Cystine Renal Calculi: New Aspects Related to Their Formation and Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crystallization Studies
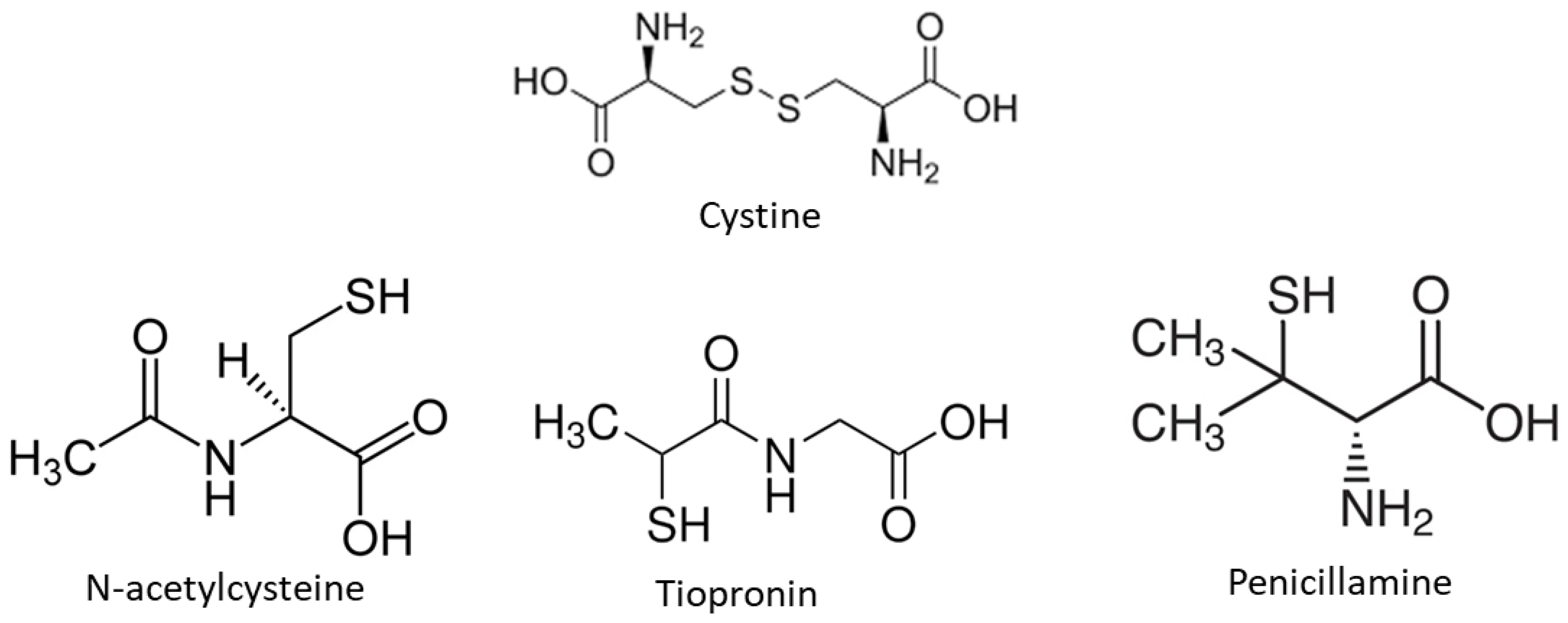
2.1.1. Reagents and Solutions
2.1.2. Turbidimetric Assay
2.1.3. Cystine Determination
2.1.4. Cystine Crystals’ Morphology
2.1.5. Statistics
2.2. Cystine Renal Stones
3. Results
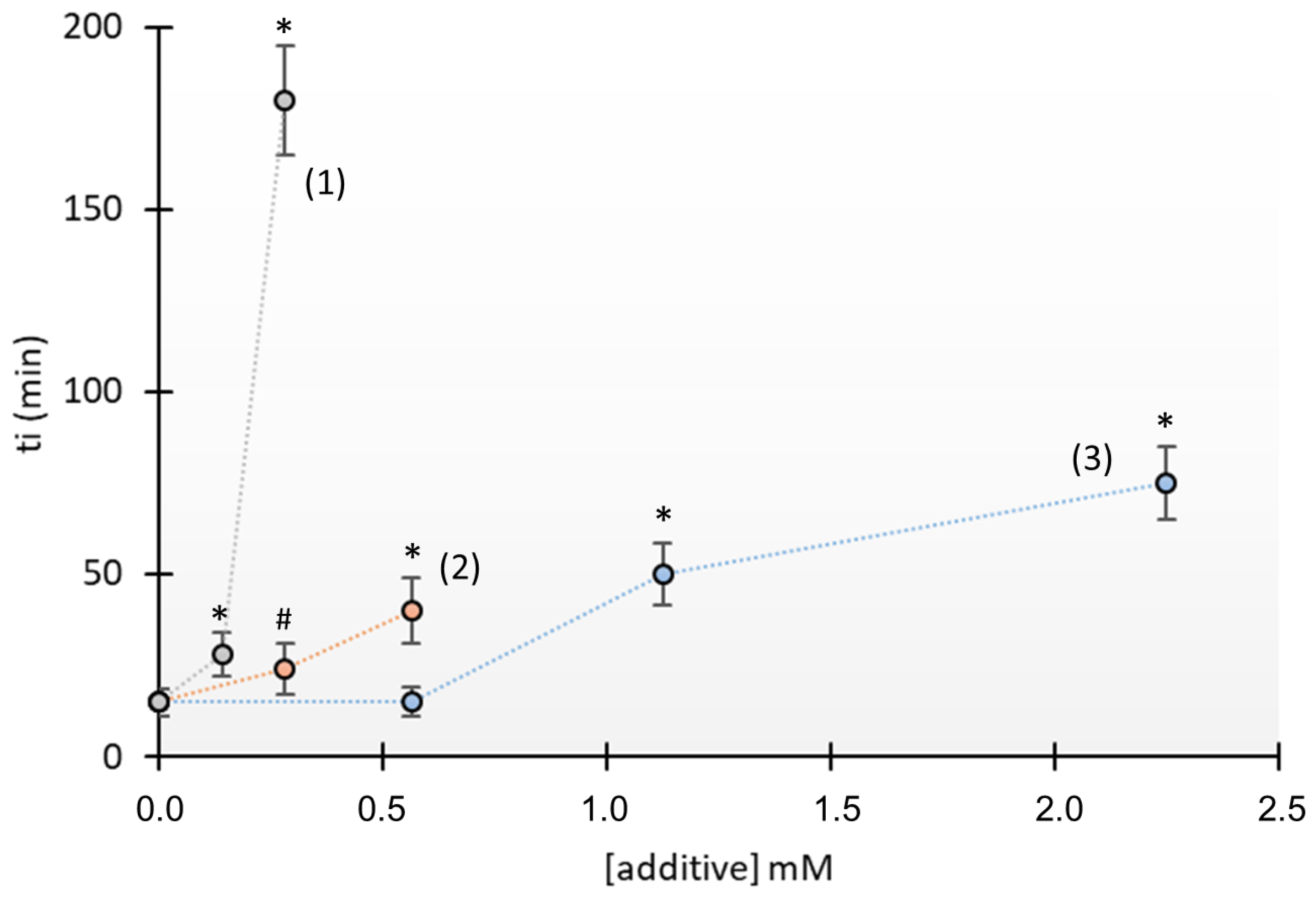
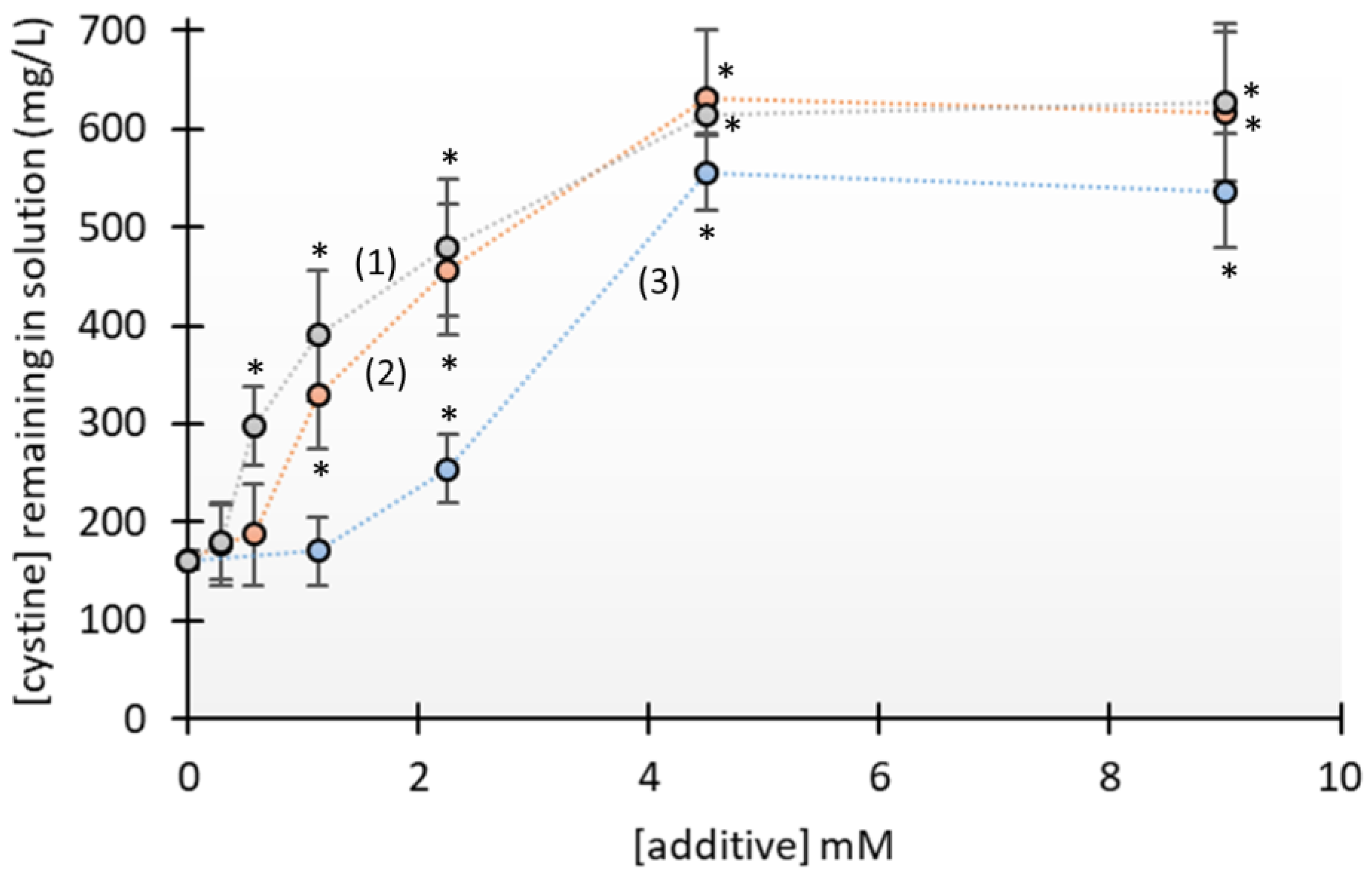
3.1. Crystallization Studies
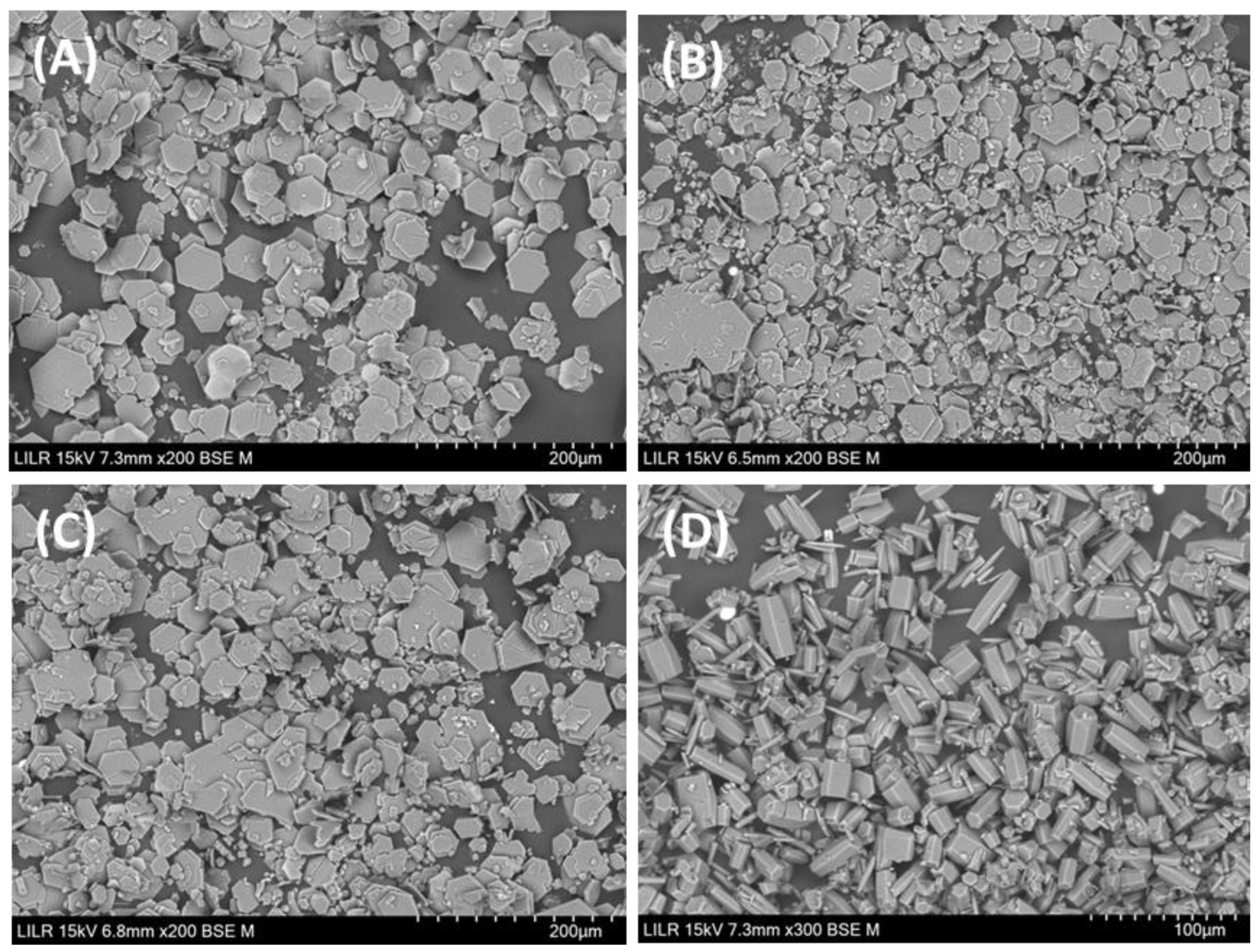
3.2. Morphology of Cystine Stones
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Türk, C.; Petřík, A.; Sarica, K.; Seitz, C.; Skolarikos, A.; Straub, M.; Knoll, T. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur. Urol. 2016, 69, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M. Component analysis of urinary calculi in the etiologic diagnosis of urolithiasis in the child. Arch Pediatr. 2000, 7, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Lieske, J.C.; Rule, A.D.; Krambeck, A.E.; Williams, J.C.; Bergstralh, E.J.; Mehta, R.A.; Moyer, T.P. Stone composition as a function of age and sex. Clin. J. Am. Soc. Nephrol. 2014, 9, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Millán, F.; Gracia, S.; Sánchez-Martín, F.; Angerri, O.; Rousaud, F.; Villavicencio, H. Un nuevo enfoque en el análisis de la litiasis urinaria en función de la combinación de sus componentes: Experiencia con 7.949 casos. Actas Urol. Esp. 2011, 35, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Moe, O.W. Kidney stones: Pathophysiology and medical management. Lancet 2006, 367, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Strologo, L.D.; Pras, E.; Pontesilli, C.; Beccia, E.; Ricci-Barbini, V.; de Sanctis, L.; Ponzone, A.; Gallucci, M.; Bisceglia, L.; Zelante, L.; et al. Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: A need for a new classification. J. Am. Soc. Nephrol. 2002, 13, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Font-Llitjos, M.; Jiménez-Vidal, M.; Bisceglia, L.; Di Perna, M.; de Sanctis, L.; Rousaud, F.; Zelante, L.; Palacín, M.; Nunes, V. New insights into cystinuria: 40 new mutations, genotype-phenotype correlation, and digenic inheritance causing partial phenotype. J. Med. Genet. 2005, 42, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Dahaoui, S.; Pichon-Pesme, V.; Howard, J.A.K.; Lecomte, C. CCD charge density study on crystals with large unit cell parameters: The case of hexagonal L-cystine. J. Phys. Chem. A 1999, 103, 6240–6250. [Google Scholar] [CrossRef]
- Poloni, L.N.; Zhu, Z.; Garcia-Vázquez, N.; Yu, A.C.; Connors, D.M.; Hu, L.; Sahota, A.; Ward, M.D.; Shtukenberg, A.G. Role of molecular recognition in L-cystine crystal growth inhibition. Cryst. Growth Des. 2017, 17, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Ejgenberg, M.; Mastai, Y. Biomimetic crystallization of L-cystine hierarchical structures. Cryst. Growth Des. 2012, 12, 4995–5001. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Y.; Aloysius, H.; Albanyan, H.; Yang, M.; Liang, J.-J.; Yu, A.; Shtukenberg, A.; Poloni, L.N.; Kholodovych, V.; et al. L-Cystine diamides as L-Cystine crystallization inhibitors for cystinuria. J. Med. Chem. 2016, 59, 7293–7298. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Albanyan, H.; Wang, Y.; Yang, Y.; Sahota, A.; Hu, L. Development of convenient crystallization inhibition assays for structure-activity relationship studies in the discovery of crystallization inhibitors. Med. Chem. Res. 2023, 32, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Girija, E.K.; Kalkura, S.N.; Ramasamy, P. Crystallization of cystine. J. Mater. Sci. Mater. Med. 1995, 6, 617–619. [Google Scholar] [CrossRef]
- Bhatta, K.M.; Prien, E.L., Jr.; Dretler, S.P. Cystine calculi—Rough and smooth: A new clinical distinction. J. Urol. 1989, 142, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Bazin, D.; Daudon, M.; André, G.; Weil, R.; Véron, E.; Matzen, G. Therapy modifies cystine kidney stones at the macroscopic scale. Do such alterations exist at the mesoscopic and nanometre scale? J. Appl. Crystallogr. 2014, 47, 719–725. [Google Scholar] [CrossRef]
- Königsberger, E.; Wang, Z.; Königsberger, L.-C. Solubility of L-cystine in nacl and artificial urine solutions. Mon. Chem. 2000, 131, 0039–0045. [Google Scholar] [CrossRef]
- Biyani, C.S.; Cartledge, J.J. Cystinuria—Diagnosis and Management. EAU-EBU Updat. Ser. 2006, 4, 175–183. [Google Scholar] [CrossRef]
- Fu, T.; Wei, T.; Liu, Y.; Jing, J.; Xu, Y.; Ou, C.; Chen, Y.; Li, J.; Li, B.; Zhu, H. Inhibition of growth ofl-cystine crystals by N-acetyl-l-cysteine. CrystEngComm 2016, 18, 8587–8590. [Google Scholar] [CrossRef]
- Mulvaney, W.P.; Quilter, T.; Mortera, A. Experiences with acetylcysteine in cystinuric patients. J. Urol. 1975, 114, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Benítez, J.; Tudela, P.; Laguna, G.; Casanueva, T.; Ruana, M.; Mata, F.; Gallardo, C. The dissolution of cystine lithiasis with n-acetylcysteine. Arch. Esp. Urol. 1995, 48, 944–948. [Google Scholar]
- Wu, J.T.; Wilson, L.W.; Christensen, S. Conversion of a qualitative screening test to a quantitative measurement of urinary cystine and homocystine. Ann. Clin. Lab. Sci. 1992, 22, 18–29. [Google Scholar] [PubMed]
- Goldfarb, D.; Coe, F.; Asplin, J. Urinary cystine excretion and capacity in patients with cystinuria. Kidney Int. 2006, 69, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-S.; Chiang, H.-Y.; Chen, H.-L.; Flores, M.; Navas-Acien, A.; Kuo, C.-C. Association of water intake and hydration status with risk of kidney stone formation based on NHANES 2009–2012 cycles. Public Health Nutr. 2022, 25, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Rodriguez, A.; Costa-Bauza, A. Efficacy of mixtures of magnesium, citrate and phytate as calcium oxalate crystallization inhibitors in urine. J. Urol. 2015, 194, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Sloand, J.A.; Izzo, J.L. Captopril reduces urinary cystine excretion in cystinuria. Arch. Intern. Med. 1987, 147, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Prot-Bertoye, C.; Lebbah, S.; Daudon, M.; Tostivint, I.; Jais, J.; Louët, A.L.; Pontoizeau, C.; Cochat, P.; Bataille, P.; Bridoux, F.; et al. Adverse events associated with currently used medical treatments for cystinuria and treatment goals: Results from a series of 442 patients in France. BJU Int. 2019, 124, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Yoshioka, T.; Miyake, O.; Okuyama, A.; Yachiku, S. Long-term study of tiopronin in patients with cystinuria. Hinyokika Kiyo 2003, 49, 115–120. [Google Scholar] [PubMed]
- Mårtensson, J.; Denneberg, T.; Lindell, A.; Textorius, O. Sulfur amino acid metabolism in cystinuria: A biochemical and clinical study of patients. Kidney Int. 1990, 37, 143–149. [Google Scholar] [CrossRef] [PubMed]


| Patient | Rough Stones | Smooth Stones |
|---|---|---|
| Patient 1 | 2 | 2 |
| Patient 2 | 2 | 1 |
| Patient 3 | 2 | 2 |
| Patient 4 | 2 | 0 |
| Patient 5 | 2 | 7 |
| Patient 6 | 1 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grases, F.; Tomàs Nadal, F.; Julià Florit, F.; Costa-Bauza, A. Cystine Renal Calculi: New Aspects Related to Their Formation and Development. J. Clin. Med. 2024, 13, 2837. https://doi.org/10.3390/jcm13102837
Grases F, Tomàs Nadal F, Julià Florit F, Costa-Bauza A. Cystine Renal Calculi: New Aspects Related to Their Formation and Development. Journal of Clinical Medicine. 2024; 13(10):2837. https://doi.org/10.3390/jcm13102837
Chicago/Turabian StyleGrases, Felix, Francisca Tomàs Nadal, Francesca Julià Florit, and Antonia Costa-Bauza. 2024. "Cystine Renal Calculi: New Aspects Related to Their Formation and Development" Journal of Clinical Medicine 13, no. 10: 2837. https://doi.org/10.3390/jcm13102837
APA StyleGrases, F., Tomàs Nadal, F., Julià Florit, F., & Costa-Bauza, A. (2024). Cystine Renal Calculi: New Aspects Related to Their Formation and Development. Journal of Clinical Medicine, 13(10), 2837. https://doi.org/10.3390/jcm13102837





