The Clinical Analysis of Checkpoint Inhibitor Pneumonitis with Different Severities in Lung Cancer Patients: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
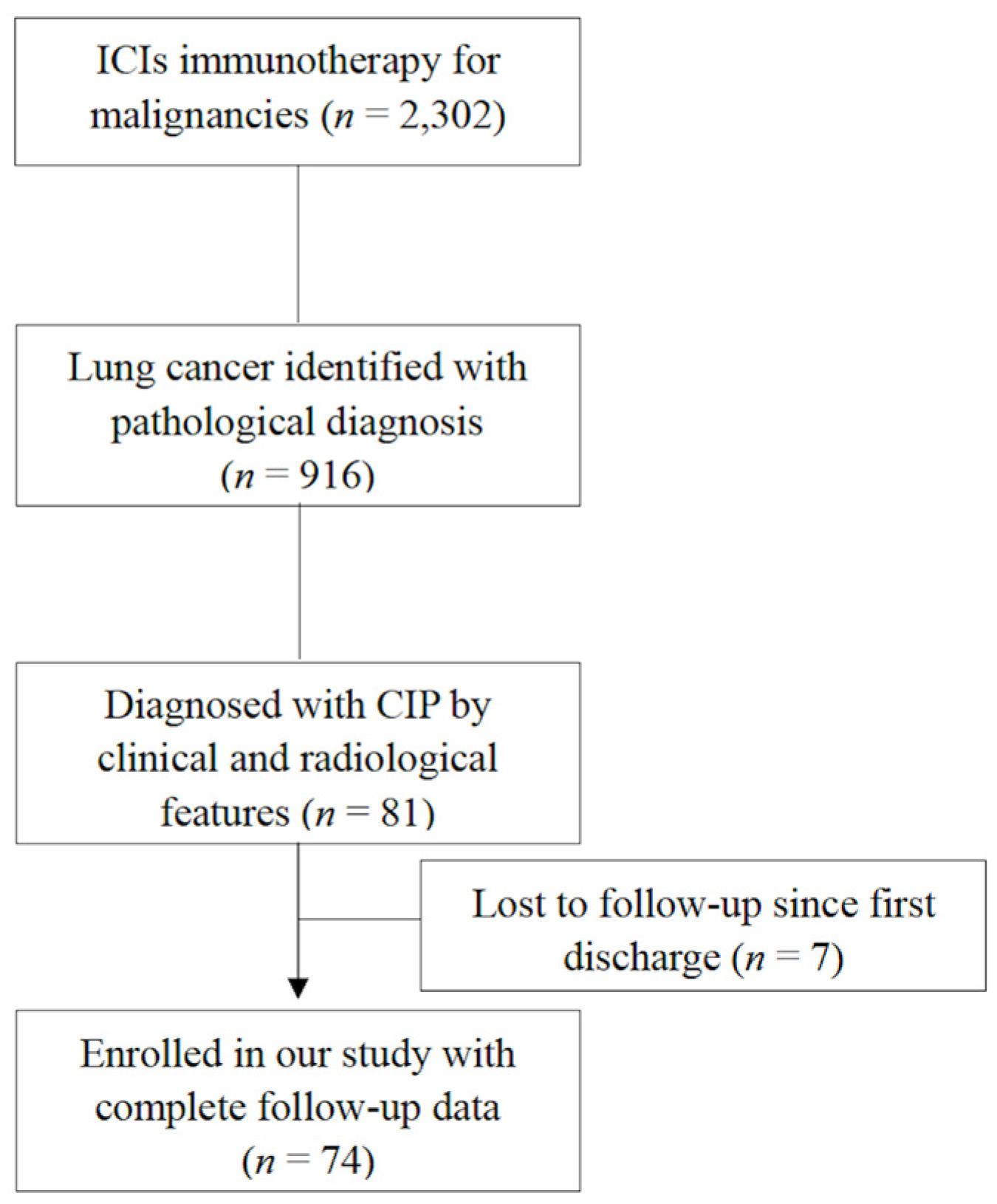
2.1. Patients
2.2. Definitions
2.3. Ethics
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of All Enrolled CIP Patients
3.2. Clinical Characteristics of Patients in the Low-Grade CIPs Group vs. the High-Grade CIPs Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishino, M.; Giobbie-Hurder, A.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jin, B.; Chen, J.; Wang, H.; Lin, S.; Dang, J.; Li, G. Comparative risk of serious and fatal treatment-related adverse events caused by 19 immune checkpoint inhibitors used in cancer treatment: A network meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920940927. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, H.; Tang, L.; Feng, Y.; Tao, Y.; Huang, L.; Lou, N.; Shi, Y. Association of immune-related adverse events and efficacy in advanced non-small-cell lung cancer: A systematic review and meta-analysis. Immunotherapy 2023, 15, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, Y.; Chen, C.; Wei, A.; Li, W. Association between immune-related adverse events and immunotherapy efficacy in non-small-cell lung cancer: A meta-analysis. Front. Pharmacol. 2023, 14, 1190001. [Google Scholar] [CrossRef] [PubMed]
- Rashdan, S.; Minna, J.D.; Gerber, D.E. Diagnosis and management of pulmonary toxicity associated with cancer immunotherapy. Lancet Respir. Med. 2018, 6, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Allouchery, M.; Lombard, T.; Martin, M.; Rouby, F.; Sassier, M.; Bertin, C.; Atzenhoffer, M.; Miremont-Salame, G.; Perault-Pochat, M.C.; Puyade, M.; et al. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥ 2 immune-related adverse events in patients with cancer. J. Immunother. Cancer 2020, 8, e001622. [Google Scholar] [CrossRef]
- Pang, L.; Xie, M.; Ma, X.; Huang, A.; Song, J.; Yao, J.; Deng, H.; Zhang, D.; Zang, X.; Ren, F.; et al. Clinical characteristics and therapeutic effects of checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. BMC Cancer 2023, 23, 203. [Google Scholar] [CrossRef]
- Tan, P.; Huang, W.; He, X.; Lv, F.; Cui, Y.; Du, S. Risk factors for refractory immune checkpoint inhibitor-related pneumonitis in patients with lung cancer. J. Immunother. 2023, 46, 64–73. [Google Scholar] [CrossRef]
- Abers, M.S.; Lionakis, M.S. Infectious complications of immune checkpoint inhibitors. Infect. Dis. Clin. North. Am. 2020, 34, 235–243. [Google Scholar] [CrossRef]
- Atchley, W.T.; Alvarez, C.; Saxena-Beem, S.; Schwartz, T.A.; Ishizawar, R.C.; Patel, K.P.; Rivera, M.P. Immune checkpoint inhibitor-related pneumonitis in lung cancer: Real-world incidence, risk factors, and management practices across six health care centers in North Carolina. Chest 2021, 160, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.; Rizvi, H.; Fuentes, P.; Luo, J.; Schoenfeld, A.; Lin, H.; Postow, M.; Callahan, M.; Voss, M.H.; Shah, N.J.; et al. Success and failure of additional immune modulators in steroid- refractory/resistant pneumonitis related to immune checkpoint blockade. J. Immunother. Cancer 2021, 9, e001884. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2002, 165, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-related adverse events (irAEs): Diagnosis, management, and clinical pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Porcu, M.; De Silva, P.; Solinas, C.; Battaglia, A.; Schena, M.; Scartozzi, M.; Bron, D.; Suri, J.S.; Willard-Gallo, K.; Sangiolo, D.; et al. Immunotherapy associated pulmonary toxicity: Biology behind clinical and radiological features. Cancers 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Chiari, R.; Ricciuti, B.; Metro, G.; Perrone, F.; Tiseo, M.; Bersanelli, M.; Bordi, P.; Santini, D.; Giusti, R.; et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin. Lung Cancer 2019, 20, 237–247 e231. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, S.; Fujimoto, D.; Morimoto, T.; Kawachi, H.; Ito, M.; Sato, Y.; Nagata, K.; Nakagawa, A.; Otsuka, K.; Uehara, K.; et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: A prospective cohort Study. J. Thorac. Oncol. 2017, 12, 1798–1805. [Google Scholar] [CrossRef]
- Toi, Y.; Sugawara, S.; Sugisaka, J.; Ono, H.; Kawashima, Y.; Aiba, T.; Kawana, S.; Saito, R.; Aso, M.; Tsurumi, K.; et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019, 5, 376–383. [Google Scholar] [CrossRef]
- Duma, N.; Abdel-Ghani, A.; Yadav, S.; Hoversten, K.P.; Reed, C.T.; Sitek, A.N.; Enninga, E.A.L.; Paludo, J.; Aguilera, J.V.; Leventakos, K.; et al. Sex differences in tolerability to anti-programmed cell death protein 1 therapy in patients with metastatic melanoma and non-small cell lung cancer: Are we all equal? Oncologist 2019, 24, e1148–e1155. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Wang, J.; Li, K.; Chen, X.; Heng, J.; Gao, Q.; Ye, Y.; Zhang, Z.; Liu, Y.; et al. Association between sex and immune-related adverse events during immune checkpoint inhibitor therapy. J. Natl. Cancer Inst. 2021, 113, 1396–1404. [Google Scholar] [CrossRef]
- Yang, K.; Li, J.; Sun, Z.; Bai, C.; Zhao, L. Effect of age on the risk of immune-related adverse events in patients receiving immune checkpoint inhibitors. Clin. Exp. Med. 2023, 23, 3907–3918. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Deng, H.; Yang, Y.; Wu, J.; Qiu, G.; Li, S.; Xie, X.; Liu, M.; Xie, Z.; Qin, Y.; et al. Peripheral blood biomarkers for early diagnosis, severity, and prognosis of checkpoint inhibitor-related pneumonitis in patients with lung cancer. Front. Oncol. 2021, 11, 698832. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, B.; Valaperti, A.; Bezel, P.; Steiner, U.C.; Scholtze, D.; Wieser, S.; Vonow-Eisenring, M.; Widmer, A.; Kohler, M.; Franzen, D. Analysis of cytokines in serum and bronchoalveolar lavage fluid in patients with immune-checkpoint inhibitor-associated pneumonitis: A cross-sectional case-control study. J. Cancer Res. Clin. Oncol. 2022, 148, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Satarker, S.; Tom, A.A.; Shaji, R.A.; Alosious, A.; Luvis, M.; Nampoothiri, M. JAK-STAT pathway inhibition and their implications in COVID-19 therapy. Postgrad. Med. 2021, 133, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. Management of immunotherapy-related toxicities, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2019, 17, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kim, J.; Lee, J.S.; Kim, Y.J.; Kim, S.H.; Lee, Y.J.; Cho, Y.J.; Yoon, H.I.; Lee, J.H.; Lee, C.T.; et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 2018, 125, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Awad, M.M.; Sholl, L.M.; Maattala, J.A.; Taibi, M.; Hatabu, H.; Ott, P.A.; Armand, P.F.; Hodi, F.S. PD-1 Inhibitor-related pneumonitis in advanced cancer patients: Radiographic patterns and clinical course. Clin. Cancer Res. 2016, 22, 6051–6060. [Google Scholar] [CrossRef]
- Delaunay, M.; Cadranel, J.; Lusque, A.; Meyer, N.; Gounant, V.; Moro-Sibilot, D.; Michot, J.M.; Raimbourg, J.; Girard, N.; Guisier, F.; et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur. Respir. J. 2017, 50, 1700050. [Google Scholar] [CrossRef]
- Ravaglia, C.; Doglioni, C.; Chilosi, M.; Piciucchi, S.; Dubini, A.; Rossi, G.; Pedica, F.; Puglisi, S.; Donati, L.; Tomassetti, S.; et al. Clinical, radiological and pathological findings in patients with persistent lung disease following SARS-CoV-2 infection. Eur. Respir. J. 2022, 60, 2102411. [Google Scholar] [CrossRef]
- Machnicki, S.; Patel, D.; Singh, A.; Talwar, A.; Mina, B.; Oks, M.; Makkar, P.; Naidich, D.; Mehta, A.; Hill, N.S.; et al. The usefulness of chest CT imaging in patients with suspected or diagnosed COVID-19: A review of literature. Chest 2021, 160, 652–670. [Google Scholar] [CrossRef]
- Carlicchi, E.; Gemma, P.; Poerio, A.; Caminati, A.; Vanzulli, A.; Zompatori, M. Chest-CT mimics of COVID-19 pneumonia-a review article. Emerg. Radiol. 2021, 28, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Picasso, R.; Cozzi, A.; Picasso, V.; Zaottini, F.; Pistoia, F.; Perissi, S.; Martinoli, C. Immune checkpoint inhibitor-related pneumonitis and COVID-19: A case-matched comparison of CT findings. Radiol. Med. 2023, 128, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, M.; Yuan, J.; Wang, F.; Wang, Z.; Li, J.; Zhang, M.; Xing, L.; Wei, J.; Peng, L.; et al. Laboratory diagnosis and monitoring the viral shedding of SARS-CoV-2 infection. Innovation 2020, 1, 100061. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Ascierto, P.A.; Patelli, G.; Danesi, R.; Vanzulli, A.; Sandomenico, F.; Tarsia, P.; Cattelan, A.; Comes, A.; De Laurentiis, M.; et al. Drug-induced interstitial lung disease during cancer therapies: Expert opinion on diagnosis and treatment. ESMO Open 2022, 7, 100404. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; Committee, E.G. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Picchi, H.; Mateus, C.; Chouaid, C.; Besse, B.; Marabelle, A.; Michot, J.M.; Champiat, S.; Voisin, A.L.; Lambotte, O. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: Reactivation of tuberculosis after anti PD-1 treatment. Clin. Microbiol. Infect. 2018, 24, 216–218. [Google Scholar] [CrossRef]
- Xing, Q.; Zhang, Z.; Zhu, B.; Lin, Q.; Shen, L.; Li, F.; Xia, Z.; Zhao, Z. Case Report: Treatment for steroid-refractory immune-related myocarditis with tofacitinib. Front. Immunol. 2022, 13, 944013. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Bretagne, M.; Arrondeau, J.; Zahr, N.; Ederhy, S.; Abbar, B.; Pinna, B.; Allenbach, Y.; Mira, J.P.; Moslehi, J.; et al. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: Proof of concept. J. Immunother. Cancer 2022, 10, e004699. [Google Scholar] [CrossRef]
- Salem, J.E.; Bretagne, M.; Abbar, B.; Leonard-Louis, S.; Ederhy, S.; Redheuil, A.; Boussouar, S.; Nguyen, L.S.; Procureur, A.; Stein, F.; et al. Abatacept/Ruxolitinib and screening for concomitant respiratory muscle failure to mitigate fatality of immune-checkpoint inhibitor myocarditis. Cancer Discov. 2023, 13, 1100–1115. [Google Scholar] [CrossRef]


| Management | Group 1 * (n = 63) | Group 2 ** (n = 11) | χ2 or t Value | p Value |
|---|---|---|---|---|
| Duration of methylprednisolone > 80 mg/d (days) | 3.14 | 8.82 | 3.39 | 0.001 |
| Initial dosage of prednisone > 1 mg/kg/d (n/%) | 35/55.6% | 9/81.8% | 2.68 | 0.18 |
| Duration of prednisolone > 1 mg/kg/d (days) | 9.7 | 18.5 | 3.26 | 0.002 |
| Tocilizumab (n/%) | 5/7.9% | 4/36.4% | 7.08 | 0.02 |
| IVIg (n/%) | 5/7.9% | 10/90.9% | 9.57 | 0.004 |
| Antibiotics administration (n/%) | 41/65.1% | 11/100% | 5.47 | 0.03 |
| Anti-fungal administration (n/%) | 5/7.9% | 5/45.5% | 11.3 | 0.005 |
| Characteristics | Low-Grade CIP (n = 31) | High-Grade CIP (n = 43) | χ2 or t Value | p Value |
|---|---|---|---|---|
| Age (years) * | 61.5 ± 10.1 | 65.8 ± 6.5 | 2.21 | 0.03 |
| Sex (male%) | 77.4% | 79.1% | 0.03 | 0.87 |
| Smoking history (n/%) ** | 18/58.1% | 30/69.8% | 0.75 | 0.39 |
| ECOG before ICIs therapy (n/%) | ||||
| 0 | 18/58.1% | 12/27.9% | ||
| 1 | 13/41.9% | 28/65.1% | 7.95 | 0.047 |
| 2 | 0 | 2/4.7% | ||
| 3 | 0 | 1/2.3% | ||
| Underlying diseases (n%) | ||||
| none | 17/54.8% | 26/60.5% | ||
| autoimmune diseases | 0 | 1/2.3% | 1.22 | 0.75 |
| other malignancies | 7/22.6% | 7/16.3% | ||
| diabetes | 7/22.6% | 9/20.9% | ||
| Pathological patterns of lung cancer (n/%) | ||||
| adenocarcinoma | 13/41.9% | 19/44.2% | ||
| squamous cell cancer | 10/32.2% | 21/48.8% | 6.25 | 0.18 |
| small cell lung cancer | 4/12.9% | 2/4.7% | ||
| others | 4/12.9% | 1/2.3% | ||
| Pre-existing ILDs (n/%) | 4/12.9% | 11/25.6% | 1.62 | 0.20 |
| Surgical resection (n/%) | ||||
| none | 28/90.3% | 36/83.7% | ||
| lobe resection | 3/9.7% | 4/9.3% | 2.26 | 0.32 |
| wedge resection | 0 | 3/7% | ||
| Non-surgical treatment (n/%) | ||||
| radiotherapy (n/%) | 11/35.5% | 12/27.9% | 0.48 | 0.49 |
| molecular targeting therapy | 7/22.6% | 4/9.3% | 0.02 | 0.11 |
| chemotherapy | 27/87.1% | 37/86.0% | 0.02 | 0.90 |
| ICIs therapy (n/%) | ||||
| first line | 14/45.2% | 22/51.2% | ||
| second line | 10/32.2% | 9/20.9% | ||
| more than the second line | 4/12.9% | 3/7% | 7.42 | 0.19 |
| new adjuvant therapy | 0 | 6/14% | ||
| maintenance therapy | 2/6.5% | 3/7% | ||
| post-surgery adjuvant therapy | 1/3.2% | 0 | ||
| With other irAEs (n/%) | 7/22.6% | 12/27.9% | 0.27 | 0.61 |
| Characteristics | Low-Grade CIP (n = 31) | High-Grade CIP (n = 43) | χ2 or t Value | p Value |
|---|---|---|---|---|
| ECOG as suffering from CIPs (n/%) | ||||
| 0 | 2/6.5% | 1/2.3% | ||
| 1 | 20/64.5% | 9/20.9% | ||
| 2 | 5/16.1% | 11/25.6% | 17.9 | 0.001 |
| 3 | 4/12.9% | 20/46.5% | ||
| 4 | 0 | 2/4.7% | ||
| Fever | 9/29% | 28/65.1% | 9.38 | 0.002 |
| Laboratory analysis * | ||||
| albumin (g/L) | 37.9 ± 4.6 | 35.2 ± 4.2 | 2.59 | 0.01 |
| D-dimer (mg/L) | 1.7 ± 2.2 | 5.1 ± 7.1 | 2.36 | 0.02 |
| CRP (mg/L) | 45.8 ± 48.1 | 68.5 ± 56.8 | 1.53 | 0.13 |
| ESR (mm/h) | 56.6 ± 32.3 | 63 ± 33.1 | 0.63 | 0.53 |
| LDH (U/L) | 286.1 ± 103.7 | 350.1 ± 157 | 1.74 | 0.09 |
| Radiological patterns of CIP (n/%) | ||||
| NSIP pattern | 12/38.7% | 21/48.8% | ||
| OP pattern | 15/48.4% | 15/34.9% | 6.34 | 0.09 |
| NSIP overlap OP pattern | 0 | 5/11.6% | ||
| Others | 4/12.9% | 2/4.7% | ||
| Chest CT features (n/%) | ||||
| CIP in both lungs | 28/90.3% | 42/97.6% | 4.98 | 0.08 |
| Emphysema | 10/32.2% | 21/48.8% | 2.03 | 0.15 |
| lymphadenopathy ** | 6/19.4% | 23/53.5% | 8.81 | 0.003 |
| pleural abnormalities | ||||
| pleural thickening | 16/51.6% | 22/51.2% | ||
| pleural effusion | 5/16.1% | 18/41.9% | 10.39 | 0.006 |
| normal | 10/32.2% | 3/7% | ||
| Complicated with infectious disease during CIP management (n/%) | 2/6.5% | 14/32.6% | 14.62 | 0.02 |
| Initial dosage of corticosteroids (>1 mg/kg/d ***, n/%) | 20/64.5% | 24/55.8% | 0.57 | 0.45 |
| Tocilizumab (n/%) | 11/35.5% | 13/30.2% | 0.23 | 0.63 |
| IVIg (n/%) | 7/22.6% | 8/18.6% | 0.18 | 0.68 |
| Outcomes (n/%) | ||||
| cured | 0 | 3/7% | ||
| improved | 24/77.4% | 30/72.1% | ||
| stable | 3/9.7% | 3/7% | 3.21 | 0.52 |
| progressed | 0 | 1/2.3% | ||
| dead | 4/12.9% | 6/14% | ||
| All-cause mortality (n/%) | 4/12.9% | 14/32.6% | 3.78 | 0.05 |
| ICIs rechallenge | 9/29% | 6/14% | 2.53 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Chen, R.; Xu, Y.; Fang, N.; Shao, C.; Xu, K.; Wang, M. The Clinical Analysis of Checkpoint Inhibitor Pneumonitis with Different Severities in Lung Cancer Patients: A Retrospective Study. J. Clin. Med. 2024, 13, 255. https://doi.org/10.3390/jcm13010255
Huang H, Chen R, Xu Y, Fang N, Shao C, Xu K, Wang M. The Clinical Analysis of Checkpoint Inhibitor Pneumonitis with Different Severities in Lung Cancer Patients: A Retrospective Study. Journal of Clinical Medicine. 2024; 13(1):255. https://doi.org/10.3390/jcm13010255
Chicago/Turabian StyleHuang, Hui, Ruxuan Chen, Yan Xu, Nan Fang, Chi Shao, Kai Xu, and Mengzhao Wang. 2024. "The Clinical Analysis of Checkpoint Inhibitor Pneumonitis with Different Severities in Lung Cancer Patients: A Retrospective Study" Journal of Clinical Medicine 13, no. 1: 255. https://doi.org/10.3390/jcm13010255
APA StyleHuang, H., Chen, R., Xu, Y., Fang, N., Shao, C., Xu, K., & Wang, M. (2024). The Clinical Analysis of Checkpoint Inhibitor Pneumonitis with Different Severities in Lung Cancer Patients: A Retrospective Study. Journal of Clinical Medicine, 13(1), 255. https://doi.org/10.3390/jcm13010255







