A 2-Year Audit on Antibiotic Resistance Patterns from a Urology Department in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Source of Isolates
2.3. Antimicrobial Susceptibility Testing
2.4. Statistical Analysis
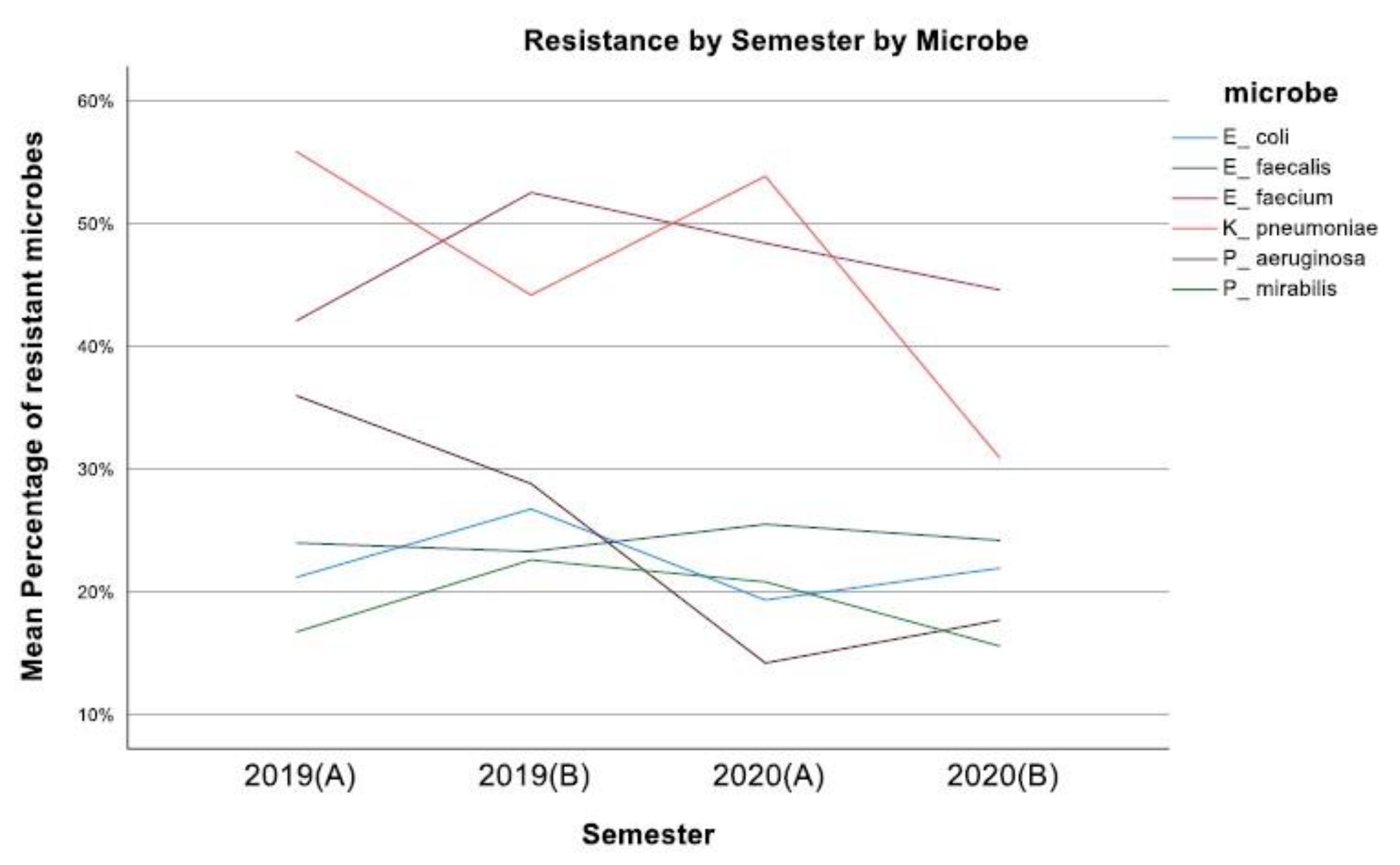
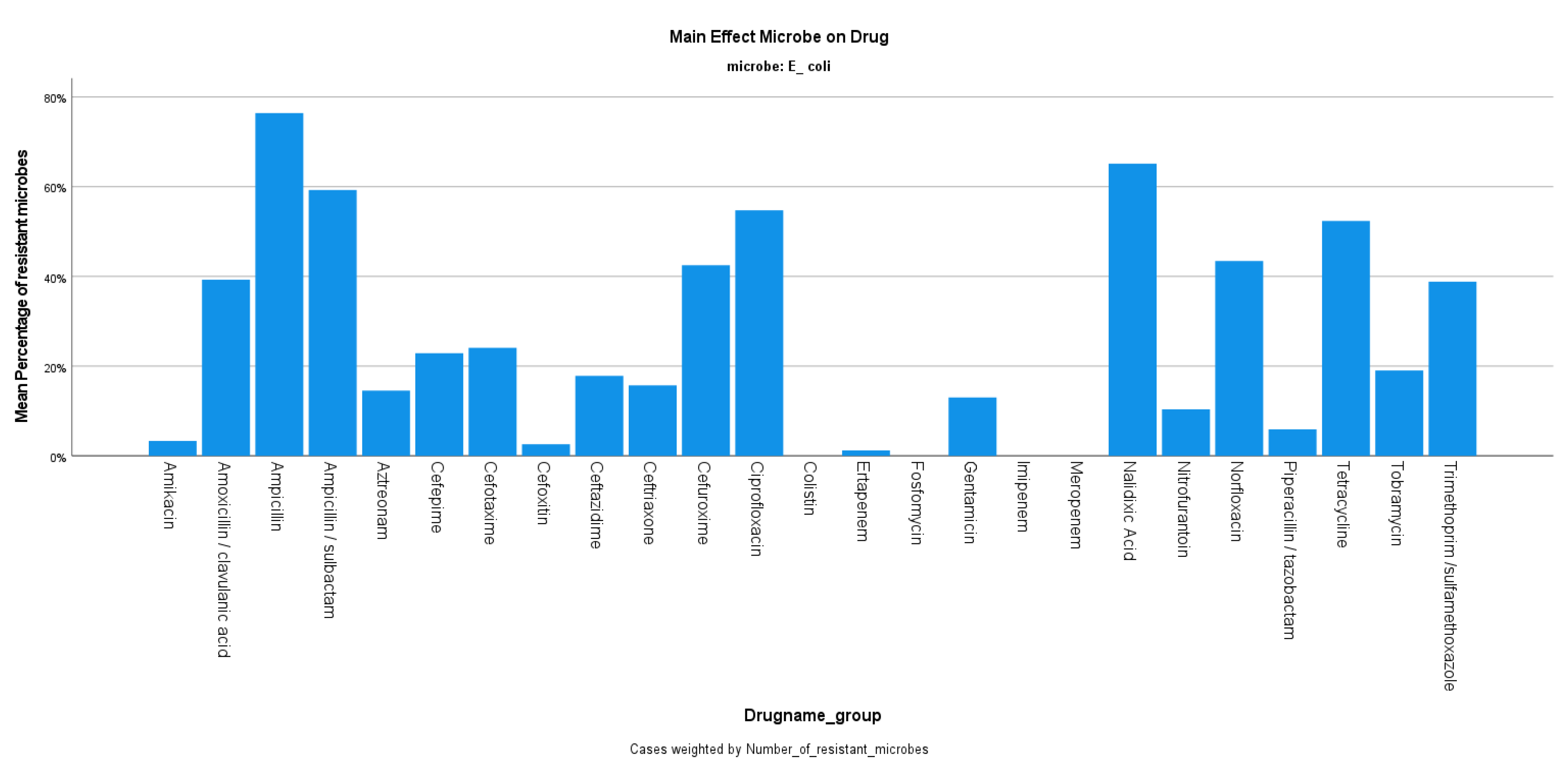
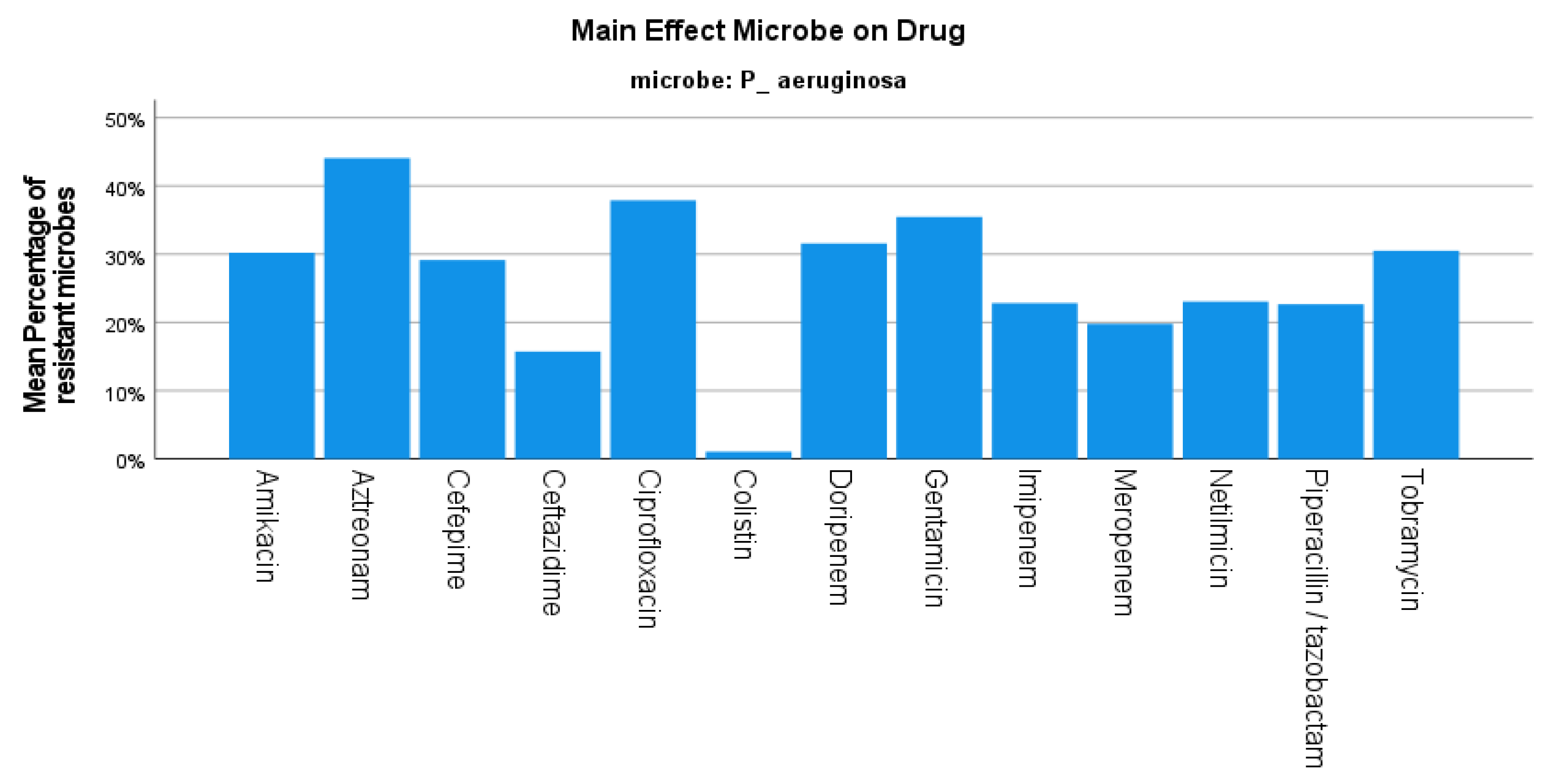
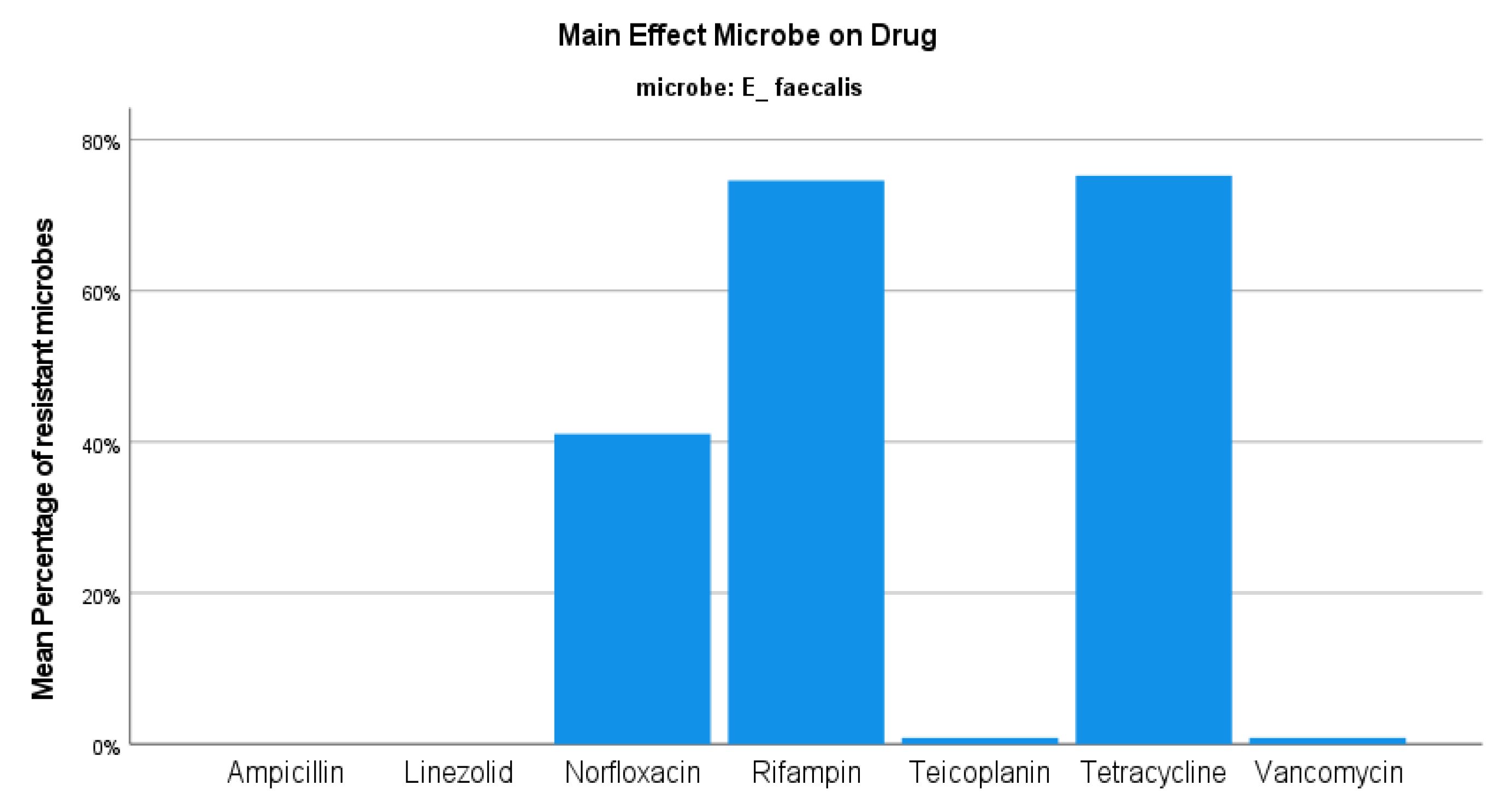
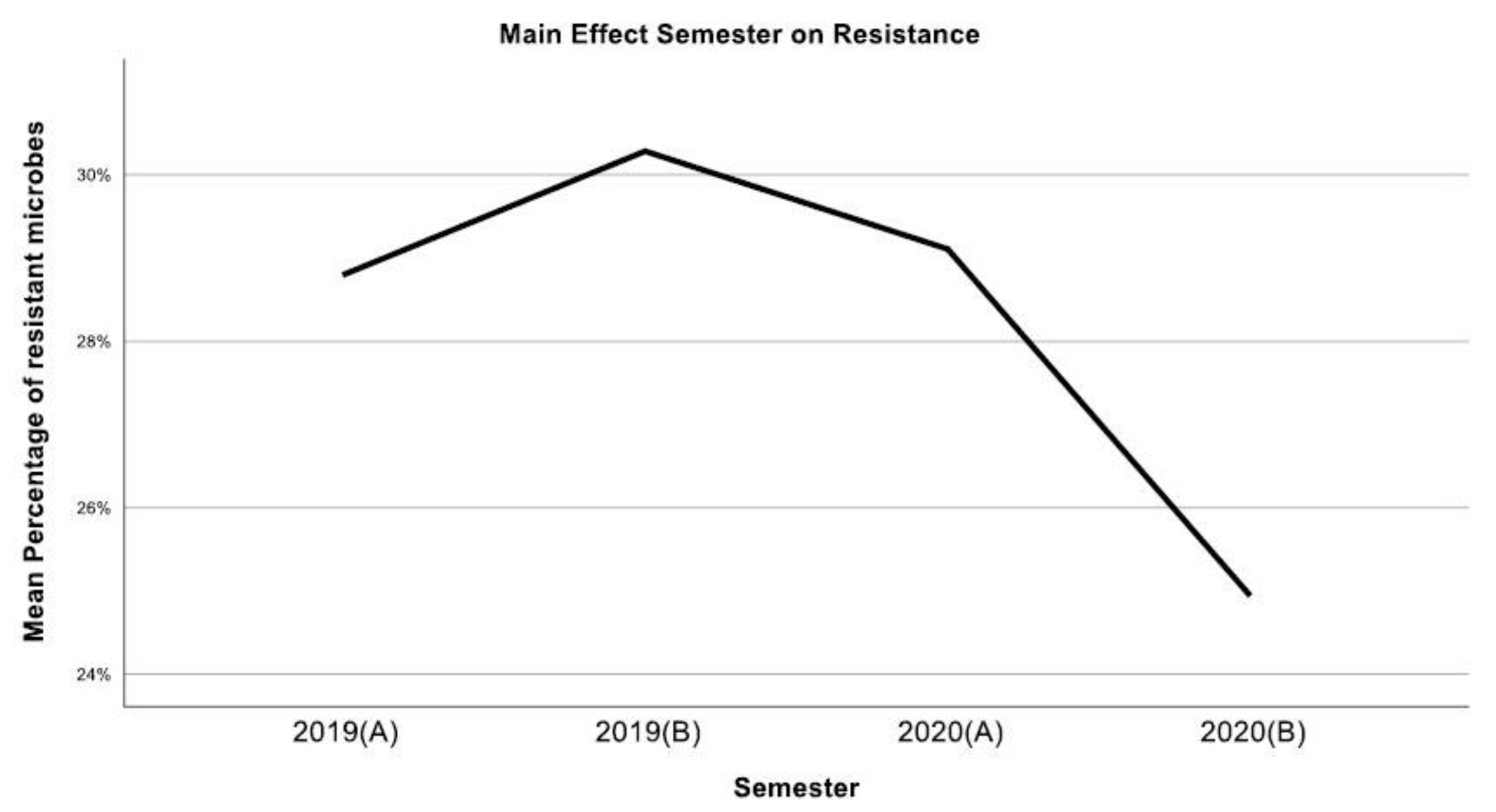
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zowawi, H.M.; Harris, P.N.A.; Roberts, M.J.; Tambyah, P.A.; Schembri, M.A.; Pezzani, M.D.; Williamson, D.A.; Paterson, D.L. The Emerging Threat of Multidrug-Resistant Gram-Negative Bacteria in Urology. Nat. Rev. Urol. 2015, 12, 570–584. [Google Scholar]
- Kandil, H.; Cramp, E.; Vaghela, T. Trends in Antibiotic Resistance in Urologic Practice. Eur. Urol. Focus 2016, 2, 363–373. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P. Extended-spectrum beta-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Deininger, S.; Gründler, T.; Deininger, S.H.M.; Lütcke, K.; Lütcke, H.; Agbesi, J.; Ladzaka, W.; Gyamfi, E.; Wichlas, F.; Hofmann, V.; et al. The Antimicrobial Resistance (AMR) Rates of Uropathogens in a Rural Western African Area—A Retrospective Single-Center Study from Kpando, Ghana. Antibiotics 2022, 11, 1808. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Barter, D.M.; Laxminarayan, R. Economic Burden of Antibiotic Resistance: How Much Do We Really Know? Clin. Microbiol. Infect. 2014, 20, 973–980. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021; World Health Organization (WHO): Geneva, Switzerland, 2021.
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2020; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- Potron, A.; Poirel, L.; Nordmann, P. Emerging Broad-Spectrum Resistance in Pseudomonas Aeruginosa and Acinetobacter Baumannii: Mechanisms and Epidemiology. Int. J. Antimicrob. Agents 2015, 45, 568–585. [Google Scholar] [PubMed]
- Karakonstantis, S.; Kalemaki, D. Antimicrobial Overuse and Misuse in the Community in Greece and Link to Antimicrobial Resistance Using Methicillin-Resistant S. Aureus as an Example. J. Infect. Public Health 2019, 12, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Adamis, G.; Tsonou, P.; Moustaka, E.; Katerelos, P.; Gargalianos, P. Consumption of Antibiotics for Community-Acquired Infections by Adults in Greece: A Cross-Sectional Study. Am. J. Infect. Control. 2016, 44, 1741–1743. [Google Scholar] [CrossRef] [PubMed]
- Luyt, C.-E.; Bréchot, N.; Trouillet, J.-L.; Chastre, J. Antibiotic Stewardship in the Intensive Care Unit. Crit. Care 2014, 18, 480. [Google Scholar] [CrossRef]
- Johansen, T.E.B.; Çek, M.; Naber, K.G.; Stratchounski, L.; Svendsen, M.V.; Tenke, P. Hospital Acquired Urinary Tract Infections in Urology Departments: Pathogens, Susceptibility and Use of Antibiotics. Int. J. Antimicrob. Agents 2006, 28, 91–107. [Google Scholar] [CrossRef]
- Balows, A.; Hausler, W.J., Jr.; Herrmann, K.L.; Isenberg, H.D.; Shadomy, H.J. Manual of Clinical Microbiology. Rev. Inst. Med. Trop. São Paulo 1991, 33, 434. [Google Scholar] [CrossRef]
- Henry, D.I. Clinical Microbiology Procedures Handbook; ASM Press: Washington, DC, USA, 2004; Volume 3. [Google Scholar]
- Murray, P.R.; Baron, E.; Jorgensen, J.H.; Pfaller, M.A.; Yolken, R.H.; Hanna, B.A. Manual of Clinical Microbiology, 8th Edition. Clin. Infect. Dis. 2004, 38, 1199–1200. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of Americaa. Clin. Infect. Dis. 2019, 68, e83–e110. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Pfaller, M.A.; Carroll, K.C.; Funke, G.; Landry, M.L.; Richter, S.S.; Warnock, D.W. Manual of Clinical Microbiology, 11th ed.; ASM Press: Washington, DC, USA, 2015. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Tsakris, A.; Poulou, A.; Pournaras, S.; Voulgari, E.; Vrioni, G.; Themeli-Digalaki, K.; Petropoulou, D.; Sofianou, D. A Simple Phenotypic Method for the Differentiation of Metallo—Lactamases and Class a KPC Carbapenemases in Enterobacteriaceae Clinical Isolates. J. Antimicrob. Chemother. 2010, 65, 1664–1671. [Google Scholar] [CrossRef]
- Skarmoutsou, N.; Adamou, D.; Tryfinopoulou, K.; Xirokosta, P.; Mylona, E.; Giakkoupi, P.; Karadimas, K.; Zervogianni, A.; Martsoukou, M. Performance of NG-Test CARBA 5 immunochromatographic assay for the detection of carbapenemases among multidrug-resistant clinical strains in Greece. In Proceedings of the ECCMID 2019, Amsterdam, The Netherlands, 13–16 April 2019. [Google Scholar]
- Yong, D.; Lee, K.; Yum, J.H.; Shin, H.B.; Rossolini, G.M.; Chong, Y. Imipenem-EDTA Disk Method for Differentiation of Metallo-β-Lactamase-Producing Clinical Isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 2002, 40, 3798–3801. [Google Scholar] [CrossRef]
- Lee, K.; Lim, Y.S.; Yong, D.; Yum, J.H.; Chong, Y. Evaluation of the Hodge Test and the Imipenem-EDTA Double-Disk Synergy Test for Differentiating Metallo-β-Lactamase-Producing Isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 2003, 41, 4623–4629. [Google Scholar] [CrossRef] [PubMed]
- Flountzi, A.; Giakkoupi, P.; Tryfinopoulou, K.; Pappa, O.; Vatopoulos, A.; Martsoukou, M.; Skarmoutsou, N.; Lebessi, E.; Charisiadou, A.E.; Chatzivasileiou, E.; et al. Investigation of Klebsiella pneumoniaeclinical isolates from 2016 onwards for the putative presence of the plasmid-mediated mcr-1 gene for colistin resistance. In Proceedings of the ECCMID 2019, Amsterdam, The Netherlands, 13–16 April 2019. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 24.0; IBM Corp.: Armonk, NY, USA, 2016. [Google Scholar]
- Elhanan, G.; Sarhat, M.; Raz, R. Empiric Antibiotic Treatment and the Misuse of Culture Results and Antibiotic Sensitivities in Patients with Community-Acquired Bacteraemia due to Urinary Tract Infection. J. Infect. 1997, 35, 283–288. [Google Scholar] [CrossRef]
- Tandogdu, Z.; Cek, M.; Wagenlehner, F.; Naber, K.; Tenke, P.; van Ostrum, E.; Bjerklund Johansen, T. Resistance Patterns of Nosocomial Urinary Tract Infections in Urology Departments: 8-Year Results of the Global Prevalence of Infections in Urology Study. World J. Urol. 2014, 32, 791–801. [Google Scholar] [CrossRef]
- Nguyen, J.L.; Benigno, M.; Malhotra, D.; Khan, F.; Angulo, F.J.; Hammond, J.; Swerdlow, D.L.; Reimbaeva, M.; Emir, B.; McLaughlin, J.M. Pandemic-Related Declines in Hospitalization for Non-COVID-19-Related Illness in the United States from January through July 2020. PLoS ONE 2022, 17, e0262347. [Google Scholar] [CrossRef]
- Mareș, C.; Petca, R.-C.; Petca, A.; Popescu, R.-I.; Jinga, V. Does the COVID Pandemic Modify the Antibiotic Resistance of Uropathogens in Female Patients? A New Storm? Antibiotics 2022, 11, 376. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-Spectrum-Lactamases: A Clinical Update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Comparative Epidemiology and Resistance Trends of Common Urinary Pathogens in a Tertiary-Care Hospital: A 10-Year Surveillance Study. Medicina 2019, 55, 356. [Google Scholar] [CrossRef]
- Chibelean, C.B.; Petca, R.-C.; Mareș, C.; Popescu, R.-I.; Enikő, B.; Mehedințu, C.; Petca, A. A Clinical Perspective on the Antimicrobial Resistance Spectrum of Uropathogens in a Romanian Male Population. Microorganisms 2020, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.; Tandogdu, Z.; Bartoletti, R.; Cai, T.; Cek, M.; Kulchavenya, E.; Köves, B.; Naber, K.; Perepanova, T.; Tenke, P.; et al. The Global Prevalence of Infections in Urology Study: A Long-Term, Worldwide Surveillance Study on Urological Infections. Pathogens 2016, 5, 10. [Google Scholar] [CrossRef]
- Ong, A.; Mahobia, N.; Browning, D.; Schembri, M.; Somani, B.K. Trends in Antibiotic Resistance for over 700,000 Escherichia Coli Positive Urinary Tract Infections over Six Years (2014–2019) from a University Teaching Hospital. Cent. Eur. J. Urol. 2021, 74, 249. [Google Scholar]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- Benkő, R.; Gajdács, M.; Matuz, M.; Bodó, G.; Lázár, A.; Hajdú, E.; Papfalvi, E.; Hannauer, P.; Erdélyi, P.; Pető, Z. Prevalence and Antibiotic Resistance of ESKAPE Pathogens Isolated in the Emergency Department of a Tertiary Care Teaching Hospital in Hungary: A 5-Year Retrospective Survey. Antibiotics 2020, 9, 624. [Google Scholar] [CrossRef]
- Boccella, M.; Santella, B.; Pagliano, P.; De Filippis, A.; Casolaro, V.; Galdiero, M.; Borrelli, A.; Capunzo, M.; Boccia, G.; Franci, G. Prevalence and Antimicrobial Resistance of Enterococcus Species: A Retrospective Cohort Study in Italy. Antibiotics 2021, 10, 1552. [Google Scholar] [CrossRef]
- Buultjens, A.H.; Lam, M.M.; Ballard, S.; Monk, I.R.; Mahony, A.A.; Grabsch, E.A.; Grayson, M.L.; Pang, S.; Coombs, G.W.; Robinson, J.O.; et al. Evolutionary origins of the emergent ST796 clone of vancomycin resistant Enterococcus faecium. PeerJ 2017, 5, e2916. [Google Scholar] [CrossRef]
- Kot, B.; Grużewska, A.; Szweda, P.; Wicha, J.; Parulska, U. Antibiotic Resistance of Uropathogens Isolated from Patients Hospitalized in District Hospital in Central Poland in 2020. Antibiotics 2021, 10, 447. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA J. 2018, 16, e05182. [Google Scholar]
- Zanetti, G.; Paparella, S.; Trinchieri, A.; Prezioso, D.; Rocco, F.; Naber, K.G. Infections and Urolithiasis: Current Clinical Evidence in Prophylaxis and Antibiotic Therapy. Arch. Ital. Urol. Androl. Organo Uff. Soc. Ital. Ecogr. Urol. Nefrol. 2008, 80, 5–12. [Google Scholar]
- Chen, D.; Zhang, Y.; Huang, J.; Liang, X.; Zeng, T.; Lan, C.; Duan, X.; Zhao, Z.; Zeng, G.; Tiselius, H.-G.; et al. The Analysis of Microbial Spectrum and Antibiotic Resistance of Uropathogens Isolated from Patients with Urinary Stones. Int. J. Clin. Pract. 2018, 72, e13205. [Google Scholar] [CrossRef] [PubMed]
- Perletti, G.; Magri, V.; Cai, T.; Stamatiou, K.; Trinchieri, A.; Montanari, E. Resistance of Uropathogens to Antibacterial Agents: Emerging Threats, Trends and Treatments. Arch. Ital. Urol. Androl. Organo Uff. Soc. Ital. Ecogr. Urol. Nefrol. 2018, 90, 85–96. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzis, E.; Alba, A.B.; Cepeda, M.; Galan, J.A.; Geavlete, P.; Giannakopoulos, S.; Saltirov, I.; Sarica, K.; Skolarikos, A.; Stavridis, S.; et al. Bacterial Spectrum and Antibiotic Resistance of Urinary Tract Infections in Patients Treated for Upper Urinary Tract Calculi: A Multicenter Analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Veneziano, D.; Somani, B.K. Artificial Intelligence in the Diagnosis, Treatment and Prevention of Urinary Stones. Curr. Opin. Urol. 2020, 30, 782–787. [Google Scholar] [CrossRef]
- Goodswen, S.J.; Barratt, J.L.N.; Kennedy, P.J.; Kaufer, A.; Calarco, L.; Ellis, J.T. Machine Learning and Applications in Microbiology. FEMS Microbiol. Rev. 2021, 45, fuab015. [Google Scholar] [CrossRef] [PubMed]
- Tzelves, L.; Lazarou, L.; Feretzakis, G.; Kalles, D.; Mourmouris, P.; Loupelis, E.; Basourakos, S.; Berdempes, M.; Manolitsis, I.; Mitsogiannis, I.; et al. Using Machine Learning Techniques to Predict Antimicrobial Resistance in Stone Disease Patients. World J. Urol. 2022, 40, 1731–1736. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolitsis, I.; Feretzakis, G.; Katsimperis, S.; Angelopoulos, P.; Loupelis, E.; Skarmoutsou, N.; Tzelves, L.; Skolarikos, A. A 2-Year Audit on Antibiotic Resistance Patterns from a Urology Department in Greece. J. Clin. Med. 2023, 12, 3180. https://doi.org/10.3390/jcm12093180
Manolitsis I, Feretzakis G, Katsimperis S, Angelopoulos P, Loupelis E, Skarmoutsou N, Tzelves L, Skolarikos A. A 2-Year Audit on Antibiotic Resistance Patterns from a Urology Department in Greece. Journal of Clinical Medicine. 2023; 12(9):3180. https://doi.org/10.3390/jcm12093180
Chicago/Turabian StyleManolitsis, Ioannis, Georgios Feretzakis, Stamatios Katsimperis, Panagiotis Angelopoulos, Evangelos Loupelis, Nikoleta Skarmoutsou, Lazaros Tzelves, and Andreas Skolarikos. 2023. "A 2-Year Audit on Antibiotic Resistance Patterns from a Urology Department in Greece" Journal of Clinical Medicine 12, no. 9: 3180. https://doi.org/10.3390/jcm12093180
APA StyleManolitsis, I., Feretzakis, G., Katsimperis, S., Angelopoulos, P., Loupelis, E., Skarmoutsou, N., Tzelves, L., & Skolarikos, A. (2023). A 2-Year Audit on Antibiotic Resistance Patterns from a Urology Department in Greece. Journal of Clinical Medicine, 12(9), 3180. https://doi.org/10.3390/jcm12093180






