Association of De Ritis Ratio with Prognosis in Patients with Coronary Artery Disease and Aminotransferase Activity within and outside the Healthy Values of Reference Range
Abstract
:1. Introduction
2. Methods
2.1. Study Patients
2.2. Definition of Healthy Range of Aminotransferases
2.3. Other Definitions
2.4. Biochemical Measurements
2.5. Endpoints and Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Correlates of De Ritis Ratio
3.3. Three-Year Mortality
3.4. Mortality Discrimination by De Ritis Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Ritis, F.; Coltorti, M.; Giusti, G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin. Chim. Acta 1957, 2, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.; Cash, J. What is the real function of the liver ‘function’ tests? Ulster. Med. J. 2012, 81, 30–36. [Google Scholar] [PubMed]
- Liu, Z.; Que, S.; Xu, J.; Peng, T. Alanine aminotransferase-old biomarker and new concept: A review. Int. J. Med. Sci. 2014, 11, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Piton, A.; Poynard, T.; Imbert-Bismut, F.; Khalil, L.; Delattre, J.; Pelissier, E.; Sansonetti, N.; Opolon, P. Factors associated with serum alanine transaminase activity in healthy subjects: Consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC Group. Hepatology 1998, 27, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Prati, D.; Taioli, E.; Zanella, A.; Della Torre, E.; Butelli, S.; Del Vecchio, E.; Vianello, L.; Zanuso, F.; Mozzi, F.; Milani, S.; et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann. Intern. Med. 2002, 137, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnejad, M.; Pourshams, A.; Malekzadeh, R.; Mohamadkhani, A.; Rajabiani, A.; Asgari, A.A.; Alimohamadi, S.M.; Razjooyan, H.; Mamar-Abadi, M. Healthy ranges of serum alanine aminotransferase levels in Iranian blood donors. World J. Gastroenterol. 2003, 9, 2322–2324. [Google Scholar] [CrossRef] [PubMed]
- Kariv, R.; Leshno, M.; Beth-Or, A.; Strul, H.; Blendis, L.; Kokia, E.; Noff, D.; Zelber-Sagie, S.; Sheinberg, B.; Oren, R.; et al. Re-evaluation of serum alanine aminotransferase upper normal limit and its modulating factors in a large-scale population study. Liver Int. 2006, 26, 445–450. [Google Scholar] [CrossRef]
- Ceriotti, F.; Henny, J.; Queralto, J.; Ziyu, S.; Ozarda, Y.; Chen, B.; Boyd, J.C.; Panteghini, M.; Intervals, I.C.O.R.; Decision, L.; et al. Common reference intervals for aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) in serum: Results from an IFCC multicenter study. Clin. Chem. Lab. Med. 2010, 48, 1593–1601. [Google Scholar] [CrossRef]
- Lee, J.K.; Shim, J.H.; Lee, H.C.; Lee, S.H.; Kim, K.M.; Lim, Y.S.; Chung, Y.H.; Lee, Y.S.; Suh, D.J. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology 2010, 51, 1577–1583. [Google Scholar] [CrossRef]
- Kang, H.S.; Um, S.H.; Seo, Y.S.; An, H.; Lee, K.G.; Hyun, J.J.; Kim, E.S.; Park, S.C.; Keum, B.; Kim, J.H.; et al. Healthy range for serum ALT and the clinical significance of “unhealthy” normal ALT levels in the Korean population. J. Gastroenterol. Hepatol. 2011, 26, 292–299. [Google Scholar] [CrossRef]
- Wu, W.C.; Wu, C.Y.; Wang, Y.J.; Hung, H.H.; Yang, H.I.; Kao, W.Y.; Su, C.W.; Wu, J.C.; Chan, W.L.; Lin, H.C.; et al. Updated thresholds for serum alanine aminotransferase level in a large-scale population study composed of 34 346 subjects. Aliment. Pharmacol. Ther. 2012, 36, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.H.; Shi, K.Q.; Fan, Y.C.; Liu, W.Y.; Lin, X.F.; Li, L.F.; Chen, Y.P. Upper limits of normal for serum alanine aminotransferase levels in Chinese Han population. PLoS ONE 2012, 7, e43736. [Google Scholar] [CrossRef] [PubMed]
- Park, H.N.; Sinn, D.H.; Gwak, G.Y.; Kim, J.E.; Rhee, S.Y.; Eo, S.J.; Kim, Y.J.; Choi, M.S.; Lee, J.H.; Koh, K.C.; et al. Upper normal threshold of serum alanine aminotransferase in identifying individuals at risk for chronic liver disease. Liver Int. 2012, 32, 937–944. [Google Scholar] [CrossRef]
- Ruhl, C.E.; Everhart, J.E. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology 2012, 55, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Jun, D.W.; Kwak, M.J.; Park, Q.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; Yoon, B.C.; Choi, H.S. Upper limit of normal serum alanine and aspartate aminotransferase levels in Korea. J. Gastroenterol. Hepatol. 2013, 28, 522–529. [Google Scholar] [CrossRef]
- Al-hamoudi, W.; Ali, S.; Hegab, B.; Elsiesy, H.; Hashim, A.; Al-Sofayan, M.; Khalaf, H.; Al-Bahili, H.; Al-Masri, N.; Al-Sebayel, M.; et al. Revising the upper limit of normal for levels of serum alanine aminotransferase in a Middle Eastern population with normal liver histology. Dig. Dis. Sci. 2013, 58, 2369–2375. [Google Scholar] [CrossRef]
- Tanaka, K.; Hyogo, H.; Ono, M.; Takahashi, H.; Kitajima, Y.; Ono, N.; Eguchi, T.; Fujimoto, K.; Chayama, K.; Saibara, T.; et al. Upper limit of normal serum alanine aminotransferase levels in Japanese subjects. Hepatol. Res. 2014, 44, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, C.Y.; Li, Y.X.; Pan, Y.; Niu, J.Q.; He, S.M. Determination of the upper cut-off values of serum alanine aminotransferase and aspartate aminotransferase in Chinese. World J. Gastroenterol. 2015, 21, 2419–2424. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Saraf, N.; Saigal, S.; Gautam, D.; Lipi, L.; Soin, A.S. Estimation of normal values of serum transaminases based on liver histology in healthy Asian Indians. J. Gastroenterol. Hepatol. 2015, 30, 763–766. [Google Scholar] [CrossRef]
- Mohan, P.; Sundar, V.; Bhaskar, E.; Anthony, S. Estimation of Upper Limit of Normal for Serum Alanine Transaminase in Healthy South Indian Population. Indian J. Clin. Biochem. 2017, 32, 337–342. [Google Scholar] [CrossRef]
- Martin-Rodriguez, J.L.; Gonzalez-Cantero, J.; Gonzalez-Cantero, A.; Arrebola, J.P.; Gonzalez-Calvin, J.L. Diagnostic accuracy of serum alanine aminotransferase as biomarker for nonalcoholic fatty liver disease and insulin resistance in healthy subjects, using 3T MR spectroscopy. Medicine 2017, 96, e6770. [Google Scholar] [CrossRef] [PubMed]
- Najmy, S.; Duseja, A.; Pal, A.; Sachdev, S.; Sharma, R.R.; Marwah, N.; Chawla, Y. Redefining the Normal Values of Serum Aminotransferases in Healthy Indian Males. J. Clin. Exp. Hepatol. 2019, 9, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Degertekin, B.; Tozun, N.; Demir, F.; Soylemez, G.; Yapali, S.; Bozkurt, U.; Gurtay, E.; Seymenoglu, T.H.; Mutlu, D.; Toraman, M. Determination of the upper limits of normal serum alanine aminotransferase (ALT) level in healthy Turkish population. Hepatol. Forum. 2020, 1, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Pelusi, S.; Bianco, C.; Ceriotti, F.; Berzuini, A.; Iogna Prat, L.; Trotti, R.; Malvestiti, F.; D’Ambrosio, R.; Lampertico, P.; et al. Definition of Healthy Ranges for Alanine Aminotransferase Levels: A 2021 Update. Hepatol. Commun. 2021, 5, 1824–1832. [Google Scholar] [CrossRef]
- Huong, N.T.C.; Karimzadeh, S.; Thanh, N.T.; Thuan, T.M.; Sabbah, G.M.; Ismaeil, K.; An, D.N.T.; Huong, L.T.; Huy, N.T.; Thi Le Hoa, P. Updated upper limit of normal for serum alanine aminotransferase value in Vietnamese population. BMJ Open Gastroenterol. 2022, 9, e000870. [Google Scholar] [CrossRef]
- Ruhl, C.E.; Everhart, J.E. The association of low serum alanine aminotransferase activity with mortality in the US population. Am. J. Epidemiol. 2013, 178, 1702–1711. [Google Scholar] [CrossRef]
- Xie, K.; Chen, C.H.; Tsai, S.P.; Lu, P.J.; Wu, H.; Zeng, Y.; Ye, Y.; Tu, H.; Wen, C.; Huang, M.; et al. Loss of Life Expectancy by 10 Years or More From Elevated Aspartate Aminotransferase: Finding Aspartate Aminotransferase a Better Mortality Predictor for All-Cause and Liver-Related than Alanine Aminotransferase. Am. J. Gastroenterol. 2019, 114, 1478–1487. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Holdenrieder, S.; Cassese, S.; Xhepa, E.; Fusaro, M.; Laugwitz, K.L.; Schunkert, H.; Kastrati, A. Aspartate aminotransferase and mortality in patients with ischemic heart disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2335–2342. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Holdenrieder, S.; Colleran, R.; Cassese, S.; Xhepa, E.; Fusaro, M.; Laugwitz, K.L.; Schunkert, H.; Kastrati, A. Inverse association of alanine aminotransferase within normal range with prognosis in patients with coronary artery disease. Clin. Chim. Acta 2019, 496, 55–61. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Holdenrieder, S.; Kastrati, A. Prognostic value of De Ritis ratio with aspartate aminotransferase and alanine aminotransferase within the reference range. Clin. Chim. Acta 2023, 538, 46–52. [Google Scholar] [CrossRef]
- Sandler, H.; Dodge, H.T. The use of single plane angiocardiograms for the calculation of left ventricular volume in man. Am. Heart J. 1968, 75, 325–334. [Google Scholar] [CrossRef]
- Pottel, H.; Bjork, J.; Courbebaisse, M.; Couzi, L.; Ebert, N.; Eriksen, B.O.; Dalton, R.N.; Dubourg, L.; Gaillard, F.; Garrouste, C.; et al. Development and Validation of a Modified Full Age Spectrum Creatinine-Based Equation to Estimate Glomerular Filtration Rate: A Cross-sectional Analysis of Pooled Data. Ann. Intern. Med. 2021, 174, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, M. Ueber den Niederschlag welchen Pikrinsaüre in normalen Harn erzeugt und über eine neue reaction des Kreatinins. Z. Physiol. Chem. 1886, 10, 391–400. [Google Scholar]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D. Circulating Markers of Liver Function and Cardiovascular Disease Risk. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Brancati, F.L.; Diehl, A.M. The prevalence and etiology of elevated aminotransferase levels in the United States. Am. J. Gastroenterol. 2003, 98, 960–967. [Google Scholar] [CrossRef]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Ackerman, Z.; Maaravi, Y.; Ben-Dov, I.Z.; Ein-Mor, E.; Stessman, J. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J. Am. Geriatr. Soc. 2006, 54, 1719–1724. [Google Scholar] [CrossRef]
- Hovinen, S.M.; Pitkala, K.H.; Tilvis, R.S.; Strandberg, T.E. Alanine aminotransferase activity and mortality in older people. J. Am. Geriatr. Soc. 2010, 58, 1399–1401. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Blyth, F.M.; Creasey, H.M.; Handelsman, D.J.; Naganathan, V.; Sambrook, P.N.; Seibel, M.J.; Waite, L.M.; Cumming, R.G. The association of alanine transaminase with aging, frailty, and mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 712–717. [Google Scholar] [CrossRef]
- Delanghe, J.R.; De Buyzere, M.L. Also low enzyme activities have a clinical meaning! Clin. Chim. Acta 2019, 496, 142. [Google Scholar] [CrossRef] [PubMed]
- Thapar, M.; Russo, M.W.; Bonkovsky, H.L. Statins and liver injury. Gastroenterol. Hepatol. (N. Y.) 2013, 9, 605–606. [Google Scholar] [PubMed]
- Jalali, M.; Rahimlou, M.; Mahmoodi, M.; Moosavian, S.P.; Symonds, M.E.; Jalali, R.; Zare, M.; Imanieh, M.H.; Stasi, C. The effects of metformin administration on liver enzymes and body composition in non-diabetic patients with non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis: An up-to date systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 159, 104799. [Google Scholar] [PubMed]
- Arora, N.; Goldhaber, S.Z. Anticoagulants and transaminase elevation. Circulation 2006, 113, e698–e702. [Google Scholar] [CrossRef]

| Characteristic | AST and ALT in Reference Range (n = 3392) | p Value | |
|---|---|---|---|
| AST and ALT in the Healthy Range (n = 1697) | AST and/or ALT outside the Healthy Range (n = 1695) | ||
| De Ritis ratio (median [25th–75th percentile]) | 0.94 [0.79–1.12] | 0.93 [0.67–1.33] | 0.700 |
| De Ritis ratio (median [5th–95th percentile]) | 0.94 [0.61–1.41] | 0.93 [0.45–1.96] | 0.700 |
| Age (years) | 67.1 [60.1–73.7] | 68.0 [60.2–75.1] | 0.080 |
| Women | 289 (17.0) | 407 (24.0) | <0.001 |
| Type 2 diabetes | 425 (25.0) | 424 (25.0) | 0.984 |
| Arterial hypertension | 1265 (74.5) | 1223 (72.2) | 0.115 |
| Hypercholesterolemia | 1228 (72.4) | 1180 (69.6) | 0.078 |
| Body mass index (kg/m2) | 26.9 [24.7–29.5] | 26.7 [24.4–29.4] | 0.100 |
| Current smoker | 258 (15.2) | 265 (15.6) | 0.728 |
| Previous myocardial infarction | 422 (24.9) | 439 (25.9) | 0.490 |
| Previous coronary artery bypass surgery | 221 (13.0) | 220 (13.0) | 0.970 |
| Extent of coronary artery disease | 0.970 | ||
| One vessel | 405 (23.9) | 410 (24.2) | |
| Two vessels | 521 (30.7) | 521 (30.7) | |
| Three vessels | 771 (45.4) | 764 (45.1) | |
| Multivessel disease | 1292 (76.1) | 1285 (75.8) | 0.826 |
| Atrial fibrillation | 220 (13.0) | 274 (16.2) | 0.008 |
| C-reactive protein (mg/L) | 1.93 [0.82–5.37] | 2.70 [0.98–8.94] | <0.001 |
| Baseline cardiac troponin T (µg/L) * | 0.00 [0.00–0.01] | 0.00 [0.00–0.01] | <0.001 |
| Aspartate aminotransferase (U/L) | 21.0 [18.4–24.0] | 22.9 [13.0–30.0] | <0.001 |
| Alanine aminotransferase (U/L) | 22.2 [18.5–26.7] | 22.0 [13.6–34.0] | 0.100 |
| Alkaline phosphatase (U/L) | 68.0 [55.8–82.3] | 73.5 [60.1–92.1] | <0.001 |
| Gamma-glutamyl transferase (U/L) | 31.4 [23.2–47.8] | 37.0 [25.0–62.8] | <0.001 |
| LDL-cholesterol (mg/dL) | 106 [83–135] | 111 [85–140] | 0.010 |
| HDL-cholesterol (mg/dL) | 48 [40–58] | 48 [40–59] | 0.700 |
| Serum creatinine (mg/dL) | 0.95 [0.80–1.10] | 1.00 [0.85–1.20] | <0.001 |
| Glomerular filtration rate (mL/min/1.73 m2) | 74 [61–85] | 70 [55–82] | <0.001 |
| Glucose on admission (mg/dL) | 105 [95–121] | 103 [92–120] | 0.005 |
| Glucated hemoglobin (%) | 6.3 [5.9–7.0] | 6.3 [5.8–7.3] | 0.900 |
| Left ventricular ejection fraction (%) ** | 60 [50–64] | 59.5 [50–65] | 0.400 |
| Outcome | AST and ALT in the Healthy Range (n = 1697) | AST and/or ALT outside the Healthy Range (n = 1695) | ||||||
|---|---|---|---|---|---|---|---|---|
| De Ritis Ratio | HR [95% CI] | p Value | De Ritis Ratio | HR [95% CI] | p Value | |||
| ≤Median (n = 848) | >Median (n = 849) | ≤Median (n = 847) | >Median (n = 848) | |||||
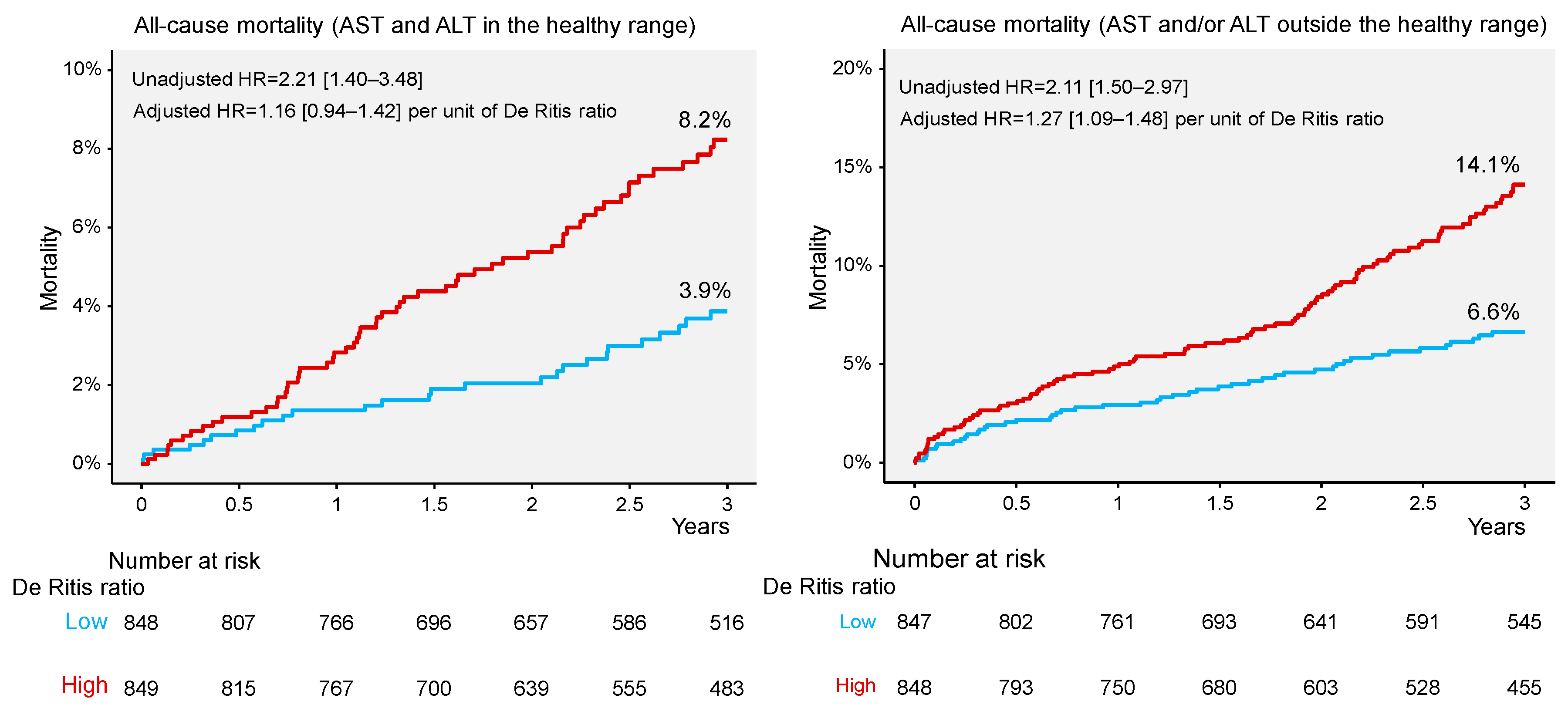
| All-cause mortality | 27 (3.9) | 59 (8.2) | 2.21 [1.40–3.48] | <0.001 | 49 (6.6) | 99 (14.1) | 2.11 [1.50–2.97] | <0.001 |
| Cardiac mortality | 15 (2.1) | 23 (3.2) | 1.54 [0.80–2.95] | 0.190 | 30 (4.2) | 53 (7.7) | 1.84 [1.18–2.88] | 0.008 |
| Noncardiac mortality | 12 (1.8) | 36 (5.2) | 3.05 [1.59–5.86] | <0.001 | 19 (2.6) | 46 (7.0) | 2.53 [1.48–4.32] | <0.001 |
| Variable | All-Cause Mortality | |||
|---|---|---|---|---|
| Aminotransferase Levels in the Healthy Range | Aminotransferase Levels outside the Healthy Range | |||
| Hazard Ratio [95% Confidence Interval] | p Value | Hazard Ratio [95% Confidence Interval] | p Value | |
| De Ritis ratio (for 1 unit higher) | 1.16 [0.94–1.42] | 0.159 | 1.27 [1.09–1.48] | 0.002 |
| Age (for 10-year increment) | 1.84 [1.33–2.56] | <0.001 | 1.39 [1.11–1.74] | 0.004 |
| Women | 0.58 [0.30–1.12] | 0.104 | 1.26 [0.86–1.84] | 0.253 |
| Arterial hypertension | 0.64 [0.39–1.04] | 0.074 | 0.70 [0.49–1.04] | 0.052 |
| Body mass index (for 5 kg/m2 higher) | 0.91 [0.69–1.21] | 0.550 | 0.79 [0.64–0.98] | 0.033 |
| Diabetes mellitus | 1.79 [1.01–2.84] | 0.047 | 1.59 [1.05–2.40] | 0.027 |
| Current smoking | 1.52 [0.79–2.93] | 0.211 | 1.64 [1.01–2.66] | 0.045 |
| Atrial fibrillation | 2.36 [1.44–3.85] | <0.001 | 1.31 [0.89–1.93] | 0.174 |
| Multivessel disease | 1.17 [0.66–2.08] | 0.601 | 1.04 [0.66–1.64] | 0.860 |
| Previous coronary artery bypass surgery | 0.45 [0.22–0.93] | 0.030 | 1.60 [1.07–2.40] | 0.023 |
| C-reactive protein (for 5 mg/L higher) | 1.03 [1.01–1.05] | <0.001 | 1.02 [1.01–1.04] | <0.001 |
| Estimated glomerular filtration rate (for 30 mL/min lower) | 1.87 [1.21–2.90] | 0.005 | 1.92 [1.42–2.60] | <0.001 |
| Baseline cardiac troponin T (for 5 ULN higher) | 1.00 [0.96–1.03] | 0.851 | 1.00 [0.98–1.02] | 0.964 |
| Gamma-glutamyl transferase (for 10 U/L higher) | 1.00 [0.96–1.04] | 0.923 | 1.02 [1.01–1.04] | 0.020 |
| Low-density lipoprotein-cholesterol (for 10 mg/dL higher) | 1.02 [0.97–1.05] | 0.529 | 1.03 [0.98–1.06] | 0.578 |
| High-density lipoprotein-cholesterol (for 10 mg/dL higher) | 0.95 [0.85–1.06] | 0.287 | 0.95 [0.84–1.07] | 0.387 |
| Glucose on admission (10 mg/dL higher) | 1.02 [0.98–1.07] | 0.391 | 1.00 [0.95–1.05] | 0.999 |
| Left ventricular ejection fraction (for 10% lower) | 1.40 [1.19–1.65] | <0.001 | 1.33 [1.19–1.49] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndrepepa, G.; Cassese, S.; Scalamogna, M.; Lahu, S.; Aytekin, A.; Xhepa, E.; Schunkert, H.; Kastrati, A. Association of De Ritis Ratio with Prognosis in Patients with Coronary Artery Disease and Aminotransferase Activity within and outside the Healthy Values of Reference Range. J. Clin. Med. 2023, 12, 3174. https://doi.org/10.3390/jcm12093174
Ndrepepa G, Cassese S, Scalamogna M, Lahu S, Aytekin A, Xhepa E, Schunkert H, Kastrati A. Association of De Ritis Ratio with Prognosis in Patients with Coronary Artery Disease and Aminotransferase Activity within and outside the Healthy Values of Reference Range. Journal of Clinical Medicine. 2023; 12(9):3174. https://doi.org/10.3390/jcm12093174
Chicago/Turabian StyleNdrepepa, Gjin, Salvatore Cassese, Maria Scalamogna, Shqipdona Lahu, Alp Aytekin, Erion Xhepa, Heribert Schunkert, and Adnan Kastrati. 2023. "Association of De Ritis Ratio with Prognosis in Patients with Coronary Artery Disease and Aminotransferase Activity within and outside the Healthy Values of Reference Range" Journal of Clinical Medicine 12, no. 9: 3174. https://doi.org/10.3390/jcm12093174
APA StyleNdrepepa, G., Cassese, S., Scalamogna, M., Lahu, S., Aytekin, A., Xhepa, E., Schunkert, H., & Kastrati, A. (2023). Association of De Ritis Ratio with Prognosis in Patients with Coronary Artery Disease and Aminotransferase Activity within and outside the Healthy Values of Reference Range. Journal of Clinical Medicine, 12(9), 3174. https://doi.org/10.3390/jcm12093174








