Impact of High-Intensity Statin on Early Neurologic Deterioration in Patients with Single Small Subcortical Infarction
Abstract
1. Introduction
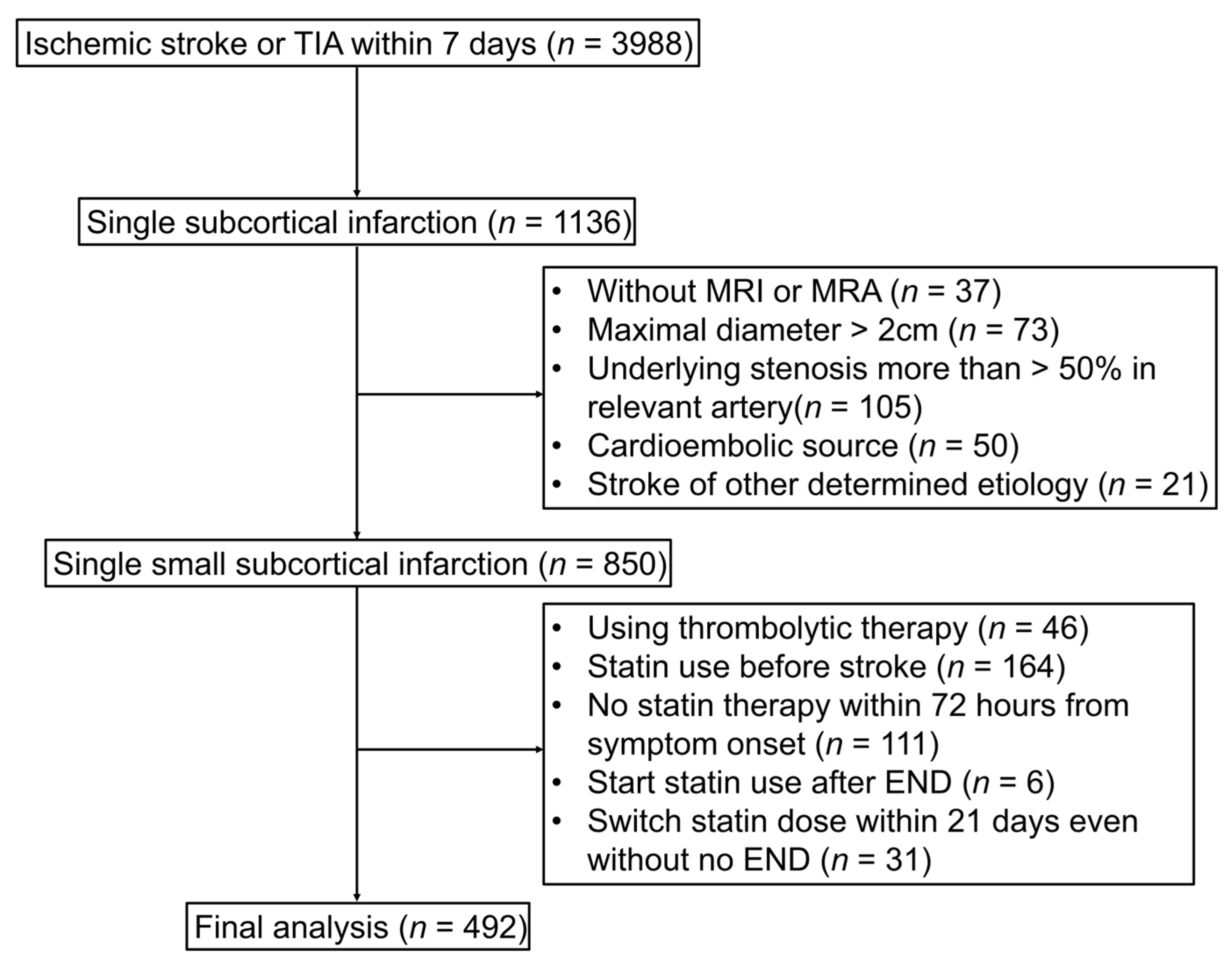
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data Collection
2.3. Follow-Up and Outcomes
2.4. Imaging Analysis
2.5. Definition of END and Collection
2.6. Statin Therapy Protocol
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Predictors of END
3.3. Statin Initiation Time from Symptom Onset
3.4. Functional Outcome at 3 Months
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vynckier, J.; Maamari, B.; Grunder, L.; Goeldlin, M.B.; Meinel, T.R.; Kaesmacher, J.; Hakim, A.; Arnold, M.; Gralla, J.; Seiffge, D.J.; et al. Early neurologic deterioration in lacunar stroke: Clinical and imaging predictors and association with long-term outcome. Neurology 2021, 97, e1437–e1446. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.M. Lacunes: Small, deep cerebral infarcts. Neurology 1965, 15, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Nannoni, S.; Del Bene, A.; Palumbo, V.; Inzitari, D. Branch atheromatous disease: A clinically meaningful, yet unproven concept. Cereb. Dis. 2016, 41, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, A.; Palumbo, V.; Lamassa, M.; Saia, V.; Piccardi, B.; Inzitari, D. Progressive lacunar stroke: Review of mechanisms, prognostic features, and putative treatments. Int. J. Stroke 2012, 7, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G.; Kim, B.J.; Yang, M.H.; Han, M.K.; Bae, H.J. Neuroimaging markers for early neurologic deterioration in single small subcortical infarction. Stroke 2015, 46, 687–691. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Brown, B.G.; Ganz, P.; Vogel, R.A.; Crowe, T.; Howard, G.; Cooper, C.J.; Brodie, B.; et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. JAMA 2004, 291, 1071–1080. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Callahan, A.; Goldstein, L.B.; Hennerici, M.; Rudolph, A.E.; Sillesen, H.; Simunovic, L.; Szarek, M.; Welch, K.M.A.; et al. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 2006, 355, 549–559. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ohara, T.; Hamanaka, M.; Hosomi, A.; Tamura, A.; Akiguchi, I. Characteristics of intracranial branch atheromatous disease and its association with progressive motor deficits. J. Neurol. Sci. 2011, 304, 78–82. [Google Scholar] [CrossRef]
- Weimar, C.; Mieck, T.; Buchthal, J.; Ehrenfeld, C.E.; Schmid, E.; Diener, H.C. Neurologic worsening during the acute phase of ischemic stroke. Arch. Neurol. 2005, 62, 393–397. [Google Scholar] [CrossRef]
- Hong, K.-S.; Kang, D.-W.; Koo, J.-S.; Yu, K.-H.; Han, M.-K.; Cho, Y.-J.; Park, J.-M.; Bae, H.-J.; Lee, B.-C. Impact of neurological and medical complications on 3-month outcomes in acute ischaemic stroke. Eur. J. Neurol. 2008, 15, 1324–1331. [Google Scholar] [CrossRef]
- Park, T.H.; Lee, J.-K.; Park, M.-S.; Park, S.-S.; Hong, K.-S.; Ryu, W.-S.; Kim, D.-E.; Park, M.S.; Choi, K.-H.; Kim, J.-T.; et al. Neurological deterioration in patients with acute ischemic stroke or transient ischemic attack. Neurology 2020, 95, e2178–e2191. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Zhu, T.; Ren, L.; Zhang, L.; Shao, Y.; Wan, L.; Li, Y.; Liang, D.; Zheng, H.; Liu, X.; Zhang, N. Comparison of plaque characteristics of small and large subcortical infarctions in the middle cerebral artery territory using high-resolution magnetic resonance vessel wall imaging. Quant. Imaging Med. Surg. 2021, 11, 57–66. [Google Scholar] [CrossRef]
- Helleberg, B.H.; Ellekjaer, H.; Indredavik, B. Outcomes after early neurological deterioration and transitory deterioration in acute ischemic stroke patients. Cereb. Dis. 2016, 42, 378–386. [Google Scholar] [CrossRef]
- Vahidy, F.S.; Hicks, W.J.; Acosta, I.; Hallevi, H.; Peng, H.; Pandurengan, R.; Gonzales, N.R.; Barreto, A.D.; Martin-Schild, S.; Wu, T.-C.; et al. Neurofluctuation in patients with subcortical ischemic stroke. Neurology 2014, 83, 398–405. [Google Scholar] [CrossRef]
- Steinke, W.; Ley, S.C. Lacunar stroke is the major cause of progressive motor deficits. Stroke 2002, 33, 1510–1516. [Google Scholar] [CrossRef]
- Berberich, A.; Schneider, C.; Reiff, T.; Gumbinger, C.; Ringleb, P.A. Dual antiplatelet therapy improves functional outcome in patients with progressive lacunar strokes. Stroke 2019, 50, 1007–1009. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Sandercock, P.A.; Dennis, M.S.; Starr, J. Is breakdown of the blood–brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003, 34, 806–812. [Google Scholar] [CrossRef]
- Serena, J.; Leira, R.; Castillo, J.; Pumar, J.M.; Castellanos, M.; Dávalos, A. Neurological deterioration in acute lacunar infarctions: The role of excitatory and inhibitory neurotransmitters. Stroke 2001, 32, 1154–1161. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, K.Y.; Koh, S.H.; Park, C.Y.; Kim, H.Y.; Lee, Y.J.; Kim, H.T.; Kim, J.; Kim, M.-H.; Kim, K.S.; et al. The role of matrix metalloproteinase 9 in early neurological worsening of acute lacunar infarction. Eur. Neurol. 2006, 55, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.O.; Budoff, M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Cimino, M.; Gelosa, P.; Gianella, A.; Nobili, E.; Tremoli, E.; Sironi, L. Statins: Multiple mechanisms of action in the ischemic brain. Neuroscientist 2007, 13, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.; Yeh, E.T. The pleiotropic effects of statins. Curr. Opin. Cardiol. 2005, 20, 541–546. [Google Scholar] [CrossRef]
- Cappellari, M.; Bovi, P.; Moretto, G.; Zini, A.; Nencini, P.; Sessa, M.; Furlan, M.; Pezzini, A.; Orlandi, G.; Paciaroni, M.; et al. The THRombolysis and statins (THRaST) study. Neurology 2013, 80, 655–661. [Google Scholar] [CrossRef]
- Flint, A.C.; Kamel, H.; Navi, B.B.; Rao, V.A.; Faigeles, B.S.; Conell, C.; Klingman, J.G.; Sidney, S.; Hills, N.K.; Sorel, M.; et al. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke 2012, 43, 147–154. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Pearce, L.A.; Bazan, C.; Catanese, L.; McClure, L.A.; Sharma, M.; Marti-Fabregas, J.; Anderson, D.C.; Kase, C.S.; Hart, R.G.; et al. Microbleeds in the Secondary Prevention of Small Subcortical Strokes Trial: Stroke, mortality, and treatment interactions. Ann. Neurol. 2017, 82, 196–207. [Google Scholar] [CrossRef]
- Helenius, J.; Mayasi, Y.; Henninger, N. White matter hyperintensity lesion burden is associated with the infarct volume and 90-day outcome in small subcortical infarcts. Acta Neurol. Scand. 2017, 135, 585–592. [Google Scholar] [CrossRef]

| Non-END (n = 390) | END (n = 102) | p Value | |
|---|---|---|---|
| Age, y, mean ± SD | 66.4 ± 11.8 | 70.3 ± 12.1 | 0.003 |
| Male, n (%) | 241 (61.8%) | 56 (54.9%) | 0.249 |
| NIHSS score on admission, median (IQR) | 2.0 [1.0; 4.0] | 3.0 [2.0; 5.0] | 0.032 |
| Onset to arrival time, h, median (IQR) | 16.2 [5.1; 31.2] | 11.4 [4.9; 23.3] | 0.046 |
| Vascular risk factors, n (%) | |||
| Hypertension | 248 (63.6%) | 63 (61.8%) | 0.822 |
| Diabetes mellitus | 115 (29.5%) | 29 (28.4%) | 0.931 |
| Dyslipidemia | 67 (17.2%) | 19 (18.6%) | 0.844 |
| Coronary artery disease | 9 (2.3%) | 4 (3.9%) | 0.577 |
| Smoking | 170 (43.6%) | 41 (40.2%) | 0.614 |
| Prior stroke or TIA | 36 (9.2%) | 12 (11.8%) | 0.562 |
| Laboratory findings, mean ± SD | |||
| Fasting glucose, mg/dL | 117.0 ± 41.4 | 117.2 ± 40.9 | 0.952 |
| LDL-C, mg/dL | 122.4 ± 36.4 | 127.7 ± 38.6 | 0.198 |
| HDL-C, mg/dL | 45.7 ± 12.6 | 45.0 ± 11.4 | 0.617 |
| Triglyceride, mg/dL | 137.6 ± 74.0 | 132.2 ± 83.4 | 0.523 |
| Total cholesterol, mg/dL | 184.4 ± 41.2 | 188.0 ± 40.0 | 0.426 |
| CRP, mg/dL | 0.7 ± 1.6 | 0.7 ± 1.6 | 0.981 |
| Systolic blood pressure, mm Hg | 156.6 ± 26.8 | 158.8 ± 23.8 | 0.445 |
| Diastolic blood pressure, mm Hg | 89.7 ± 16.3 | 90.3 ± 14.8 | 0.708 |
| Prior medication, n (%) | |||
| Antiplatelet | 57 (14.6%) | 17 (16.7%) | 0.719 |
| Antihypertensive treatment | 145 (37.2%) | 35 (34.3%) | 0.675 |
| Neuroimaging analysis | |||
| Branch atheromatous lesion | 114 (29.2%) | 59 (57.8%) | <0.001 |
| Parent artery stenosis (0–50%) | 132 (33.8%) | 38 (37.3%) | 0.598 |
| Location of SSSI | 0.168 | ||
| Anterior circulation | 201 (51.5%) | 61 (59.8%) | |
| Posterior circulation | 189 (48.5%) | 41 (40.2%) | |
| Regimen of antiplatelet in acute phase, n (%) | 0.215 | ||
| No antiplatelet | 13 (3.3%) | 6 (5.9%) | |
| Single antiplatelet | 157 (40.3%) | 33 (32.4%) | |
| Dual antiplatelet | 220 (56.4%) | 63 (61.8%) | |
| Initiation time of statin from symptom onset | 0.020 | ||
| ≤24 h | 221 (56.7%) | 71 (69.6%) | |
| 24–48 h | 117 (30.0%) | 26 (25.5%) | |
| 48–72 h | 52 (13.3%) | 5 (4.9%) | |
| Intensity of statin therapy in acute phase, n (%) | 0.004 | ||
| Moderate intensity | 60 (15.4%) | 29 (28.4%) | |
| High intensity | 330 (84.6%) | 73 (71.6%) |
| Crude OR | Adjusted OR | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age, years | 1.03 (1.01–1.05) | <0.001 | 1.02 (1.00–1.05) | 0.017 |
| Male | 0.75 (0.48–1.17) | 0.206 | ||
| NIHSS score on admission | 1.10 (1.01–1.19) | 0.030 | 1.05 (0.96–1.16) | 0.263 |
| Onset to arrival time, hours | 0.98 (0.96–0.99) | 0.005 | 0.97 (0.94–1.00) | 0.116 |
| Hypertension | 0.92 (0.59–1.46) | 0.734 | ||
| Diabetes mellitus | 0.95 (0.58–1.53) | 0.835 | ||
| Dyslipidemia | 1.10 (0.61–1.91) | 0.732 | ||
| Coronary artery disease | 1.73 (0.46–5.43) | 0.371 | ||
| Smoking | 0.87 (0.56–1.35) | 0.538 | ||
| Prior stroke or TIA | 1.31 (0.63–2.56) | 0.444 | ||
| Fasting glucose | 1.00 (0.99–1.01) | 0.952 | ||
| LDL-C | 1.00 (0.99–1.01) | 0.198 | 1.00 (0.99–1.01) | 0.144 |
| HDL-C | 0.99 (0.98–1.01) | 0.615 | ||
| Triglyceride | 1.00 (0.99–1.00) | 0.522 | ||
| Total cholesterol | 1.00 (0.99–1.01) | 0.425 | ||
| CRP | 1.00 (0.86–1.13) | 0.981 | ||
| Systolic blood pressure | 1.00 (0.99–1.01) | 0.444 | ||
| Diastolic blood pressure | 1.00 (0.99–1.02) | 0.707 | ||
| Prior medication, antiplatelet | 1.17 (0.63–2.07) | 0.606 | ||
| Prior medication, antihypertensive | 0.88 (0.55–1.39) | 0.593 | ||
| Branch atheromatous lesion | 3.32 (2.13–5.23) | <0.001 | 3.49 (2.16–5.74) | <0.001 |
| Parent artery stenosis (0–50%) | 1.16 (0.73–1.82) | 0.519 | ||
| Location of SSSI | 0.137 | 0.375 | ||
| Anterior circulation (reference) vs. posterior circulation | 0.71 (0.46–1.11) | 0.80 (0.49–1.30) | ||
| Regimen of antiplatelet in acute phase | 0.221 | |||
| No antiplatelet | Reference | - | ||
| Single antiplatelet | 0.46 (0.17–1.37) | 0.137 | ||
| Dual antiplatelet | 0.62 (0.23–1.83) | 0.353 | ||
| Initiation time of statin from symptom onset | 0.026 | 0.887 | ||
| ≤24 h | Reference | - | Reference | - |
| 24–48 h | 0.69 (0.41–1.13) | 0.150 | 1.01 (0.45–2.35) | 0.966 |
| 48–72 h | 0.30 (0.10–0.71) | 0.013 | 0.75 (0.13–4.17) | 0.750 |
| Intensity of statin therapy in acute phase | 0.002 | 0.004 | ||
| Moderate intensity (reference) vs. high intensity | 0.46 (0.28–0.77) | 0.44 (0.25–0.77) |
| Crude OR | Adjusted OR | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age, years | 1.03 (1.00–1.06) | 0.045 | 1.02 (0.98–1.05) | 0.327 |
| Male | 0.89 (0.50–1.60) | 0.703 | ||
| NIHSS score on admission | 1.15 (1.03–1.29) | 0.011 | 1.14 (0.99–1.30) | 0.052 |
| Onset to arrival time, hours | 0.98 (0.96–0.99) | 0.038 | 0.97 (0.93–1.01) | 0.176 |
| Hypertension | 0.91 (0.50–1.66) | 0.746 | ||
| Diabetes mellitus | 0.99 (0.51–1.87) | 0.980 | ||
| Dyslipidemia | 1.48 (0.62–3.32) | 0.352 | ||
| Coronary artery disease | 0.90 (0.04–6.22) | 0.925 | ||
| Smoking | 1.03 (0.56–1.85) | 0.923 | ||
| Prior stroke or TIA | 1.03 (0.36–2.55) | 0.947 | ||
| Fasting glucose | 1.00 (0.99–1.01) | 0.095 | 1.00 (1.00–1.01) | 0.102 |
| LDL-C | 1.00 (0.99–1.01) | 0.211 | ||
| HDL-C | 1.00 (0.98–1.02) | 0.950 | ||
| Triglyceride | 0.99 (0.99–1.00) | 0.976 | ||
| Total cholesterol | 1.00 (0.99–1.01) | 0.584 | ||
| CRP | 1.01 (0.83–1.18) | 0.877 | ||
| Systolic blood pressure | 1.00 (0.99–1.01) | 0.462 | ||
| Diastolic blood pressure | 1.00 (0.99–1.02) | 0.331 | ||
| Prior medication, antiplatelet | 0.73 (0.31–1.54) | 0.434 | ||
| Prior medication, antihypertensive | 0.98 (0.53–1.78) | 0.962 | ||
| Branch atheromatous lesion | 3.26 (1.78–6.01) | <0.001 | 2.71 (1.35–5.51) | 0.005 |
| Parent artery stenosis (0–50%) | 1.08 (0.58–1.97) | 0.804 | ||
| Location of SSSI | 0.060 | 0.099 | ||
| Anterior circulation (reference) vs. posterior circulation | 0.55 (0.29–1.01) | 0.55 (0.26–1.10) | ||
| Regimen of antiplatelet in acute phase | 0.568 | |||
| No antiplatelet | Reference | - | ||
| Single antiplatelet | 0.46 (0.14–1.61) | 0.197 | ||
| Dual antiplatelet | 0.49 (0.16–1.70) | 0.235 | ||
| Initiation time of statin from symptom onset | 0.051 | 0.920 | ||
| ≤24 h | Reference | - | Reference | - |
| 24–48 h | 0.74 (0.36–1.43) | 0.381 | 0.96 (0.33–2.81) | 0.950 |
| 48–72 h | 0.31 (0.07–0.93) | 0.064 | 0.88 (0.09–7.28) | 0.907 |
| Intensity of statin therapy in acute phase | 0.002 | 0.004 | ||
| Moderate intensity (reference) vs. high intensity | 0.40 (0.22–0.73) | 0.39 (0.20–0.75) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.H.; Park, H.; Hong, J.-H.; Yoo, J.; Lee, H.; Kim, H.A.; Sohn, S.-I. Impact of High-Intensity Statin on Early Neurologic Deterioration in Patients with Single Small Subcortical Infarction. J. Clin. Med. 2023, 12, 3260. https://doi.org/10.3390/jcm12093260
Jang SH, Park H, Hong J-H, Yoo J, Lee H, Kim HA, Sohn S-I. Impact of High-Intensity Statin on Early Neurologic Deterioration in Patients with Single Small Subcortical Infarction. Journal of Clinical Medicine. 2023; 12(9):3260. https://doi.org/10.3390/jcm12093260
Chicago/Turabian StyleJang, Seong Hwa, Hyungjong Park, Jeong-Ho Hong, Joonsang Yoo, Hyung Lee, Hyun Ah Kim, and Sung-Il Sohn. 2023. "Impact of High-Intensity Statin on Early Neurologic Deterioration in Patients with Single Small Subcortical Infarction" Journal of Clinical Medicine 12, no. 9: 3260. https://doi.org/10.3390/jcm12093260
APA StyleJang, S. H., Park, H., Hong, J.-H., Yoo, J., Lee, H., Kim, H. A., & Sohn, S.-I. (2023). Impact of High-Intensity Statin on Early Neurologic Deterioration in Patients with Single Small Subcortical Infarction. Journal of Clinical Medicine, 12(9), 3260. https://doi.org/10.3390/jcm12093260






