Outcome of Surgery for Ischemic Mitral Regurgitation Depends on the Type and Timing of the Coronary Revascularization
Abstract
1. Introduction
2. Methods
2.1. Study Population and Definition of IMR
2.2. Data Collection and Variable Selection
2.3. Outcome Measures
2.4. Statistical Analysis
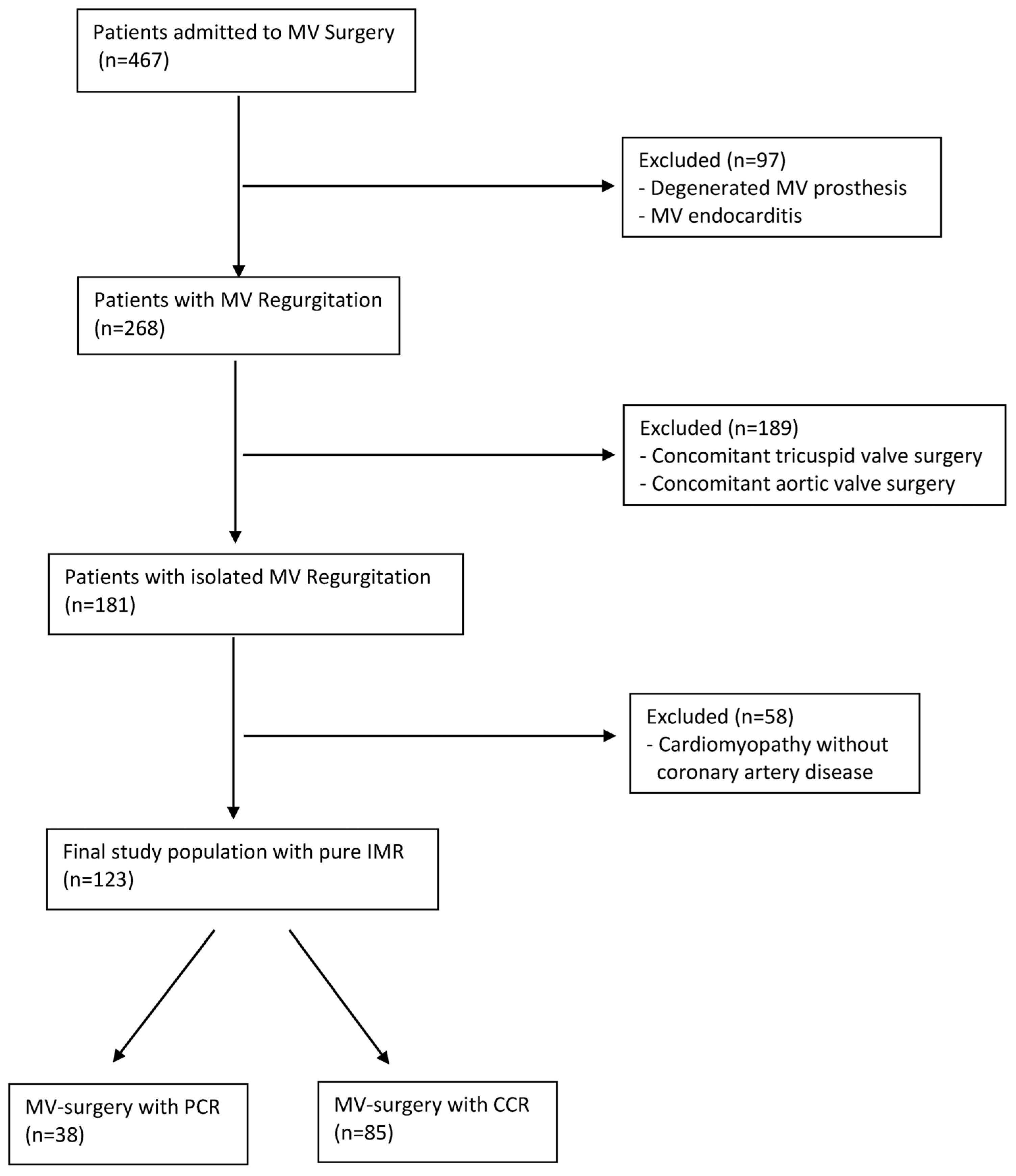
3. Results
3.1. Demographic Data
3.2. Perioperative Outcome
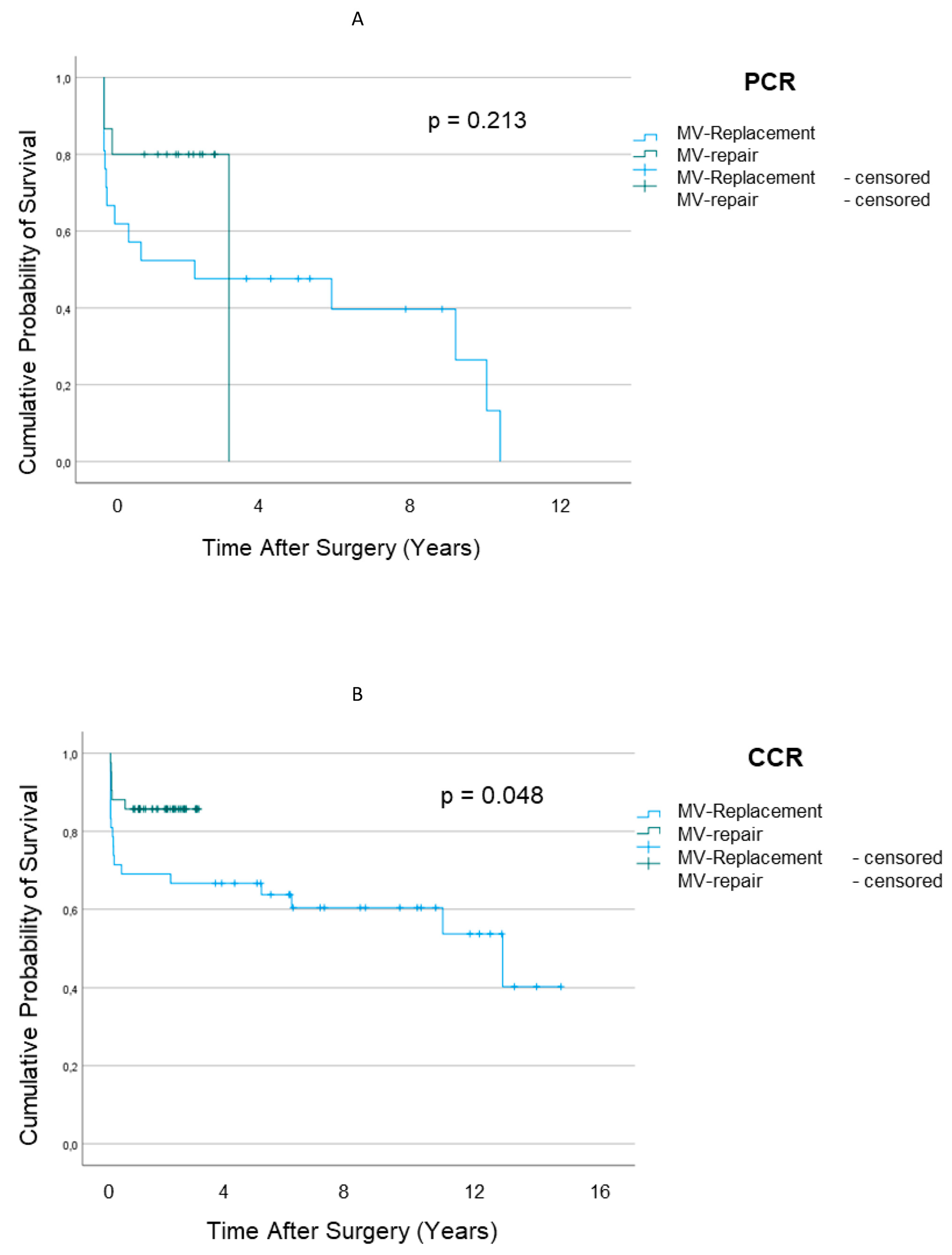
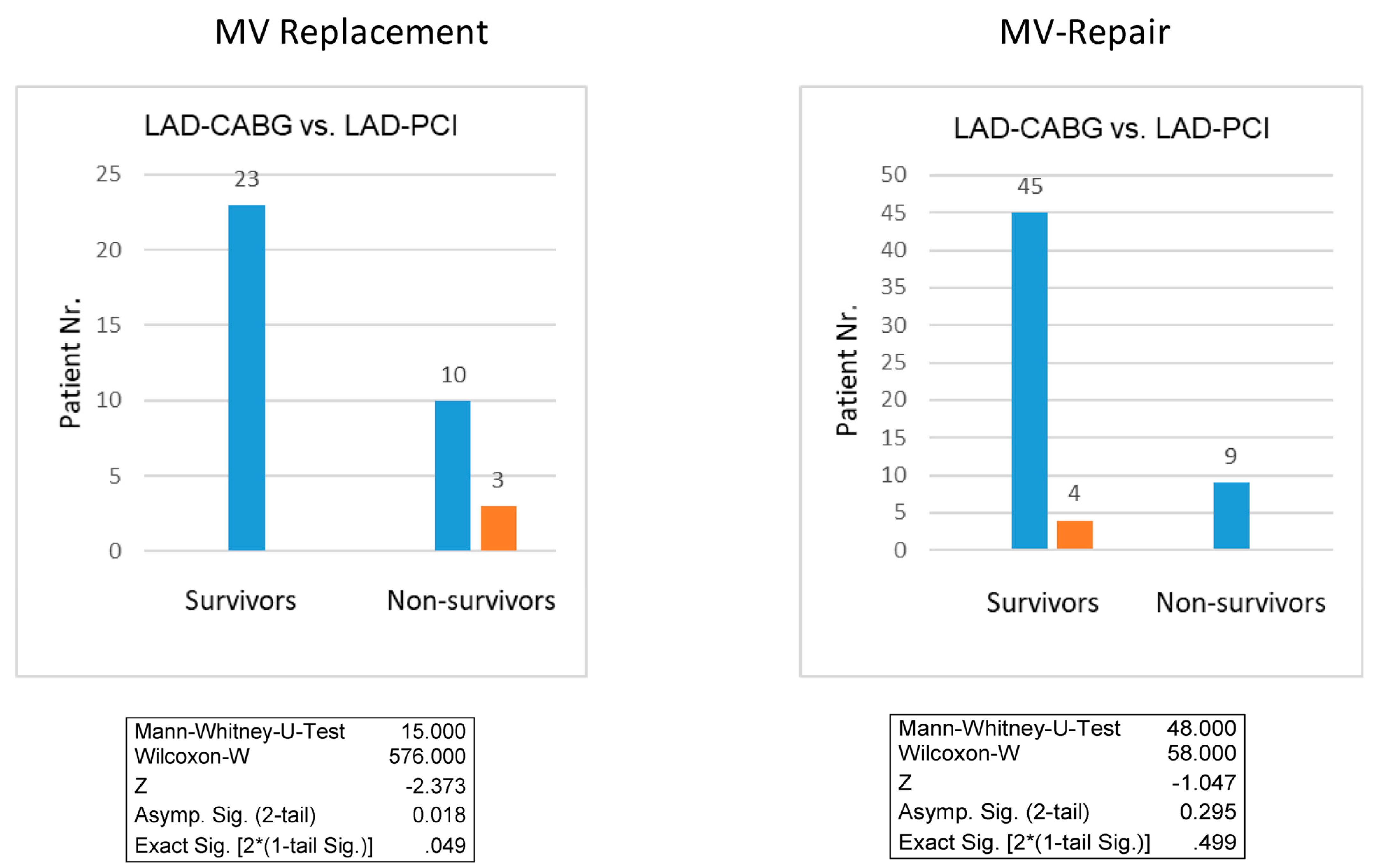
3.3. Survival Outcomes
4. Discussion
5. Limitations
6. Conclusions
7. Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hickey, M.S.; Smith, L.R.; Muhlbaier, L.H.; Harrell, F.E.; Reves, J.G.; Hinohara, T.; Califf, R.M.; Pryor, D.B.; Rankin, J.S. Current prognosis of ischemic mitral regurgitation. Implications for future management. Circulation 1988, 78, I51–I59. [Google Scholar] [PubMed]
- Tcheng, J.E.; Jackman, J.D., Jr.; Nelson, C.L.; Gardner, L.H.; Smith, L.R.; Rankin, J.S.; Califf, R.M.; Stack, R.S. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann. Intern. Med. 1992, 117, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Rydén, T.; Bech-Hanssen, O.; Brandrup-Wognsen, G.; Nilsson, F.; Svensson, S.; Jeppsson, A. The importance of grade 2 ischemic mitral regurgitation in coronary artery bypass grafting. Eur. J. Cardio-Thorac. Surg. 2001, 20, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Galloway, A.C.; Grossi, E.A.; Spencer, F.C.; Colvin, S.B. Operative therapy for mitral insufficiency from coronary artery disease. Semin. Thorac. Cardiovasc. Surg. 1995, 7, 227–232. [Google Scholar]
- Miller, D.C. Ischemic mitral regurgitation redux--to repair or to replace? J. Thorac. Cardiovasc. Surg. 2001, 122, 1059–1062. [Google Scholar] [CrossRef]
- Grigioni, F.; Enriquez-Sarano, M.; Zehr, K.J.; Bailey, K.; Tajik, A. Ischemic mitral regurgitation: Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001, 103, 1759–1764. [Google Scholar] [CrossRef]
- Bursi, F.; Enriquez-Sarano, M.; Jacobsen, S.J.; Roger, V.L. Mitral regurgitation after myocardial infarction: A review. Am. J. Med. 2006, 119, 103–112. [Google Scholar] [CrossRef]
- Grigioni, F.; Detaint, D.; Avierinos, J.F.; Scott, C.; Tajik, J.; Enriquez-Sarano, M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 260–267. [Google Scholar] [CrossRef]
- Nappi, F.; Antoniou, G.A.; Nenna, A.; Michler, R.; Benedetto, U.; Singh, S.S.A.; Gambardella, I.C.; Chello, M. Treatment options for ischemic mitral regurgitation: A meta-analysis. J. Thorac. Cardiovasc. Surg. 2022, 163, 607–622.e14. [Google Scholar] [CrossRef]
- Christenson, J.T.; Simonet, F.; Bloch, A.; Maurice, J.; Velebit, V.; Schmuziger, M. Should a mild to moderate ischemic mitral valve regurgitation in patients with poor left ventricular function be repaired or not? J. Heart Valve Dis. 1995, 4, 484–488. [Google Scholar]
- Duarte, I.G.; Shen, Y.; MacDonald, M.J.; Jones, E.L.; Craver, J.M.; Guyton, R.A. Treatment of moderate mitral regurgitation and coronary disease by coronary bypass alone: Late results. Ann. Thorac. Surg. 1999, 68, 426–430. [Google Scholar] [CrossRef]
- Dion, R.; Benetis, R.; Elias, B.; Guennaoui, T.; Raphael, D.; Van Dyck, M.; Noirhomme, P.; Van Overschelde, J.L. Mitral valve procedures in ischemic regurgitation. J. Heart Valve Dis. 1995, 4 (Suppl. S2), S124–S129. [Google Scholar]
- Hendren, W.G.; Nemec, J.J.; Lytle, B.W.; Loop, F.D.; Taylor, P.C.; Stewart, R.W.; Cosgrove, D.M. Mitral valve repair for ischemic mitral insufficiency. Ann. Thorac. Surg. 1991, 52, 1246–1251. [Google Scholar] [CrossRef]
- David, T.E.; Uden, D.E.; Strauss, H.D. The importance of the mitral apparatus in left ventricular function after correction of mitral regurgitation. Circulation 1983, 68, II76–II82. [Google Scholar]
- Galloway, A.C.; Colvin, S.B.; Baumann, F.G.; Grossi, E.; Ribakove, G.; Harty, S.; Spencer, F. A comparison of mitral valve reconstruction with mitral valve replacement: Intermediate-term results. Ann. Thorac. Surg. 1989, 47, 655–662. [Google Scholar] [CrossRef]
- Benedetto, U.; Melina, G.; Roscitano, A.; Fiorani, B.; Capuano, F.; Sclafani, G.; Comito, C.; di Nucci, G.D.; Sinatra, R. Does combined mitral valve surgery improve survival when compared to revascularization alone in patients with ischemic mitral regurgitation? A meta-analysis on 2479 patients. J. Cardiovasc. Med. 2009, 10, 109–114. [Google Scholar] [CrossRef]
- Oury, J.H.; Cleveland, J.C.; Duran, C.G.; Angell, W.W. Ischemic mitral valve disease: Classification and systemic approach to management. J. Card Surg. 1994, 9, 262–273. [Google Scholar] [CrossRef]
- Nishino, S.; Watanabe, N.; Gi, T.; Kuriyama, N.; Shibata, Y.; Asada, Y. Longitudinal Evaluation of Mitral Valve Leaflet Remodeling After Acute Myocardial Infarction: Serial Quantitation of Valve Geometry Using Real-Time 3-Dimensional Echocardiography. Circ. Cardiovasc. Imaging 2020, 13, e011396. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Grossi, E.A.; Goldberg, J.D.; LaPietra, A.; Ye, X.; Zakow, P.; Sussman, M.; Delianides, J.; Culliford, A.T.; Esposito, R.A.; Ribakove, G.H.; et al. Ischemic mitral valve reconstruction and replacement: Comparison of long-term survival and complications. J. Thorac. Cardiovasc. Surg. 2001, 122, 1107–1124. [Google Scholar] [CrossRef]
- Michler, R.E.; Smith, P.K.; Parides, M.K.; Ailawadi, G.; Thourani, V.; Moskowitz, A.J.; Acker, M.A.; Hung, J.W.; Chang, H.L.; Perrault, L.P.; et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N. Engl. J. Med. 2016, 374, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Pinson, C.W.; Cobanoglu, A.; Metzdorff, M.T.; Grunkemeier, G.L.; Kay, P.H.; Starr, A. Late surgical results for ischemic mitral regurgitation. Role of wall motion score and severity of regurgitation. J. Thorac. Cardiovasc. Surg. 1984, 88 Pt 1, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, H.; Siniawski, H.; Hetzer, R. Mitral valve reconstruction and replacement for ischemic mitral insufficiency: Seven years’ follow up. J. Heart Valve Dis. 1999, 8, 536–542. [Google Scholar] [PubMed]
- Pienta, M.J.; Theurer, P.; He, C.; Clark, M.; Haft, J.; Bolling, S.F.; Willekes, C.; Nemeh, H.; Prager, R.L.; Romano, M.A.; et al. Contemporary Management of Ischemic Mitral Regurgitation at Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2023, 115, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Fattouch, K.; Guccione, F.; Sampognaro, R.; Panzarella, G.; Corrado, E.; Navarra, E.; Calvaruso, D.; Ruvolo, G. Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: A randomized trial. J. Thorac. Cardiovasc. Surg. 2009, 138, 278–285. [Google Scholar] [CrossRef]
- Chan, K.M.; Punjabi, P.P.; Flather, M.; Wage, R.; Symmonds, K.; Roussin, I.; Pepper, J.R. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: Final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation 2012, 126, 2502–2510. [Google Scholar] [CrossRef]
- Trento, A.; Goland, S.; De Robertis, M.A.; Czer, L.S. Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation. J. Thorac. Cardiovasc. Surg. 2009, 138, 286–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Zhao, H. Efficacy of mitral valve repair as an adjunct procedure to coronary artery bypass grafting in moderate ischemic mitral regurgitation: A meta-analysis of randomized trials. J. Card Surg. 2015, 30, 623–630. [Google Scholar] [CrossRef]
- Lamas, G.A.; Mitchell, G.F.; Flaker, G.C.; Smith, S.C.; Gersh, B.J.; Basta, L.; Moyé, L.; Braunwald, E.; Pfeffer, M.A. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation 1997, 96, 827–833. [Google Scholar] [CrossRef]
- Trichon, B.H.; Glower, D.D.; Shaw, L.K.; Cabell, C.H.; Anstrom, K.J.; Felker, G.M.; O’connor, C.M. Survival after coronary revascularization, with and without mitral valve surgery, in patients with ischemic mitral regurgitation. Circulation 2003, 108 (Suppl. S1), II103–II110. [Google Scholar] [CrossRef]
- Gillinov, A.M.; Wierup, P.N.; Blackstone, E.H.; Bishay, E.S.; Cosgrove, D.M.; White, J.; McCarthy, P.M. Is repair preferable to replacement for ischemic mitral regurgitation? J. Thorac. Cardiovasc. Surg. 2001, 122, 1125–1141. [Google Scholar] [CrossRef]
- Goldstein, D.; Moskowitz, A.J.; Gelijns, A.C.; Ailawadi, G.; Parides, M.K.; Perrault, L.P.; Hung, J.W.; Voisine, P.; Dagenais, F.; Gillinov, A.M.; et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N. Engl. J. Med. 2016, 374, 344–353. [Google Scholar] [CrossRef]
- Calafiore, A.M.; Gallina, S.; Di Mauro, M.; Gaeta, F.; Iacò, A.L.; D’Alessandro, S.; Mazzei, V.; Di Giammarco, G. Mitral valve procedure in dilated cardiomyopathy: Repair or replacement? Ann. Thorac. Surg. 2001, 71, 1146–1152. [Google Scholar] [CrossRef]
- Gillinov, A.M. Is ischemic mitral regurgitation an indication for surgical repair or replacement? Heart Fail Rev. 2006, 11, 231–239. [Google Scholar] [CrossRef]
- Cohn, L.H.; Rizzo, R.J.; Adams, D.H.; Couper, G.; Sullivan, T.; Collinsjr, J.; Aranki, S. The effect of pathophysiology on the surgical treatment of ischemic mitral regurgitation: Operative and late risks of repair versus replacement. Eur. J. Cardiothorac. Surg. 1995, 9, 568–574. [Google Scholar] [CrossRef]
- Kay, G.L.; Kay, J.H.; Zubiate, P.; Yokoyama, T.; Mendez, M. Mitral valve repair for mitral regurgitation secondary to coronary artery disease. Circulation 1986, 74 Pt 2, I88–I98. [Google Scholar]
- Lillehei, C.W.; Levy, M.J.; Bonnabeau, R.C., Jr. Mitral valve replacement with preservation of papillary muscles and chordae tendineae. J. Thorac. Cardiovasc. Surg. 1964, 47, 532–543. [Google Scholar] [CrossRef]
- Duchnowski, P. Risk Factors of Sudden Cardiac Arrest during the Postoperative Period in Patients Undergoing Heart Valve Surgery. J. Clin. Med. 2022, 11, 7098. [Google Scholar] [CrossRef]
- Vassileva, C.M.; Boley, T.; Markwell, S.; Hazelrigg, S. Meta-analysis of short-term and long-term survival following repair versus replacement for ischemic mitral regurgitation. Eur. J. Cardiothorac. Surg. 2011, 39, 295–303. [Google Scholar] [CrossRef]

| CCR | PCR | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Nr. Patients | 87 (69.6%) | 38 (30.4%) | |||||
| Demographics | |||||||
| Age | 67.69 | ± | 10.31 | 69.94 | ± | 8.68 | 0.244 |
| BMI | 26.73 | ± | 3.87 | 27.97 | ± | 4.05 | 0.047 |
| Male Gender | 31 | (36.47) | 12 | (31.57) | 0.685 | ||
| Previous Cardiac Therapy | |||||||
| CABG | 0 | (0.0) | 10 | (26.31) | 0.0001 | ||
| PCI | 0 | (0.0) | 25 | (65.78) | 0.0001 | ||
| CABG + PCI | 0 | (0.0) | 3 | (7.89) | 0.028 | ||
| Clinical Symptoms | |||||||
| Angina Pectoris | 17 | (20.00) | 3 | (7.89) | 0.179 | ||
| Dyspnea | 60 | (70.59) | 31 | (81.58) | 0.267 | ||
| Acute Heart Failure | 8 | (9.41) | 4 | (10.52) | 0.540 | ||
| Laboratory Data | |||||||
| Troponin (ng/l) | 1614.81 | ± | 4451.41 | 507 | ± | 151.56 | 0.784 |
| CK-MB (U/l) | 62.05 | ± | 69.65 | 35.28 | ± | 60.60 | 0.813 |
| Echocardiography | |||||||
| Pre-OP LV-EF (%) | 49.91 | ± | 10.48 | 44.16 | ± | 12.44 | 0.746 |
| MR Grade I-II | 14 | (16.71) | 4 | (10.53) | 0.428 | ||
| MR Grade III-IV | 71 | (83.29) | 34 | (89.47) | 0.428 | ||
| Predominant Carpentier Type I | 32 | (37.65) | 13 | (34.21) | 0.840 | ||
| Predominant Carpentier Type II | 34 | (40.00) | 14 | (36.84) | 0.842 | ||
| Predominant Carpentier Type IIIa | 16 | (18.82) | 7 | (18.42) | 1.000 | ||
| Predominant Carpentier Type IIIb | 2 | (2.35) | 3 | (7.89) | 0.322 | ||
| Coronary Angiography | |||||||
| Left Main-CAD | 8 | (9.41) | 1 | (2.63) | 0.272 | ||
| 1-Vessel-CAD | 29 | (34.12) | 10 | (26.32) | 0.530 | ||
| 2-Vessel-CAD | 25 | (29.41) | 8 | (21.05) | 0.385 | ||
| 3-Vessel-CAD | 31 | (36.47) | 20 | (52.63) | 0.115 | ||
| LAD | 55 | (64.70) | 15 | (39.47) | 0.011 | ||
| D 1 | 12 | (14.12) | 3 | (7.89) | 0.389 | ||
| D 2 | 1 | (1.18) | 0 | (0.00) | 1.000 | ||
| IM | 6 | (7.06) | 1 | (2.63) | 0.435 | ||
| (L)CX | 29 | (34.12) | 11 | (28.95) | 0.678 | ||
| OM 1 | 8 | (9.41) | 1 | (2.63) | 0.272 | ||
| OM 2 | 4 | (4.71) | 4 | (10.53) | 0.251 | ||
| RCA | 39 | (45.88) | 15 | (39.47) | 0.559 | ||
| PDA | 2 | (2.35) | 1 | (2.63) | 1.000 | ||
| (R)AM | 4 | (4.71) | 2 | (5.26) | 1.000 | ||
| CCR | PCR | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 87 (69.6) | 38 (30.4) | ||||||
| Elective | 62 | (72.94) | 30 | (78.95) | 0.655 | ||
| Urgent | 15 | (17.65) | 6 | (15.79) | 1.000 | ||
| Emergent | 8 | (9.41) | 2 | (5.26) | 0.722 | ||
| First Sternotomy and First CPB | 73 | (85.88) | 25 | (65.79) | 0.015 | ||
| Redo-Sternotomy and Re-CPB | 12 | (14.11) | 13 | (34.21) | 0.015 | ||
| MV repair | 42 | (48.3) | 15 | (39.47) | 0.334 | ||
| MV replacement | 45 | (51.7) | 23 | (60.52) | 0.334 | ||
| - Biological Prosthesis | 28 | (31.86) | 13 | (34.21) | 0.836 | ||
| - Mechanical Prosthesis | 17 | (19.84) | 10 | (26.32) | 0.350 | ||
| Coronary Revascularization (total) | |||||||
| LAD System | 64 | (75.29) | 10 | (26.31) | 0.0005 | ||
| (L)CX System | 8 | (9.41) | 1 | (2.63) | 0.277 | ||
| RCA System | 23 | (27.05) | 6 | (15.78) | 0.252 | ||
| Coronary Surgery (CABG) | 72 | (84.78) | 18 | (47.37) | 0.00003 | ||
| - 1 Bypass | 28 | (32.94) | 14 | (36.84) | 0.685 | ||
| - 2 Bypasses | 19 | (21.18) | 2 | (5.26) | 0.033 | ||
| - 3 Bypasses | 25 | (29.41) | 2 | (5.26) | 0.002 | ||
| Coronary Intervention (PCI) | 13 | (15.29) | 4 | (10.53) | 0.580 | ||
| - 1-Stent | 10 | (11.76) | 3 | (7.89) | 0.372 | ||
| - 2-Stent | 3 | (3.53) | 0 | (0.00) | 0.552 | ||
| - 3-Stents | 0 | (0.00) | 1 | (2.63) | 0.309 | ||
| Post-OP MR I | 23 | (31.08) | 8 | (25) | 0.654 | ||
| Post-OP LV-EF (%) | 47.59 | ± | 10.78 | 46.61 | ± | 9.64 | 0.553 |
| Hospital Stay (days) | 19.32 | ± | 13.76 | 22.34 | ± | 18.26 | 0.419 |
| MACCE at Hospital Discharge | 29 | (34.11) | 24 | (63.57) | 0.003 | ||
| Mortality at Hospital Discharge | 17 | (19.54) | 10 | (26.32) | 0.399 | ||
| Follow-Up Time (years) | 11.6 | ± | 0.69 | 4.68 | ± | 0.73 | 0.016 |
| MACCE at Follow-up | 29 | (34.11) | 25 | (65.79) | 0.0008 | ||
| Mortality at Follow-up | 24 | (21.17) | 19 | (42.10) | 0.016 | ||
| Cardiac Causes of Death | 15 | (17.13) | 9 | (23.79) | 0.452 | ||
| Cardiogenic Shock | 11 | (12.6) | 5 | (13.2) | 1.000 | ||
| - Myocardial Infarction | 2 | (2.40) | 2 | (5.31) | 0.580 | ||
| Cardiac Bleeding | 1 | (1.20) | 0 | (0.0) | 1.000 | ||
| Cardiac Failure with MOF | 1 | (1.20) | 2 | (5.71) | 0.225 | ||
| Non-Cardiac Causes of Death | 8 | (9.41) | 9 | (23.68) | 0.045 | ||
| Septical Shock | 5 | (6.02) | 6 | (11.42) | 0.456 | ||
| Gastro-intestinal Bleeding | 0 | (0.0) | 1 | (2.86) | 0.309 | ||
| Pneumonia | 1 | (1.20) | 0 | (0.0) | 1.000 | ||
| Stroke | 1 | (1.20) | 2 | (5.71) | 0.225 | ||
| Univariable Analysis | Multivariable Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | Coeff. | OR | 95% CI | p-Value | Coeff. | OR | 95% CI | p-Value | |||
| MV replacement | |||||||||||
| LAD | Arterial | −1.522 | 0.218 | 0.058 | 0.819 | 0.024 | −0.883 | 0.413 | 0.179 | 0.954 | 0.038 |
| Venous | −1.606 | 0.201 | 0.038 | 1.062 | 0.059 | −0.759 | 0.468 | 0.157 | 1.394 | 0.173 | |
| PCI | −1.058 | 0.347 | 0.045 | 2.669 | 0.309 | 0.778 | 2.176 | 0.285 | 16.62 | 0.453 | |
| OM | Venous | 2.898 | 18.14 | 1.639 | 20.818 | 0.018 | −0.402 | 0.669 | 0.151 | 2.965 | 0.597 |
| PCI | −0.528 | 0.590 | 0.073 | 4.767 | 0.621 | −0.007 | 0.993 | 0.134 | 7.331 | 0.994 | |
| RCA | Arterial | −14.594 | 0.000 | 0.000 | 0.984 | ||||||
| Venous | 0162 | 1.176 | 0.462 | 2.993 | 0.734 | ||||||
| PCI | 0.289 | 1.335 | 0.302 | 5.901 | 0.703 | ||||||
| PDA | Arterial | −0.615 | 0.541 | 0.000 | 0.984 | ||||||
| Venous | 0.874 | 2.396 | 0.302 | 0.997 | |||||||
| MV repair | |||||||||||
| LAD | Arterial | 0.730 | 2.076 | 0.504 | 8.553 | 0.312 | |||||
| PCI | 0.778 | 2.176 | 0.285 | 16.623 | 0.453 | ||||||
| OM | Arterial | −13.434 | 1.728 | 0.000 | 0.050 | −13.100 | 0.000 | 0.000 | 0.000 | 0.991 | |
| Venous | −27.556 | 0.000 | 0.000 | 0.547 | 1.728 | 0.216 | 13.830 | 0.606 | |||
| PCI | 1.296 | 3.656 | 0.000 | 1.296 | |||||||
| RCA | Arterial | −12.998 | 0.000 | 0.000 | 0.988 | ||||||
| Venous | 1.308 | 3.699 | 0.732 | 18.690 | 0.114 | ||||||
| PCI | 1.844 | 6.322 | 1.258 | 31.778 | 0.025 | 0.874 | 2.396 | 0.000 | 0.000 | 0.997 | |
| PDA | Arterial | −10.374 | 0.000 | 0.000 | 0.957 | ||||||
| Venous | 0.753 | 2.124 | 0.790 | 5.711 | 0.136 | ||||||
| PCI | −0.652 | 0.521 | 0.000 | 0.000 | 0.998 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrási, T.B.; Glück, A.C.; Ben Taieb, O.; Talipov, I.; Abudureheman, N.; Volevski, L.; Vasiloi, I. Outcome of Surgery for Ischemic Mitral Regurgitation Depends on the Type and Timing of the Coronary Revascularization. J. Clin. Med. 2023, 12, 3182. https://doi.org/10.3390/jcm12093182
Andrási TB, Glück AC, Ben Taieb O, Talipov I, Abudureheman N, Volevski L, Vasiloi I. Outcome of Surgery for Ischemic Mitral Regurgitation Depends on the Type and Timing of the Coronary Revascularization. Journal of Clinical Medicine. 2023; 12(9):3182. https://doi.org/10.3390/jcm12093182
Chicago/Turabian StyleAndrási, Terézia B., Alannah C. Glück, Olfa Ben Taieb, Ildar Talipov, Nunijiati Abudureheman, Lachezar Volevski, and Ion Vasiloi. 2023. "Outcome of Surgery for Ischemic Mitral Regurgitation Depends on the Type and Timing of the Coronary Revascularization" Journal of Clinical Medicine 12, no. 9: 3182. https://doi.org/10.3390/jcm12093182
APA StyleAndrási, T. B., Glück, A. C., Ben Taieb, O., Talipov, I., Abudureheman, N., Volevski, L., & Vasiloi, I. (2023). Outcome of Surgery for Ischemic Mitral Regurgitation Depends on the Type and Timing of the Coronary Revascularization. Journal of Clinical Medicine, 12(9), 3182. https://doi.org/10.3390/jcm12093182





