Optimal Antithrombotic Strategies in Cardiogenic Shock
Abstract
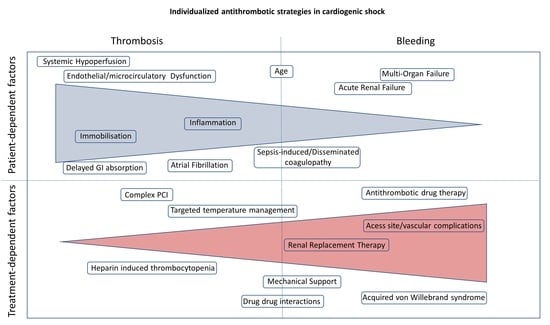
1. Introduction
2. Definition and Pathophysiology
3. Characteristics of Antithrombotic Substances
3.1. Aspirin
3.2. P2Y12 Receptor Inhibitors
3.2.1. Clopidogrel
3.2.2. Prasugrel
3.2.3. Ticagrelor
3.2.4. Cangrelor
3.3. Glycoprotein IIb/IIIa Receptor Inhibitors (GPI)
3.4. Unfractionated Heparin (UFH)
3.5. Low-Molecular-Weight Heparins (LMWHs) and Fondaparinux
3.6. Bivalirudin
3.7. Argatroban
4. Antithrombotic Therapy in Special Situations (Figure 2)
4.1. Mechanical Circulatory Support (MCS)
4.1.1. Extracorporeal Membrane Oxygenation (ECMO)

4.1.2. Impella
4.2. Hemofiltration
4.3. Hypothermia/Targeted Temperature Management (TTM)
5. Atrial Fibrillation
6. Management of Antithrombotic Therapy in CS Patients with Bleeding
7. Bridging to Destination Therapy/Surgery
8. Conclusions and Gaps in Evidence
Author Contributions
Funding
Conflicts of Interest
References
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This Statement Was Endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J. Am. Coll. Cardiol. 2022, 79, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; de Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Akin, I.; Behnes, M.; Rassaf, T.; Mahabadi, A.A.; Lehmann, R.; Eitel, I.; Graf, T.; Seidler, T.; et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2023, 389, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Jobs, A.; Lurz, P.; Feistritzer, H.-J.; De Waha-Thiele, S.; Meyer-Saraei, R.; Montalescot, G.; Huber, K.; Noc, M.; Windecker, S.; et al. Frequency and Impact of Bleeding on Outcome in Patients with Cardiogenic Shock. JACC Cardiovasc. Interv. 2020, 13, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Tilemann, L.; Mohr, S.K.; Preusch, M.; Chorianopoulos, E.; Giannitsis, E.; Katus, H.A.; Müller, O.J. Platelet Function Monitoring for Stent Thrombosis in Critically III Patients with an Acute Coronary Syndrome. J. Intervent. Cardiol. 2018, 31, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zeymer, U.; Hohlfeld, T.; Vom Dahl, J.; Erbel, R.; Münzel, T.; Zahn, R.; Roitenberg, A.; Breitenstein, S.; Pap, Á.F.; Trenk, D. Prospective, Randomised Trial of the Time Dependent Antiplatelet Effects of 500 Mg and 250 Mg Acetylsalicylic Acid i. v. and 300 Mg p. o. in ACS (ACUTE). Thromb. Haemost. 2017, 117, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Orban, M.; Kleeberger, J.; Ouarrak, T.; Freund, A.; Feistritzer, H.-J.; Fuernau, G.; Geisler, T.; Huber, K.; Dudek, D.; Noc, M.; et al. Clopidogrel vs. Prasugrel vs. Ticagrelor in Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Pooled IABP-SHOCK II and CULPRIT-SHOCK Trial Sub-Analysis. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 1493–1503. [Google Scholar] [CrossRef]
- Gouffran, G.; Rosencher, J.; Bougouin, W.; Jakamy, R.; Joffre, J.; Lamhaut, L.; Dumas, F.; Cariou, A.; Varenne, O. Stent Thrombosis after Primary Percutaneous Coronary Intervention in Comatose Survivors of Out-of-Hospital Cardiac Arrest: Are the New P2Y12 Inhibitors Really More Effective than Clopidogrel? Resuscitation 2016, 98, 73–78. [Google Scholar] [CrossRef]
- Jiménez-Brítez, G.; Freixa, X.; Flores-Umanzor, E.; San Antonio, R.; Caixal, G.; Garcia, J.; Hernandez-Enriquez, M.; Andrea, R.; Regueiro, A.; Masotti, M.; et al. Out-of-Hospital Cardiac Arrest and Stent Thrombosis: Ticagrelor versus Clopidogrel in Patients with Primary Percutaneous Coronary Intervention under Mild Therapeutic Hypothermia. Resuscitation 2017, 114, 141–145. [Google Scholar] [CrossRef]
- Steblovnik, K.; Blinc, A.; Mijovski, M.B.; Fister, M.; Mikuz, U.; Noc, M. Ticagrelor Versus Clopidogrel in Comatose Survivors of Out-of-Hospital Cardiac Arrest Undergoing Percutaneous Coronary Intervention and Hypothermia: A Randomized Study. Circulation 2016, 134, 2128–2130. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, R.; Al-Turbak, H.; Osborne, C.; Hibbert, B.; So, D.Y.F.; Le May, M.R. Superiority of Ticagrelor Over Clopidogrel in Patients After Cardiac Arrest Undergoing Therapeutic Hypothermia. Can. J. Cardiol. 2014, 30, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.; Christoph, M.; Schmeinck, S.; Schmieder, K.; Steiding, K.; Schoener, L.; Pfluecke, C.; Quick, S.; Mues, C.; Jellinghaus, S.; et al. High Rates of Prasugrel and Ticagrelor Non-Responder in Patients Treated with Therapeutic Hypothermia after Cardiac Arrest. Resuscitation 2014, 85, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, S.H.; Kandlakunta, H.; Kuchkuntla, A.R.; West, C.P.; Murad, M.H.; Wang, Z.; Kochar, A.; Rab, S.T.; Gersh, B.J.; Holmes, D.R.; et al. Newer P2Y12 Inhibitors vs Clopidogrel in Acute Myocardial Infarction With Cardiac Arrest or Cardiogenic Shock: A Systematic Review and Meta-Analysis. Mayo Clin. Proc. 2022, 97, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Parodi, G.; Xanthopoulou, I.; Bellandi, B.; Gkizas, V.; Valenti, R.; Karanikas, S.; Migliorini, A.; Angelidis, C.; Abbate, R.; Patsilinakos, S.; et al. Ticagrelor Crushed Tablets Administration in STEMI Patients: The MOJITO Study. J. Am. Coll. Cardiol. 2015, 65, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Rollini, F.; Franchi, F.; Hu, J.; Kureti, M.; Aggarwal, N.; Durairaj, A.; Park, Y.; Seawell, M.; Cox-Alomar, P.; Zenni, M.M.; et al. Crushed Prasugrel Tablets in Patients with STEMI Undergoing Primary Percutaneous Coronary Intervention: The CRUSH Study. J. Am. Coll. Cardiol. 2016, 67, 1994–2004. [Google Scholar] [CrossRef]
- Meine, T.J.; Roe, M.T.; Chen, A.Y.; Patel, M.R.; Washam, J.B.; Ohman, E.M.; Peacock, W.F.; Pollack, C.V.; Gibler, W.B.; Peterson, E.D.; et al. Association of Intravenous Morphine Use and Outcomes in Acute Coronary Syndromes: Results from the CRUSADE Quality Improvement Initiative. Am. Heart J. 2005, 149, 1043–1049. [Google Scholar] [CrossRef]
- Montalescot, G.; van ’t Hof, A.W.; Lapostolle, F.; Silvain, J.; Lassen, J.F.; Bolognese, L.; Cantor, W.J.; Cequier, A.; Chettibi, M.; Goodman, S.G.; et al. Prehospital Ticagrelor in ST-Segment Elevation Myocardial Infarction. N. Engl. J. Med. 2014, 371, 1016–1027. [Google Scholar] [CrossRef]
- Farag, M.; Spinthakis, N.; Srinivasan, M.; Sullivan, K.; Wellsted, D.; Gorog, D.A. Morphine Analgesia Pre-PPCI Is Associated with Prothrombotic State, Reduced Spontaneous Reperfusion and Greater Infarct Size. Thromb. Haemost. 2018, 118, 601–612. [Google Scholar] [CrossRef]
- Hobl, E.-L.; Stimpfl, T.; Ebner, J.; Schoergenhofer, C.; Derhaschnig, U.; Sunder-Plassmann, R.; Jilma-Stohlawetz, P.; Mannhalter, C.; Posch, M.; Jilma, B. Morphine Decreases Clopidogrel Concentrations and Effects: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Cardiol. 2014, 63, 630–635. [Google Scholar] [CrossRef]
- Hobl, E.-L.; Reiter, B.; Schoergenhofer, C.; Schwameis, M.; Derhaschnig, U.; Kubica, J.; Stimpfl, T.; Jilma, B. Morphine Decreases Ticagrelor Concentrations but Not Its Antiplatelet Effects: A Randomized Trial in Healthy Volunteers. Eur. J. Clin. Invest. 2016, 46, 7–14. [Google Scholar] [CrossRef]
- Kubica, J.; Adamski, P.; Ostrowska, M.; Sikora, J.; Kubica, J.M.; Sroka, W.D.; Stankowska, K.; Buszko, K.; Navarese, E.P.; Jilma, B.; et al. Morphine Delays and Attenuates Ticagrelor Exposure and Action in Patients with Myocardial Infarction: The Randomized, Double-Blind, Placebo-Controlled IMPRESSION Trial. Eur. Heart J. 2016, 37, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Tavenier, A.H.; Hermanides, R.S.; Ottervanger, J.P.; Tolsma, R.; van Beurden, A.; Slingerland, R.J.; Ter Horst, P.G.J.; Gosselink, A.T.M.; Dambrink, J.-H.E.; van Leeuwen, M.A.H.; et al. Impact of Opioids on P2Y12 Receptor Inhibition in Patients with ST-Elevation Myocardial Infarction Who Are Pre-Treated with Crushed Ticagrelor: Opioids aNd Crushed Ticagrelor In Myocardial Infarction Evaluation (ON-TIME 3) Trial. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; Rollini, F.; Park, Y.; Hu, J.; Kureti, M.; Rivas Rios, J.; Faz, G.; Yaranov, D.; Been, L.; Pineda, A.M.; et al. Effects of Methylnaltrexone on Ticagrelor-Induced Antiplatelet Effects in Coronary Artery Disease Patients Treated With Morphine. JACC Cardiovasc. Interv. 2019, 12, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Niezgoda, P.; Barańska, M.; Buszko, K.; Skibińska, N.; Sroka, W.; Pstrągowski, K.; Siller-Matula, J.; Bernd, J.; Gorog, D.; et al. METoclopramide Administration as a Strategy to Overcome MORPHine-ticagrelOr Interaction in PatientS with Unstable Angina PectorIS-The METAMORPHOSIS Trial. Thromb. Haemost. 2018, 118, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Geisler, T.; Langer, H.; Wydymus, M.; Göhring, K.; Zürn, C.; Bigalke, B.; Stellos, K.; May, A.E.; Gawaz, M. Low Response to Clopidogrel Is Associated with Cardiovascular Outcome after Coronary Stent Implantation. Eur. Heart J. 2006, 27, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Varenhorst, C.; James, S.; Erlinge, D.; Braun, O.O.; Jakubowski, J.A.; Sugidachi, A.; Winters, K.J.; Siegbahn, A. Prasugrel Achieves Greater and Faster P2Y12receptor-Mediated Platelet Inhibition than Clopidogrel Due to More Efficient Generation of Its Active Metabolite in Aspirin-Treated Patients with Coronary Artery Disease. Eur. Heart J. 2008, 29, 21–30. [Google Scholar] [CrossRef]
- Available online: https://www.Ema.Europa.Eu/En/Documents/Product-Information/Kengrexal-Epar-Product-Information_de.Pdf (accessed on 28 November 2023).
- Bhatt, D.L.; Stone, G.W.; Mahaffey, K.W.; Gibson, C.M.; Steg, P.G.; Hamm, C.W.; Price, M.J.; Leonardi, S.; Gallup, D.; Bramucci, E.; et al. Effect of Platelet Inhibition with Cangrelor during PCI on Ischemic Events. N. Engl. J. Med. 2013, 368, 1303–1313. [Google Scholar] [CrossRef]
- Steg, P.G.; Bhatt, D.L.; Hamm, C.W.; Stone, G.W.; Gibson, C.M.; Mahaffey, K.W.; Leonardi, S.; Liu, T.; Skerjanec, S.; Day, J.R.; et al. Effect of Cangrelor on Periprocedural Outcomes in Percutaneous Coronary Interventions: A Pooled Analysis of Patient-Level Data. Lancet 2013, 382, 1981–1992. [Google Scholar] [CrossRef]
- Droppa, M.; Spahn, P.; Takhgiriev, K.; Müller, K.A.L.; Alboji, A.; Straub, A.; Rath, D.; Jeong, Y.-H.; Gawaz, M.; Geisler, T. Periprocedural Platelet Inhibition with Cangrelor in P2Y12-Inhibitor Naïve Patients with Acute Coronary Syndromes—A Matched-Control Pharmacodynamic Comparison in Real-World Patients. Int. J. Cardiol. 2016, 223, 848–851. [Google Scholar] [CrossRef]
- Westman, P.C.; Lipinski, M.J.; Torguson, R.; Waksman, R. A Comparison of Cangrelor, Prasugrel, Ticagrelor, and Clopidogrel in Patients Undergoing Percutaneous Coronary Intervention: A Network Meta-Analysis. Cardiovasc. Revascularization Med. Mol. Interv. 2017, 18, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; Rollini, F.; Rivas, A.; Wali, M.; Briceno, M.; Agarwal, M.; Shaikh, Z.; Nawaz, A.; Silva, G.; Been, L.; et al. Platelet Inhibition with Cangrelor and Crushed Ticagrelor in Patients With ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Circulation 2019, 139, 1661–1670. [Google Scholar] [CrossRef]
- Droppa, M.; Vaduganathan, M.; Venkateswaran, R.V.; Singh, A.; Szumita, P.M.; Roberts, R.J.; Qamar, A.; Hack, L.; Rath, D.; Gawaz, M.; et al. Cangrelor in Cardiogenic Shock and after Cardiopulmonary Resuscitation: A Global, Multicenter, Matched Pair Analysis with Oral P2Y12 Inhibition from the IABP-SHOCK II Trial. Resuscitation 2019, 137, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Grimfjärd, P.; Lagerqvist, B.; Erlinge, D.; Varenhorst, C.; James, S. Clinical Use of Cangrelor: Nationwide Experience from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kordis, P.; Bozic Mijovski, M.; Berden, J.; Steblovnik, K.; Blinc, A.; Noc, M. Cangrelor for Comatose Survivors of Out-of-Hospital Cardiac Arrest Undergoing Percutaneous Coronary Intervention: The CANGRELOR-OHCA Study. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2023, 18, 1269–1271. [Google Scholar]
- Zeymer, U.; Lober, C.; Richter, S.; Olivier, C.B.; Huber, K.; Haring, B.; Schwimmbeck, P.; Andrassy, M.; Akin, I.; Cuneo, A.; et al. Cangrelor in Patients with Percutaneous Coronary Intervention for Acute Myocardial Infarction after Cardiac Arrest and/or with Cardiogenic Shock. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Firstenberg, M.S.; Price, M.J.; Tummala, P.E.; Hutyra, M.; Welsby, I.J.; Voeltz, M.D.; Chandna, H.; Ramaiah, C.; Brtko, M.; et al. Bridging Antiplatelet Therapy with Cangrelor in Patients Undergoing Cardiac Surgery: A Randomized Controlled Trial. JAMA 2012, 307, 265–274. [Google Scholar] [CrossRef]
- Hochholzer, W.; Kleiner, P.; Younas, I.; Valina, C.M.; Löffelhardt, N.; Amann, M.; Bömicke, T.; Ferenc, M.; Hauschke, D.; Trenk, D.; et al. Randomized Comparison of Oral P2Y12-Receptor Inhibitor Loading Strategies for Transitioning from Cangrelor: The ExcelsiorLOAD2 Trial. JACC Cardiovasc. Interv. 2017, 10, 121–129. [Google Scholar] [CrossRef]
- Schneider, D.J.; Agarwal, Z.; Seecheran, N.; Keating, F.K.; Gogo, P. Pharmacodynamic Effects during the Transition between Cangrelor and Ticagrelor. JACC Cardiovasc. Interv. 2014, 7, 435–442. [Google Scholar] [CrossRef]
- Schneider, D.J.; Seecheran, N.; Raza, S.S.; Keating, F.K.; Gogo, P. Pharmacodynamic Effects during the Transition between Cangrelor and Prasugrel. Coron. Artery Dis. 2015, 26, 42–48. [Google Scholar] [CrossRef]
- Gargiulo, G.; Esposito, G.; Avvedimento, M.; Nagler, M.; Minuz, P.; Campo, G.; Gragnano, F.; Manavifar, N.; Piccolo, R.; Tebaldi, M.; et al. Cangrelor, Tirofiban, and Chewed or Standard Prasugrel Regimens in Patients With ST-Segment-Elevation Myocardial Infarction: Primary Results of the FABOLUS-FASTER Trial. Circulation 2020, 142, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Karathanos, A.; Lin, Y.; Dannenberg, L.; Parco, C.; Schulze, V.; Brockmeyer, M.; Jung, C.; Heinen, Y.; Perings, S.; Zeymer, U.; et al. Routine Glycoprotein IIb/IIIa Inhibitor Therapy in ST-Segment Elevation Myocardial Infarction: A Meta-Analysis. Can. J. Cardiol. 2019, 35, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Kanic, V.; Vollrath, M.; Penko, M.; Markota, A.; Kompara, G.; Kanic, Z. GPIIb-IIIa Receptor Inhibitors in Acute Coronary Syndrome Patients Presenting with Cardiogenic Shock and/or After Cardiopulmonary Resuscitation. Heart Lung Circ. 2018, 27, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Bernat, I.; Abdelaal, E.; Plourde, G.; Bataille, Y.; Cech, J.; Pesek, J.; Koza, J.; Jirous, S.; Machaalany, J.; Déry, J.-P.; et al. Early and Late Outcomes after Primary Percutaneous Coronary Intervention by Radial or Femoral Approach in Patients Presenting in Acute ST-Elevation Myocardial Infarction and Cardiogenic Shock. Am. Heart J. 2013, 165, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Saleiro, C.; de Campos, D.; Ribeiro, J.M.; Lopes, J.; Puga, L.; Sousa, J.P.; Gomes, A.R.M.; Siserman, A.; Lourenço, C.; Gonçalves, L.; et al. Glycoprotein IIb/IIIa Inhibitor Use in Cardiogenic Shock Complicating Myocardial Infarction: The Portuguese Registry of Acute Coronary Syndromes. Rev. Port. Cardiol. 2023, 42, 113–120. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Tomassini, F.; Fiorilli, R.; Gagnor, A.; Parma, A.; Cerrato, E.; Musto, C.; Nazzaro, M.S.; Varbella, F.; Violini, R. Effect of Abciximab Therapy in Patients Undergoing Coronary Angioplasty for Acute ST-Elevation Myocardial Infarction Complicated by Cardiogenic Shock. Circ. J. 2015, 79, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Merlini, P.A.; Rossi, M.; Menozzi, A.; Buratti, S.; Brennan, D.M.; Moliterno, D.J.; Topol, E.J.; Ardissino, D. Thrombocytopenia Caused by Abciximab or Tirofiban and Its Association with Clinical Outcome in Patients Undergoing Coronary Stenting. Circulation 2004, 109, 2203–2206. [Google Scholar] [CrossRef]
- ESPRIT Investigators. Enhanced Suppression of the Platelet IIb/IIIa Receptor with Integrilin Therapy Novel Dosing Regimen of Eptifibatide in Planned Coronary Stent Implantation (ESPRIT): A Randomised, Placebo-Controlled Trial. Lancet 2000, 356, 2037–2044. [Google Scholar] [CrossRef]
- PURSUIT Trial Investigators. Inhibition of Platelet Glycoprotein IIb/IIIa with Eptifibatide in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 1998, 339, 436–443. [Google Scholar] [CrossRef]
- Hohlfelder, B.; Kelly, D.; Hoang, M.; Anger, K.E.; Sylvester, K.W.; Kaufman, R.M.; Connors, J.M. Activated Clotting Times Demonstrate Weak Correlation With Heparin Dosing in Adult Extracorporeal Membrane Oxygenation. Am. J. Ther. 2022, 29, e385–e393. [Google Scholar] [CrossRef]
- Levy, J.H.; Staudinger, T.; Steiner, M.E. How to Manage Anticoagulation during Extracorporeal Membrane Oxygenation. Intensive Care Med. 2022, 48, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Uricchio, M.N.; Ramanan, R.; Esper, S.A.; Murray, H.; Kaczorowski, D.J.; D’Aloiso, B.; Gomez, H.; Sciortino, C.; Sanchez, P.G.; Sappington, P.L.; et al. Bivalirudin Versus Unfractionated Heparin in Patients With Cardiogenic Shock Requiring Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2023, 69, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Geli, J.; Capoccia, M.; Maybauer, D.M.; Maybauer, M.O. Argatroban Anticoagulation for Adult Extracorporeal Membrane Oxygenation: A Systematic Review. J. Intensive Care Med. 2022, 37, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Menninger, L.; Körner, A.; Mirakaj, V.; Heck-Swain, K.-L.; Haeberle, H.A.; Althaus, K.; Baumgaertner, M.; Jost, W.; Schlensak, C.; Rosenberger, P.; et al. Membrane Oxygenator Longevity Was Higher in Argatroban-Treated Patients Undergoing vvECMO. Eur. J. Clin. Invest. 2023, 53, e13963. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Lincoff, A.M.; Gibson, C.M.; Stone, G.W.; McNulty, S.; Montalescot, G.; Kleiman, N.S.; Goodman, S.G.; White, H.D.; Mahaffey, K.W.; et al. Intravenous Platelet Blockade with Cangrelor during PCI. N. Engl. J. Med. 2009, 361, 2330–2341. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.A.; Stone, G.W.; McNulty, S.; White, H.D.; Lincoff, A.M.; Gibson, C.M.; Pollack, C.V.; Montalescot, G.; Mahaffey, K.W.; Kleiman, N.S.; et al. Platelet Inhibition with Cangrelor in Patients Undergoing PCI. N. Engl. J. Med. 2009, 361, 2318–2329. [Google Scholar] [CrossRef]
- Yusuf, S.; Mehta, S.R.; Chrolavicius, S.; Afzal, R.; Pogue, J.; Granger, C.B.; Budaj, A.; Peters, R.J.G.; Bassand, J.-P.; Wallentin, L.; et al. Effects of Fondaparinux on Mortality and Reinfarction in Patients with Acute ST-Segment Elevation Myocardial Infarction: The OASIS-6 Randomized Trial. JAMA 2006, 295, 1519–1530. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E. Sensitivity of Viscoelastic Tests to Platelet Function. J. Clin. Med. 2020, 9, 189. [Google Scholar] [CrossRef]
- Erdoes, G.; Koster, A.; Levy, J.H. Viscoelastic Coagulation Testing: Use and Current Limitations in Perioperative Decision-Making. Anesthesiology 2021, 135, 342–349. [Google Scholar] [CrossRef]
- Bartoli, C.R.; Kang, J.; Restle, D.J.; Zhang, D.M.; Shabahang, C.; Acker, M.A.; Atluri, P. Inhibition of ADAMTS-13 by Doxycycline Reduces von Willebrand Factor Degradation During Supraphysiological Shear Stress: Therapeutic Implications for Left Ventricular Assist Device-Associated Bleeding. JACC Heart Fail. 2015, 3, 860–869. [Google Scholar] [CrossRef]
- Pollack, C.V.; Kurz, M.A.; Hayward, N.J. EP-7041, a Factor XIa Inhibitor as a Potential Antithrombotic Strategy in Extracorporeal Membrane Oxygenation: A Brief Report. Crit. Care Explor. 2020, 2, e0196. [Google Scholar] [CrossRef] [PubMed]
- Gernhofer, Y.K.; Banks, D.A.; Golts, E.; Pretorius, V. Novel Use of Cangrelor With Heparin During Cardiopulmonary Bypass in Patients With Heparin-Induced Thrombocytopenia Who Require Cardiovascular Surgery: A Case Series. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ciolek, A.M.; Ma, K.; Garan, A.R.; Eisenberger, A.B.; Jennings, D.L. Use of Cangrelor during Venoarterial Extracorporeal Membrane Oxygenation Following Percutaneous Coronary Intervention. Artif. Organs 2020, 44, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Colvin, B.M.; Rivosecchi, R. Parenteral Antiplatelet Agents in Three Patients Receiving VA-ECMO Support to Maintain Drug-Eluting Stent Patency. J. Heart Lung Transplant. 2021, 40, S533. [Google Scholar] [CrossRef]
- Gurnani, P.K.; Bohlmann, A.; March, R.J. Prolonged Use of Eptifibatide as a Bridge to Maintain Drug-Eluting Stent Patency in a Patient Receiving Extracorporeal Membrane Oxygenation. Perfusion 2018, 33, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Baldetti, L.; Nardelli, P.; Ajello, S.; Melisurgo, G.; Calabrò, M.G.; Pieri, M.; Scandroglio, A.M. Anti-Thrombotic Therapy With Cangrelor and Bivalirudin in Venoarterial Extracorporeal Membrane Oxygenation Patients Undergoing Percutaneous Coronary Intervention: A Single-Center Experience. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2023, 69, e346–e350. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Kamran, H.; Lech, T.; Montgomery, J.; Venesy, D.; Patten, R.; Shah, S. (128)—High Thromboembolic Event Rate in Patients Supported With an Impella CP Device With an Anti-Xa Level of Less Than 0.1 u/mL. J. Heart Lung Transplant. 2018, 37, S59. [Google Scholar] [CrossRef]
- Vandenbriele, C.; Arachchillage, D.J.; Frederiks, P.; Giustino, G.; Gorog, D.A.; Gramegna, M.; Janssens, S.; Meyns, B.; Polzin, A.; Scandroglio, M.; et al. Anticoagulation for Percutaneous Ventricular Assist Device-Supported Cardiogenic Shock: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 1949–1962. [Google Scholar] [CrossRef]
- Vidal, S.; Richebé, P.; Barandon, L.; Calderon, J.; Tafer, N.; Pouquet, O.; Fournet, N.; Janvier, G. Evaluation of Continuous Veno-Venous Hemofiltration for the Treatment of Cardiogenic Shock in Conjunction with Acute Renal Failure after Cardiac Surgery. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2009, 36, 572–579. [Google Scholar] [CrossRef]
- Gorog, D.A.; Price, S.; Sibbing, D.; Baumbach, A.; Capodanno, D.; Gigante, B.; Halvorsen, S.; Huber, K.; Lettino, M.; Leonardi, S.; et al. Antithrombotic Therapy in Patients with Acute Coronary Syndrome Complicated by Cardiogenic Shock or Out-of-Hospital Cardiac Arrest: A Joint Position Paper from the European Society of Cardiology (ESC) Working Group on Thrombosis, in Association with the Acute Cardiovascular Care Association (ACCA) and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J.-Cardiovasc. Pharmacother. 2021, 7, 125–140. [Google Scholar] [CrossRef]
- Schilder, L.; Nurmohamed, S.A.; Bosch, F.H.; Purmer, I.M.; den Boer, S.S.; Kleppe, C.G.; Vervloet, M.G.; Beishuizen, A.; Girbes, A.R.J.; Ter Wee, P.M.; et al. Citrate Anticoagulation versus Systemic Heparinisation in Continuous Venovenous Hemofiltration in Critically Ill Patients with Acute Kidney Injury: A Multi-Center Randomized Clinical Trial. Crit. Care 2014, 18, 472. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mao, Z.; Kang, H.; Hu, J.; Zhou, F. Regional Citrate versus Heparin Anticoagulation for Continuous Renal Replacement Therapy in Critically Ill Patients: A Meta-Analysis with Trial Sequential Analysis of Randomized Controlled Trials. Crit. Care 2016, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Girndt, M.; Selejan, S.; Rbah, R.; Böhm, M. Tirofiban Preserves Platelet Loss during Continuous Renal Replacement Therapy in a Randomised Prospective Open-Blinded Pilot Study. Crit. Care 2008, 12, R111. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Ohman, E.M.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of Cardiogenic Shock Complicating Myocardial Infarction: An Update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Girerd, N.; Amour, J.; Besnier, E.; Nesseler, N.; Helms, J.; Delmas, C.; Sonneville, R.; Guidon, C.; Rozec, B.; et al. Effect of Moderate Hypothermia vs Normothermia on 30-Day Mortality in Patients with Cardiogenic Shock Receiving Venoarterial Extracorporeal Membrane Oxygenation: A Randomized Clinical Trial. JAMA 2022, 327, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Bian, W.; Li, Y.; Feng, X.; Song, M.; Zhou, P. Hypothermia May Reduce Mortality and Improve Neurologic Outcomes in Adult Patients Treated with VA-ECMO: A Systematic Review and Meta-Analysis. Am. J. Emerg. Med. 2023, 70, 163–170. [Google Scholar] [CrossRef]
- Sandroni, C.; Nolan, J.P.; Andersen, L.W.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Lilja, G.; Morley, P.T.; et al. ERC-ESICM Guidelines on Temperature Control after Cardiac Arrest in Adults. Intensive Care Med. 2022, 48, 261–269. [Google Scholar] [CrossRef]
- Fuernau, G.; Beck, J.; Desch, S.; Eitel, I.; Jung, C.; Erbs, S.; Mangner, N.; Lurz, P.; Fengler, K.; Jobs, A.; et al. Mild Hypothermia in Cardiogenic Shock Complicating Myocardial Infarction. Circulation 2019, 139, 448–457. [Google Scholar] [CrossRef]
- Levi, M. Hemostasis and Thrombosis in Extreme Temperatures (Hypo- and Hyperthermia). Semin. Thromb. Hemost. 2018, 44, 651–655. [Google Scholar] [CrossRef]
- Van der Pals, J.; Götberg, M.I.; Götberg, M.; Hultén, L.M.; Magnusson, M.; Jern, S.; Erlinge, D. Hypothermia in Cardiogenic Shock Reduces Systemic T-PA Release. J. Thromb. Thrombolysis 2011, 32, 72–81. [Google Scholar] [CrossRef]
- Van Poucke, S.; Stevens, K.; Marcus, A.E.; Lancé, M. Hypothermia: Effects on Platelet Function and Hemostasis. Thromb. J. 2014, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.G.; White, A.E.; Fox, S.C.; Wilcox, R.G.; Heptinstall, S. Enhanced Platelet Aggregation and Activation under Conditions of Hypothermia. Thromb. Haemost. 2007, 98, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Varenne, O.; Bougouin, W.; Rosencher, J.; Mira, J.-P.; Cariou, A. Stent Thrombosis: An Increased Adverse Event after Angioplasty Following Resuscitated Cardiac Arrest. Resuscitation 2014, 85, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Penela, D.; Magaldi, M.; Fontanals, J.; Martin, V.; Regueiro, A.; Ortiz, J.T.; Bosch, X.; Sabaté, M.; Heras, M. Hypothermia in Acute Coronary Syndrome: Brain Salvage versus Stent Thrombosis? J. Am. Coll. Cardiol. 2013, 61, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Chaudhary, R.; Mehta, K.; Agarwal, V.; Garg, J.; Freudenberger, R.; Jacobs, L.; Cox, D.; Kern, K.B.; Patel, N. Therapeutic Hypothermia and Stent Thrombosis: A Nationwide Analysis. JACC Cardiovasc. Interv. 2016, 9, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Bednar, F.; Kroupa, J.; Ondrakova, M.; Osmancik, P.; Kopa, M.; Motovska, Z. Antiplatelet Efficacy of P2Y12 Inhibitors (Prasugrel, Ticagrelor, Clopidogrel) in Patients Treated with Mild Therapeutic Hypothermia after Cardiac Arrest Due to Acute Myocardial Infarction. J. Thromb. Thrombolysis 2016, 41, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Flierl, U.; Röntgen, P.; Zauner, F.; Tongers, J.; Berliner, D.; Bauersachs, J.; Schäfer, A. Platelet Inhibition with Prasugrel in Patients with Acute Myocardial Infarction Undergoing Therapeutic Hypothermia after Cardiopulmonary Resuscitation. Thromb. Haemost. 2016, 115, 960–968. [Google Scholar] [CrossRef]
- Frelinger, A.L.; Furman, M.I.; Barnard, M.R.; Krueger, L.A.; Dae, M.W.; Michelson, A.D. Combined Effects of Mild Hypothermia and Glycoprotein IIb/IIIa Antagonists on Platelet–Platelet and Leukocyte–Platelet Aggregation. Am. J. Cardiol. 2003, 92, 1099–1101. [Google Scholar] [CrossRef]
- Feistritzer, H.-J.; Desch, S.; Zeymer, U.; Fuernau, G.; de Waha-Thiele, S.; Dudek, D.; Huber, K.; Stepinska, J.; Schneider, S.; Ouarrak, T.; et al. Prognostic Impact of Atrial Fibrillation in Acute Myocardial Infarction and Cardiogenic Shock. Circ. Cardiovasc. Interv. 2019, 12, e007661. [Google Scholar] [CrossRef]
- Kalarus, Z.; Svendsen, J.H.; Capodanno, D.; Dan, G.-A.; De Maria, E.; Gorenek, B.; Jędrzejczyk-Patej, E.; Mazurek, M.; Podolecki, T.; Sticherling, C.; et al. Cardiac Arrhythmias in the Emergency Settings of Acute Coronary Syndrome and Revascularization: An European Heart Rhythm Association (EHRA) Consensus Document, Endorsed by the European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Acute Cardiovascular Care Association (ACCA). EP Europace 2019, 21, 1603–1604. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Collet, J.-P.; Haude, M.; Byrne, R.; Chung, E.H.; Fauchier, L.; Halvorsen, S.; Lau, D.; Lopez-Cabanillas, N.; Lettino, M.; et al. 2018 Joint European Consensus Document on the Management of Antithrombotic Therapy in Atrial Fibrillation Patients Presenting with Acute Coronary Syndrome and/or Undergoing Percutaneous Cardiovascular Interventions: A Joint Consensus Document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) Endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). EP Europace 2019, 21, 192–193. [Google Scholar] [CrossRef]
- Boriani, G.; Fauchier, L.; Aguinaga, L.; Beattie, J.M.; Blomstrom Lundqvist, C.; Cohen, A.; Dan, G.-A.; Genovesi, S.; Israel, C.; Joung, B.; et al. European Heart Rhythm Association (EHRA) Consensus Document on Management of Arrhythmias and Cardiac Electronic Devices in the Critically Ill and Post-Surgery Patient, Endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin American Heart Rhythm Society (LAHRS). EP Europace 2019, 21, 7–8. [Google Scholar] [CrossRef]
- Geisler, T.; Poli, S.; Huber, K.; Rath, D.; Aidery, P.; Kristensen, S.D.; Storey, R.F.; Ball, A.; Collet, J.-P.; Berg, J.T. Resumption of Antiplatelet Therapy after Major Bleeding. Thromb. Haemost. 2023, 123, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.; Gass, J.; Weeks, P. Antiplatelet Therapy Bridging with Cangrelor in Patients With Coronary Stents: A Case Series. Ann. Pharmacother. 2019, 53, 171–177. [Google Scholar] [CrossRef]
- Pagano, D.; Milojevic, M.; Meesters, M.I.; Benedetto, U.; Bolliger, D.; von Heymann, C.; Jeppsson, A.; Koster, A.; Osnabrugge, R.L.; Ranucci, M.; et al. 2017 EACTS/EACTA Guidelines on Patient Blood Management for Adult Cardiac Surgery. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2018, 53, 79–111. [Google Scholar] [CrossRef]

| Drug | Route of Administration | Mode of Action | Plasma Half-life/Duration of Action since Last Dose | Dose | Pharmacokinetics/ Pharmacodynamics in CS | Particular Aspects in CS | References |
|---|---|---|---|---|---|---|---|
| Aspirin | Oral/IV | Irreversible COX-1 Inhibition | 20 min/7–10 days | loading dose of 150–300 mg perorally or 75–250 intravenously; 75–100 mg daily maintenance dose | Platelet inhibition by oral aspirin may be reduced during TTM | IV preferred route | [5,6,7] |
| P2Y12 receptor inhibitors | |||||||
| Clopidogrel | Oral/crushed | Irreversible P2Y12-Inhibition | 30–60 min/3–10 days | 600 mg loading dose; 75 mg daily maintenance dose | Delayed GI absorption Decreased hepatic metabolism | Due to two-step hepatic metabolism, conversion to active metabolite may be substantially impaired in CS patients with liver failure No sufficient antiplatelet effect in hypothermia Potential Interaction with CYP3A4, CYP3A5 or CYP2C19 inhibitors | [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] |
| Prasugrel | Oral/crushed | Irreversible P2Y12-Inhibition | 30–60 min/7–10 days | 60 mg loading dose; 10 mg daily maintenance dose (5 mg daily in patients >75 years of age) | Delayed GI absorption Decreased hepatic metabolism | Potential interactions with strong CYP3A4/A5 and CYP2B6 inhibitors Delayed onset with opioids Long offset of 7–10 days | |
| Ticagrelor | Oral/crushed | Reversible P2Y12-Inhibition | 6–12 h/3–5 days | 180 mg loading dose; 90 mg twice daily maintenance dose | Delayed GI absorption Decreased hepatic metabolism | Interactions with strong CYP3A4 inducers or inhibitors Delayed onset with opioids Contraindicated in liver failure | |
| Cangrelor | IV | Reversible P2Y12-Inhibition | 3–6 min/1–2 h | Bolus of 30 µg/kg IV followed by 4 µg/kg/min infusion for at least 2 h or the duration of the procedure (whichever is longer) Bridge Dose: 0.75 µg/kg/min | Not dependent on hepatic/renal metabolism in CS | No drug interactions via CYP450 metabolism. No interaction with opiates | [28,29,30,31,32,33,34,35,36,37,38,39,40,41] |
| GP IIb/IIIa inhibitors | |||||||
| Eptifibatide | IV | Blockade of the GP IIb/IIIa receptor | 2–3 h/4 h | IV bolus of 180 µg/kg followed by a continuous infusion of 2 µg/kg/min 180 µg/kg by a continuous infusion dose of 1.0 µg/kg/min in patient with 30 ≤ CrCl < 50 mL/min) | Competitive inhibition of GP IIb/IIIa receptor Rapid recovery of platelet function | Cautious use during TTM because of reduced net benefit Clearance reduced in renal impairment. Contraindicated in thrombocytopenia (<100.000 cells/mm3), severe renal impairment (<30 mL/min)/renal dialysis and severe hepatic failure, patients with prior ICH, ischemic stroke within 30 days and prior fibrinolysis | [42,43,44,45,46,47,48,49,50] |
| Tirofiban | IV | Blockade of the GP Iib/IIIa receptor | 1.5–2 h/4–8 h | Bolus of 25 µg/kg IV over 3 min, followed by an infusion of 0.15 µg/kg/min for up to 18 h. For CrCl ≤ 60 mL/min: LD, 25 µg/kg IV over 5 min followed by a maintenance infusion of 0.075 µg/kg/min continued for up to 18 h. Initial infusion rate of 0.4 μg/kg/min for 30 min followed by 0.1 µg/kg/min (CrCl < 30 mL/min use 0.05 µg/kg/min) | Cautious use during TTM because of reduced net benefit Contraindicated in thrombocytopenia (<100.000 cells/mm3), severe hepatic failure, patients with prior ICH, ischemic stroke within 30 days and prior fibrinolysis | ||
| Anticoagulants | |||||||
| UFH | IV | Thrombin inhibition and factor Xa inhibition by antithrombin complex formation/activation | 1.5 h/2–6 h | IV bolus 70–100 U/kg during PCI. IV infusion titrated to achieve an aPTT of 60–80 s (or less) depending on further indications for anticoagulation (e.g., according to ECMO/Impella protocol) | Variable response in CS | In TTM, UFH dose adjustment/reduction required under frequent aPTT/ACT monitoring Can be antagonized by protamine in case of bleeding | [5,51,52] |
| LMWH (enoxaparin) | SC/IV | Factor Xa inhibition by antithrombin complex formation/activation, little effect on thrombin | 4–8 h/12 h | Enoxaparin: During PCI: IV bolus of 0.3 mg/kg enoxaparin if the last s.c. administration was given > 8 h before balloon inflation | Less variability in drug response compared to UFH | Impaired subcutaneous absorption due to reduced tissue perfusion Monitoring of anti-Fxa activity may be necessary in critically ill patients/patients with acute renal failure | [5] |
| Direct intravenous thrombin inhibitors | |||||||
| Bivalirudin | IV | Reversible direct thrombin inhibitor | 25 min/1 h | 0.75 mg/kg IV bolus, followed by 1.75 mg/kg/h IV infusion for duration of procedure, extended duration for up 4 h after STEMI Renal impairment: No reduction of bolus dose Reduction of IV infusion dose CrCl < 30 mL/min: 1 mg/kg/h Hemodialysis: 0.25 mg/kg/h HIT (off-label): 0.15–0.2 mg/kg/h IV; adjust to aPTT 1.5–2.5 times baseline value ECMO (off-label): Individual aPTT/ACT guided protocols (e.g., [53]) | No need to titrate dose No need for routine ACT monitoring | Alternative anticoagulant in HIT No clinically relevant drug–drug interactions reported Safe and efficacious alternative to UFH in CS/ECMO | [53] |
| Argatroban | IV | Reversible direct thrombin inhibitor | 40–50 min/2–4 h | Initial dose 2 µg/kg/minute, dose adjustments according to aPTT and ACT | Rapid onset of action, fast reversibility of its anticoagulant effect, inhibition of clot-bound thrombin Hepatically cleared | Alternative anticoagulant in HIT No dosage adjustment in renal-impairment Contraindicated in patients with severe liver dysfunction Alternative to UHF in ECMO | [54,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Droppa, M.; Geisler, T. Optimal Antithrombotic Strategies in Cardiogenic Shock. J. Clin. Med. 2024, 13, 277. https://doi.org/10.3390/jcm13010277
Droppa M, Geisler T. Optimal Antithrombotic Strategies in Cardiogenic Shock. Journal of Clinical Medicine. 2024; 13(1):277. https://doi.org/10.3390/jcm13010277
Chicago/Turabian StyleDroppa, Michal, and Tobias Geisler. 2024. "Optimal Antithrombotic Strategies in Cardiogenic Shock" Journal of Clinical Medicine 13, no. 1: 277. https://doi.org/10.3390/jcm13010277
APA StyleDroppa, M., & Geisler, T. (2024). Optimal Antithrombotic Strategies in Cardiogenic Shock. Journal of Clinical Medicine, 13(1), 277. https://doi.org/10.3390/jcm13010277