Unlocking the Potential of Acetazolamide: A Literature Review of an Adjunctive Approach in Heart Failure Management
Abstract
:1. Introduction
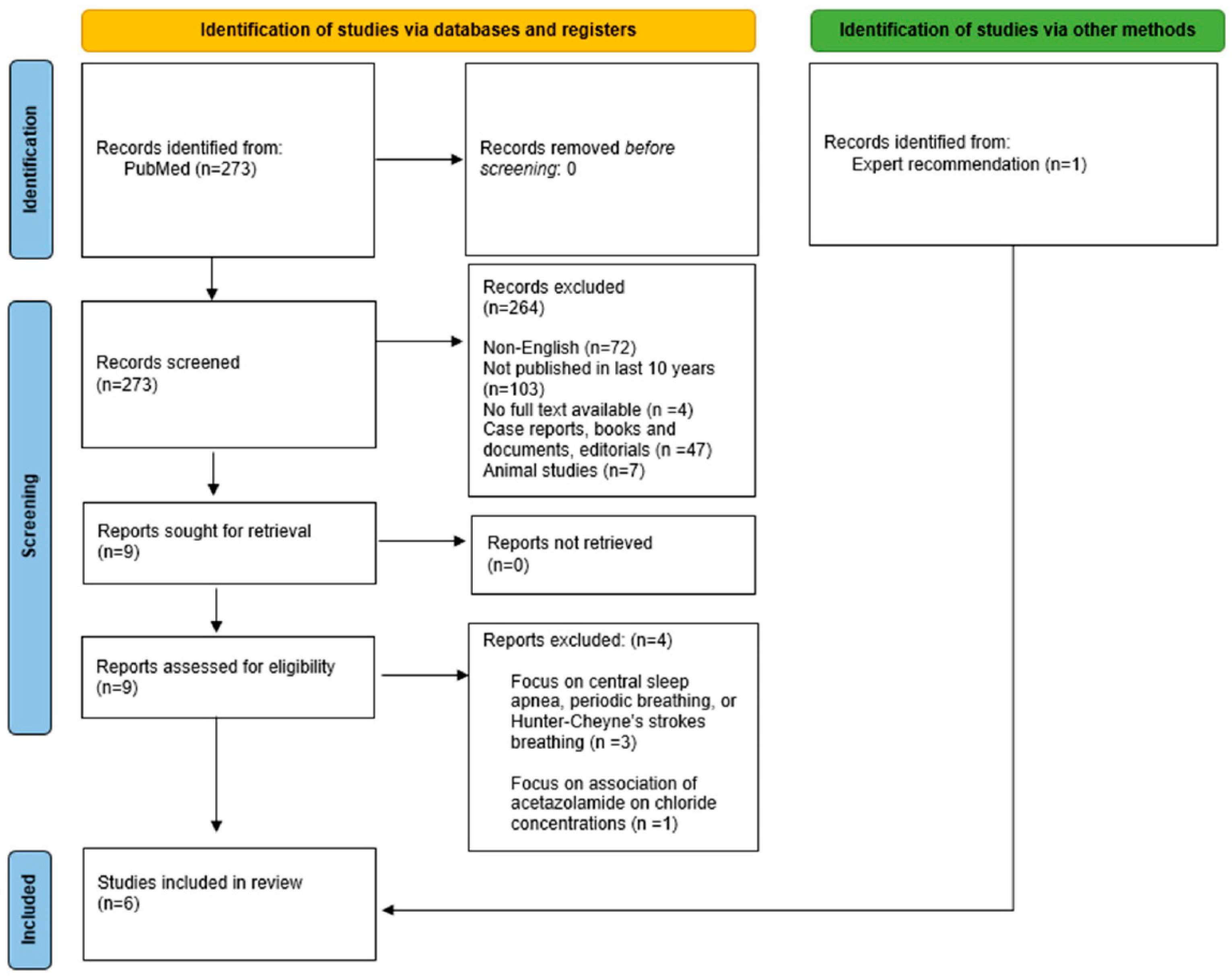
2. Methodology
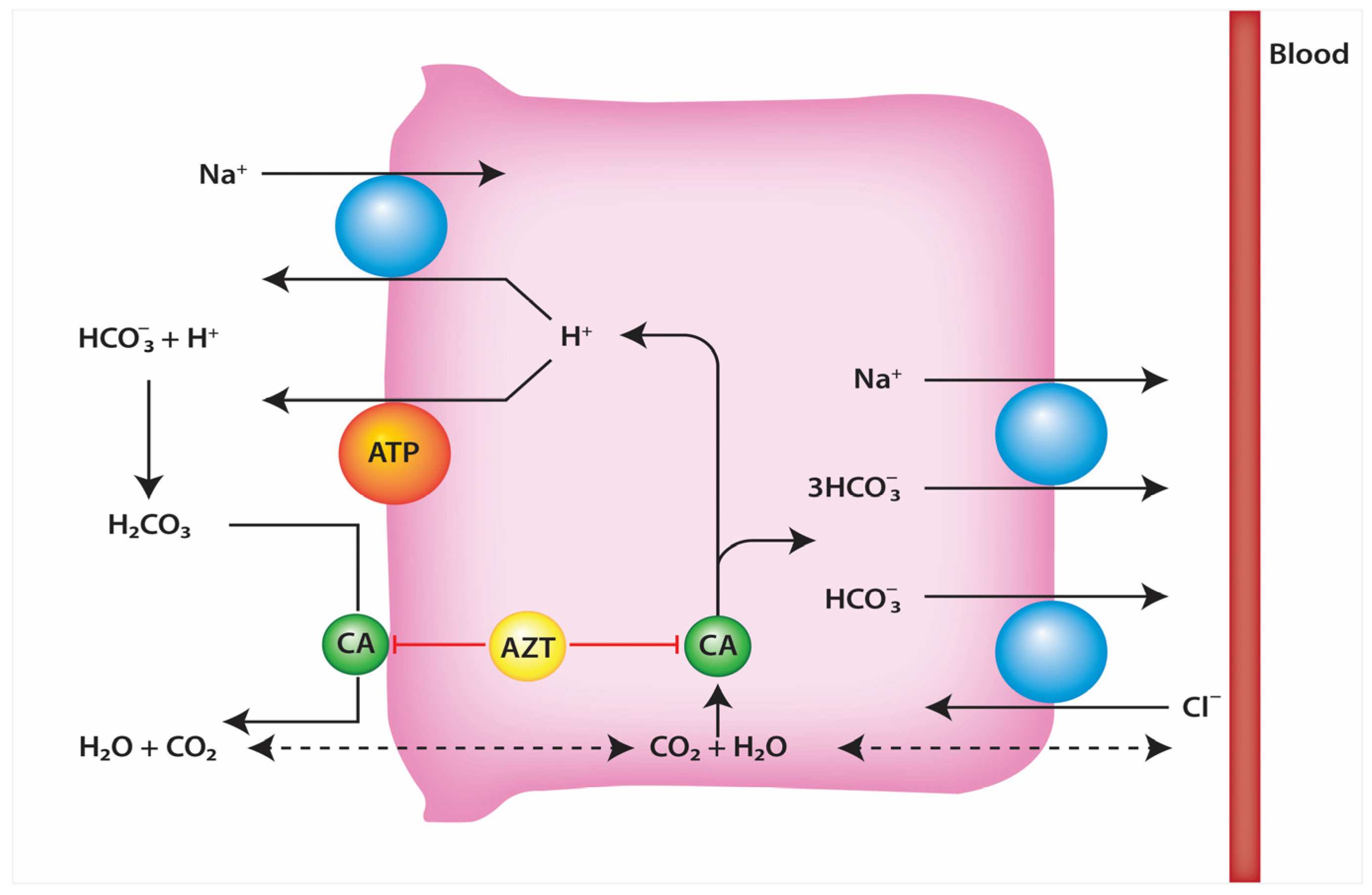
3. Characteristics of Acetazolamide
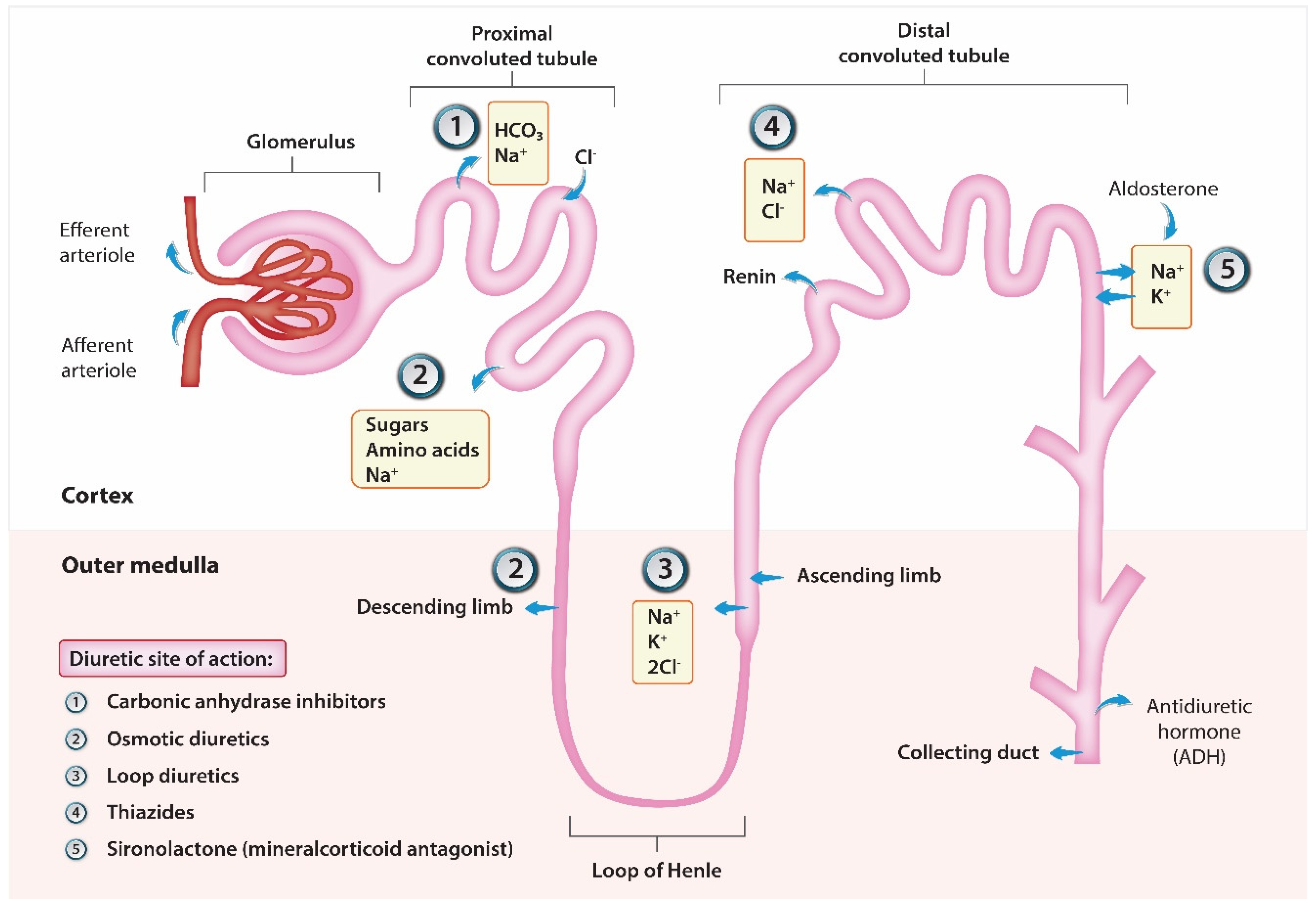
4. Diuretic Resistance
5. Study Analysis
5.1. Acetazolamide as Add-On Diuretic Therapy in Exacerbations of Chronic Heart Failure: A Pilot Study by Imiela et al., 2017 [16]
5.2. Use of Acetazolamide in the Treatment of Patients with Refractory Congestive Heart Failure by Núñez et al. (2018) [17]
5.3. DIURESIS-CHF TRIAL by Verbrugge et al., 2019 [18]
5.4. ADVOR-Trial by Mullens et al., 2022 [19]
5.5. CANDI by Martin et al. (2022) [22]
5.6. Diuretic, Natriuretic, and Chloride-Regaining Effects of Oral Acetazolamide as an Add-On Therapy for Acute Heart Failure with Volume Overload by Kosiorek et al., 2023 [23]
6. Discussion
6.1. Summary of Trial Analysis
6.2. Acetazolamide Standing Amongst Other Adjuncts
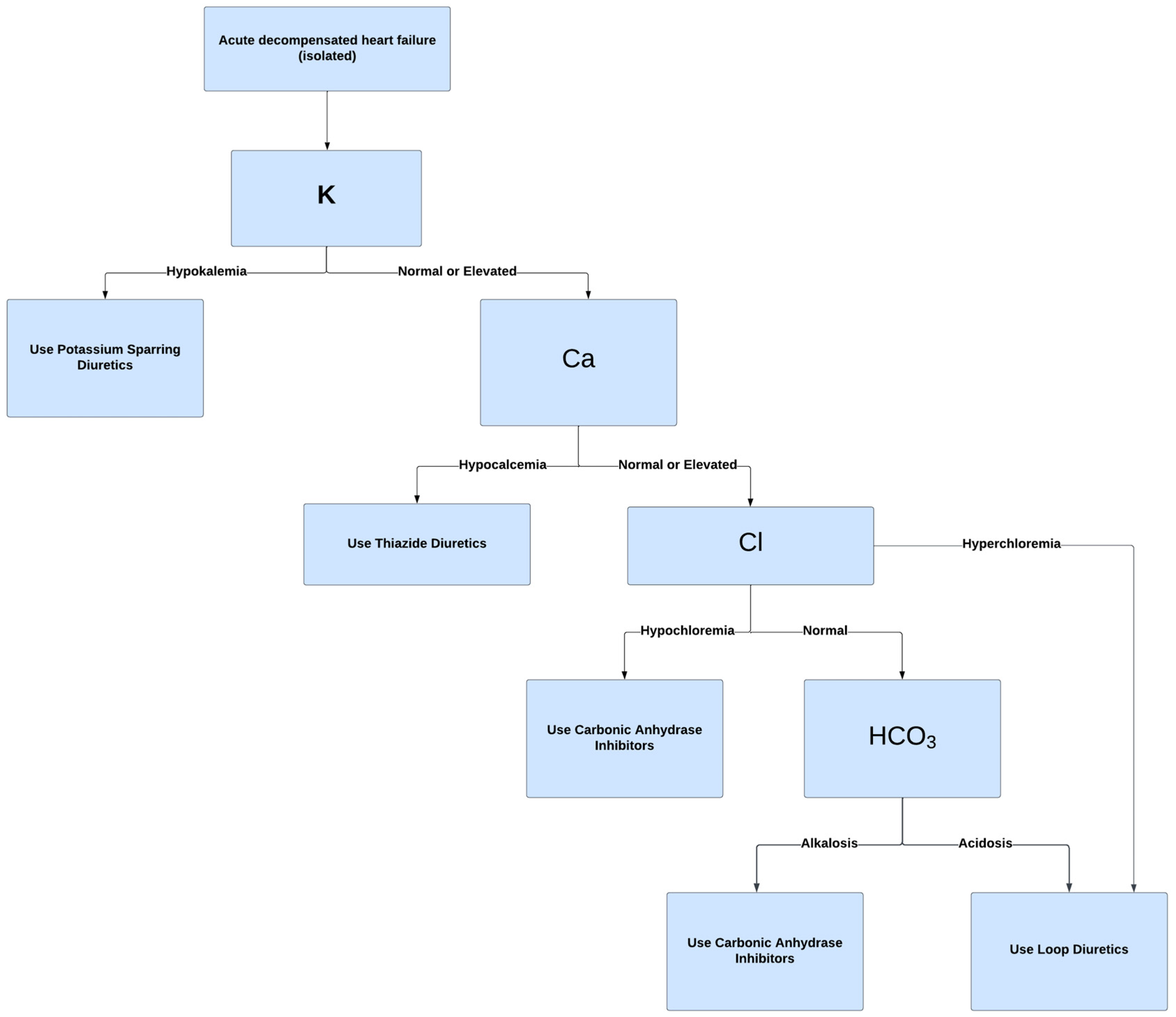
6.3. Recommendations for Acetazolamide as Adjunct Therapy
7. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Wile, D. Diuretics: A review. Ann. Clin. Biochem. 2012, 49 Pt 5, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Leaf, A.; Schwartz, W.B.; Relman, A.S. Oral administration of a potent carbonic anhydrase inhibitor (“Diamox”). I. Changes in electrolyte and acid-base balance. N. Engl. J. Med. 1954, 250, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Loon, N.R.; Wilcox, C.S. Mild metabolic alkalosis impairs the natriuretic response to bumetanide in normal human subjects. Clin. Sci. 1998, 94, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Hanberg, J.S.; Rao, V.; Ter Maaten, J.M.; Laur, O.; Brisco, M.A.; Perry Wilson, F.; Grodin, J.L.; Assefa, M.; Samuel Broughton, J.; Planavsky, N.J.; et al. Hypochloremia and Diuretic Resistance in Heart Failure: Mechanistic Insights. Circ. Heart Fail. 2016, 9, e003180. [Google Scholar] [CrossRef] [PubMed]
- Addison, J.D.; Peterson, E.J.; Meyenburg, L. Intravenous or Oral Acetazolamide for Treatment of Diuretic-Induced Alkalosis in Patients with Heart Failure. Ann. Pharmacother. 2023, 57, 1241–1247. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Testani, J.M.; Pitt, B. Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure. Hypertension 2020, 76, 1045–1054. [Google Scholar] [CrossRef]
- Almeshari, K.; Ahlstrom, N.; Capraro, F.; Wilcox, C.S. A volume-independent component to postdiuretic sodium retention in humans. J. Am. Soc. Nephrol. 1993, 3, 1878–1883. [Google Scholar] [CrossRef]
- Huang, X.; Dorhout Mees, E.; Vos, P.; Hamza, S.; Braam, B. Everything we always wanted to know about furosemide but were afraid to ask. Am. J. Physiol. Ren. Physiol. 2016, 310, F958–F971. [Google Scholar] [CrossRef]
- Amatruda, J.G.; Scherzer, R.; Rao, V.S.; Ivey-Miranda, J.B.; Shlipak, M.G.; Estrella, M.M.; Testani, J.M. Renin-Angiotensin-Aldosterone System activation and diuretic response in ambulatory patients with heart failure. Kidney Med. 2022, 4, 100465. [Google Scholar] [CrossRef]
- Trullàs, J.C.; Morales-Rull, J.L.; Casado, J.; Carrera-Izquierdo, M.; Sánchez-Marteles, M.; Conde-Martel, A.; Dávila-Ramos, M.F.; Llácer, P.; Salamanca-Bautista, P.; Pérez-Silvestre, J.; et al. Combining loop with thiazide diuretics for decompensated heart failure: The CLOROTIC trial. Eur. Heart J. 2023, 44, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Brisco-Bacik, M.A.; ter Maaten, J.M.; Houser, S.R.; Vedage, N.A.; Rao, V.; Ahmad, T.; Wilson, F.P.; Testani, J.M. Outcomes Associated with a Strategy of Adjuvant Metolazone or High-Dose Loop Diuretics in Acute Decompensated Heart Failure: A Propensity Analysis. J. Am. Heart Assoc. 2018, 7, e009149. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Anstrom, K.J.; Felker, G.M.; Givertz, M.M.; Kalogeropoulos, A.P.; Konstam, M.A.; Mann, D.L.; Margulies, K.B.; McNulty, S.E.; Mentz, R.J.; et al. Efficacy and safety of spironolactone in acute heart failure: The ATHENA-HF randomized clinical trial. JAMA Cardiol. 2017, 2, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Gattis, W.A.; O’Connor, C.M.; Adams, K.F.; Elkayam, U., Jr.; Barbagelata, A.; Ghali, J.K.; Benza, R.L.; McGrew, F.A.; Klapholz, M.; et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004, 291, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Mentz, R.J.; Cole, R.T.; Adams, K.F.; Egnaczyk, G.F.; Fiuzat, M.; Patel, C.B.; Echols, M.; Khouri, M.G.; Tauras, J.M.; et al. Efficacy and Safety of Tolvaptan in Patients Hospitalized with Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Imiela, T.; Budaj, A. Acetazolamide as Add-on Diuretic Therapy in Exacerbations of Chronic Heart Failure: A Pilot Study. Clin. Drug Investig. 2017, 37, 1175–1181. [Google Scholar] [CrossRef]
- Núñez, J.; Heredia, R.; Payá, A.; Sanchis, I.; Del Prado, S.; Miñana, G.; Santas, E.; de la Espriella, R.; Núñez, E.; Sanchis, J.; et al. Use of acetazolamide in the treatment of patients with refractory congestive heart failure. Cardiovasc. Ther. 2018, 36, e12465. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Martens, P.; Ameloot, K.; Haemels, V.; Penders, J.; Dupont, M.; Tang, W.H.; Droogné, W.; Mullens, W. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur. J. Heart Fail. 2019, 21, 1415–1422. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in acute decompensated heart failure with volume overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef]
- Meekers, E.; Dauw, J.; Martens, P.; Dhont, S.; Verbrugge, F.H.; Nijst, P.; Ter Maaten, J.M.; Damman, K.; Mebazaa, A.; Filippatos, G.; et al. Renal function and decongestion with acetazolamide in acute decompensated heart failure: The ADVOR trial. Eur. Heart J. 2023, 44, 3672–3682. [Google Scholar] [CrossRef]
- Bueno, H.; Packer, M. Acetazolamide for acute heart failure: Is ADVOR a riddle wrapped in a mystery inside an enigma? Eur. Heart J. 2023, 44, 3683–3685. [Google Scholar] [CrossRef]
- Vela Martín, P.; Matutano Munoz, A.; Sánchez Ortiz, D.; Domínguez, F.; Royuela, A.; Cobo Marcos, M. CANDI: Chlorthalidone versus acetazolamide in patients with diuretic resistance and acute heart failure [CANDI: Clortalidona frente a acetazolamida en pacientes con resistencia diurética e insuficiencia cardiaca aguda]. Rev. Esp. Cardiol. 2023, 76, 473–485. [Google Scholar] [CrossRef]
- Kosiorek, A.; Urban, S.; Detyna, J.; Biegus, J.; Hurkacz, M.; Zymliński, R. Diuretic, natriuretic, and chloride-regaining effects of oral acetazolamide as an add-on therapy for acute heart failure with volume overload: A single-center, prospective, randomized study. Pol. Arch. Intern. Med. 2023, 133, 16526. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.A.; Nnodebe, I.; Fayaz, A.; Inayat, H.; Murtaza, S.F.; Umer, M.; Zaidi, S.A.; Amin, A. Effect of acetazolamide as add-on diuretic therapy in patients with heart failure: A meta-analysis. Cureus 2023, 15, e37792. [Google Scholar] [CrossRef] [PubMed]
- Llàcer, P.; Núñez, J.; García, M.; Ruiz, R.; López, G.; Fabregate, M.; Fernández, C.; Croset, F.; Del Hoyo, B.; Gomis, A.; et al. Comparison of chlorthalidone and spironolactone as additional diuretic therapy in patients with acute heart failure and preserved ejection fraction. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 350–355. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ponikowski, P. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Möbius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients with Acute Decompensated Heart Failure (EMPAG-HF). Circulation 2022, 146, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.J.; Elvan, A.; van Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Proposal for New Classification and Practical Use of Diuretics According to Their Effects on the Serum Chloride Concentration: Rationale Based on the “Chloride Theory”. Cardiol. Ther. 2020, 9, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Zandijk AJ, L.; van Norel, M.R.; Julius FE, C.; Sepehrvand, N.; Pannu, N.; McAlister, F.A.; Voors, A.A.; Ezekowitz, J.A. Chloride in Heart Failure: The Neglected Electrolyte. JACC Heart Fail. 2021, 9, 904–915. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabina, M.; Barakat, Z.; Feliciano, A.; Lamb, A.; Alsamman, M.M. Unlocking the Potential of Acetazolamide: A Literature Review of an Adjunctive Approach in Heart Failure Management. J. Clin. Med. 2024, 13, 288. https://doi.org/10.3390/jcm13010288
Sabina M, Barakat Z, Feliciano A, Lamb A, Alsamman MM. Unlocking the Potential of Acetazolamide: A Literature Review of an Adjunctive Approach in Heart Failure Management. Journal of Clinical Medicine. 2024; 13(1):288. https://doi.org/10.3390/jcm13010288
Chicago/Turabian StyleSabina, Michael, Zein Barakat, Adrian Feliciano, Andrew Lamb, and M Mrhaf Alsamman. 2024. "Unlocking the Potential of Acetazolamide: A Literature Review of an Adjunctive Approach in Heart Failure Management" Journal of Clinical Medicine 13, no. 1: 288. https://doi.org/10.3390/jcm13010288
APA StyleSabina, M., Barakat, Z., Feliciano, A., Lamb, A., & Alsamman, M. M. (2024). Unlocking the Potential of Acetazolamide: A Literature Review of an Adjunctive Approach in Heart Failure Management. Journal of Clinical Medicine, 13(1), 288. https://doi.org/10.3390/jcm13010288





