. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.H.; Sharma, T. Stargardt Disease. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1085, pp. 139–151. [Google Scholar]
- Arrigo, A.; Romano, F.; Aragona, E.; di Nunzio, C.; Sperti, A.; Bandello, F.; Parodi, M.B. Octa-Based Identification of Different Vascular Patterns in Stargardt Disease. Transl. Vis. Sci. Technol. 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Grazioli, A.; Romano, F.; Aragona, E.; Bordato, A.; di Nunzio, C.; Sperti, A.; Bandello, F.; Parodi, M.B. Choroidal Patterns in Stargardt Disease: Correlations with Visual Acuity and Disease Progression. J. Clin. Med. 2019, 8, 1388. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, R.; Toto, L.; Borrelli, E.; Di Antonio, L.; Mattei, P.A.; Senatore, A.; Di Nicola, M.; Mariotti, C. Optical Coherence Tomography Angiography Findings in Stargardt Disease. PLoS ONE 2017, 12, e0170343. [Google Scholar] [CrossRef] [PubMed]
- Della Volpe Waizel, M.; Scholl, H.P.N.; Todorova, M.G. Microvascular and Metabolic Alterations in Retinitis Pigmentosa and Stargardt Disease. Acta Ophthalmol. 2021, 99, e1396–e1404. [Google Scholar] [CrossRef]
- Birnbach, C.D.; Järveläínen, M.; Possin, D.E.; Milam, A.H. Histopathology and Immunocytochemistry of the Neurosensory Retina in Fundus Flavimaculatus. Ophthalmology 1994, 101, 1211–1219. [Google Scholar] [CrossRef]
- Müller, P.L.; Pfau, M.; Möller, P.T.; Nadal, J.; Schmid, M.; Lindner, M.; de Sisternes, L.; Stöhr, H.; Weber, B.H.F.; Neuhaus, C.; et al. Choroidal Flow Signal in Late-Onset Stargardt Disease and Age-Related Macular Degeneration: An OCT-Angiography Study. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD122–AMD131. [Google Scholar] [CrossRef]
- Mansour, A.M.; Elnahry, A.G.; Tripathy, K.; Foster, R.E.; Mehanna, C.J.; Vishal, R.; Çavdarlı, C.; Arrigo, A.; Parodi, M.B. Analysis of Optical Coherence Angiography in Cystoid Macular Oedema Associated with Gyrate Atrophy. Eye 2021, 35, 1766–1774. [Google Scholar] [CrossRef]
- Zhioua Braham, I.; Ammous, I.; Maalej, R.; Boukari, M.; Mili Boussen, I.; Errais, K.; Zhioua, R. Multimodal Imaging of Foveoschisis and Macular Pseudohole Associated with Gyrate Atrophy: A Family Report. BMC Ophthalmol. 2018, 18, 89. [Google Scholar] [CrossRef]
- Miyata, M.; Oishi, A.; Hasegawa, T.; Ishihara, K.; Oishi, M.; Ogino, K.; Sugahara, M.; Hirashima, T.; Hata, M.; Yoshikawa, M.; et al. Choriocapillaris Flow Deficit in Bietti Crystalline Dystrophy Detected Using Optical Coherence Tomography Angiography. Br. J. Ophthalmol. 2018, 102, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Tiryaki Demir, S.; Keles Yesiltas, S.; Kacar, H.; Akbas, E.B.; Guven, D. Optical Coherence Tomography and Optical Coherence Tomography Angiography Imaging in Bietti Crystalline Dystrophy. Ophthalmic Genet. 2020, 41, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Ito, Y.; Kaneko, H.; Kataoka, K.; Ra, E.; Terasaki, H. Optical Coherence Tomography Angiography in Leber Hereditary Optic Neuropathy. Acta Ophthalmol. 2017, 95, e344–e345. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Falavarjani, K.; Tian, J.J.; Akil, H.; Garcia, G.A.; Sadda, S.R.; Sadun, A.A. Swept-Source Optical Coherence Tomography Angiography of The Optic Disk in Optic Neuropathy. Retina 2016, 36, S168–S177. [Google Scholar] [CrossRef]
- Gaier, E.D.; Gittinger, J.W.; Cestari, D.M.; Miller, J.B. Peripapillary Capillary Dilation in Leber Hereditary Optic Neuropathy Revealed by Optical Coherence Tomographic Angiography. JAMA Ophthalmol. 2016, 134, 1332–1334. [Google Scholar] [CrossRef]
- Borrelli, E.; Balasubramanian, S.; Triolo, G.; Barboni, P.; Sadda, S.V.R.; Sadun, A.A. Topographic Macular Microvascular Changes and Correlation With Visual Loss in Chronic Leber Hereditary Optic Neuropathy. Am. J. Ophthalmol. 2018, 192, 217–228. [Google Scholar] [CrossRef]
- Balducci, N.; Cascavilla, M.L.; Ciardella, A.; la Morgia, C.; Triolo, G.; Parisi, V.; Bandello, F.; Sadun, A.A.; Carelli, V.; Barboni, P. Peripapillary Vessel Density Changes in Leber’s Hereditary Optic Neuropathy: A New Biomarker. Clin. Exp. Ophthalmol. 2018, 46, 1055–1062. [Google Scholar] [CrossRef]
- Yu, J.; Xu, H.; Huang, Y.; Gu, R.; Zong, Y.; Zhu, H.; Wang, M. Changes in Retinal Perfusion in Leber’s Hereditary Optic Neuropathy: An Optical Coherence Tomography-Angiography Study. Ophthalmic Res. 2021, 64, 863–870. [Google Scholar] [CrossRef]
- Kousal, B.; Kolarova, H.; Meliska, M.; Bydzovsky, J.; Diblik, P.; Kulhanek, J.; Votruba, M.; Honzik, T.; Liskova, P. Peripapillary Microcirculation in Leber Hereditary Optic Neuropathy. Acta Ophthalmol. 2019, 97, e71–e76. [Google Scholar] [CrossRef]
- Molday, R.S.; Kellner, U.; Weber, B.H.F. X-Linked Juvenile Retinoschisis: Clinical Diagnosis, Genetic Analysis, and Molecular Mechanisms. Prog. Retin. Eye Res. 2012, 31, 195–212. [Google Scholar] [CrossRef]
- Padrón-Pérez, N.; Català-Mora, J.; Díaz, J.; Arias, L.; Prat, J.; Caminal, J.M. Swept-Source and Optical Coherence Tomography Angiography in Patients with X-Linked Retinoschisis. Eye 2018, 32, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Han, I.C.; Whitmore, S.S.; Critser, D.B.; Lee, S.Y.; DeLuca, A.P.; Daggett, H.T.; Affatigato, L.M.; Mullins, R.F.; Tucker, B.A.; Drack, A.V.; et al. Wide-Field Swept-Source OCT and Angiography in X-Linked Retinoschisis. Ophthalmol. Retina 2019, 3, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Stringa, F.; Tsamis, E.; Papayannis, A.; Chwiejczak, K.; Jalil, A.; Biswas, S.; Ahmad, H.; Stanga, P.E. Segmented Swept Source Optical Coherence Tomography Angiography Assessment of the Perifoveal Vasculature in Patients with X-Linked Juvenile Retinoschisis: A Serial Case Report. Int. Med. Case Rep. J. 2017, 10, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Arrigo, A.; Ch’Ng, S.W.; Parodi, M.B.; Manitto, M.P.; Martina, E.; Bandello, F.; Stanga, P.E. Capillary Network Alterations in X-Linked Retinoschisis Imaged on Optical Coherence Tomography Angiography. Retina 2019, 39, 1761–1767. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Al-Sheikh, M.; Akil, H.; Sadda, S.R. Image Artefacts in Swept-Source Optical Coherence Tomography Angiography. Br. J. Ophthalmol. 2017, 101, 564–568. [Google Scholar] [CrossRef]

| Authors | Study | F-UP | N. Eyes | SCP VD | DCP VD | CC VD | CH VD | ONH/RPL VD | FAZ Area |
|---|---|---|---|---|---|---|---|---|---|
| Alnawaiseh [15] | P | NA | 20 | Reduced | Reduced | Reduced | / | Reduced | Increased |
| Arrigo [16] | P | 12 MO | 68 | Reduced | Reduced | / | / | / | / |
| Atas [17] | R | NA | 26 | Reduced | Reduced | / | / | / | / |
| Attaallah [18] | P | 3 MO | 24 | Reduced | Reduced | Reduced | / | / | Increased |
| Deutsch [19] | R | 24 MO | 29 | Reduced | Reduced | Reduced | Reduced | / | Reduced |
| Giansanti [20] | R | 13 MO | 52 | Reduced | Reduced | Reduced | Reduced | / | Reduced |
| Hagag [21] | P | NA | 44 | Reduced | Reduced | / | / | / | / |
| Jauregui [22] | R | 15 MO | 28 | Reduced | Reduced | / | / | / | Increased |
| Koyanagi [23] | R | 24 MO | 73 | Both Reduced | Both Reduced | / | / | / | Increased |
| Liu [24] | R | 36 MO | 53 | / | / | Reduced | Reduced | / | / |
| Mastropasqua [25] | P | 6 MO | 20 | Both Reduced | Both Reduced | Both Reduced | / | / | / |
| Miyata [26] | P | 2 MO | 43 | / | / | Reduced | / | / | / |
| Nakajima [27] | R | NA | 38 | / | Reduced | / | / | Reduced | / |
| Nassisi [28] | R | 9 MO | 28 | Reduced | Reduced | Reduced | / | / | / |
| Parodi [29] | R | 8 MO | 32 | Reduced | Reduced | / | / | / | Increased |
| Shen [30] | P | 10 MO | 34 | Reduced | Reduced | / | / | / | / |
| Sugahara [31] | R | NA | 68 | Reduced | Reduced | / | / | / | / |
| Takagi [32] | R | 6 MO | 50 | Reduced | Reduced | / | / | / | Reduced |
| Toto [33] | R | NA | 28 | Reduced | Reduced | Reduced | / | / | / |
| Wang [34] | P | NA | 40 | Both Reduced | Both Reduced | Both Reduced | Increased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iovino, C.; Iodice, C.M.; Pisani, D.; Damiano, L.; Di Iorio, V.; Testa, F.; Simonelli, F. Clinical Applications of Optical Coherence Tomography Angiography in Inherited Retinal Diseases: An Up-to-Date Review of the Literature. J. Clin. Med. 2023, 12, 3170. https://doi.org/10.3390/jcm12093170
Iovino C, Iodice CM, Pisani D, Damiano L, Di Iorio V, Testa F, Simonelli F. Clinical Applications of Optical Coherence Tomography Angiography in Inherited Retinal Diseases: An Up-to-Date Review of the Literature. Journal of Clinical Medicine. 2023; 12(9):3170. https://doi.org/10.3390/jcm12093170
Chicago/Turabian StyleIovino, Claudio, Clemente Maria Iodice, Danila Pisani, Luciana Damiano, Valentina Di Iorio, Francesco Testa, and Francesca Simonelli. 2023. "Clinical Applications of Optical Coherence Tomography Angiography in Inherited Retinal Diseases: An Up-to-Date Review of the Literature" Journal of Clinical Medicine 12, no. 9: 3170. https://doi.org/10.3390/jcm12093170
APA StyleIovino, C., Iodice, C. M., Pisani, D., Damiano, L., Di Iorio, V., Testa, F., & Simonelli, F. (2023). Clinical Applications of Optical Coherence Tomography Angiography in Inherited Retinal Diseases: An Up-to-Date Review of the Literature. Journal of Clinical Medicine, 12(9), 3170. https://doi.org/10.3390/jcm12093170







