Good Manufacturing Practice-Compliant Cryopreserved and Thawed Native Adipose Tissue Ready for Fat Grafting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adipose Tissue Collection
2.2. Adipose Tissue Transport
2.3. Adipose Tissue Processing
2.4. Freezing Conditions Test and Cryopreservation of Native Adipose Tissue
2.5. Thawing of Cryopreserved Adipose Tissue
2.6. SVF Characterization
2.7. SVF Characterization through Multicolor Flow Cytometry
2.8. Colony-Forming Unit (CFU-F) Assay
2.9. Statistical Analyses
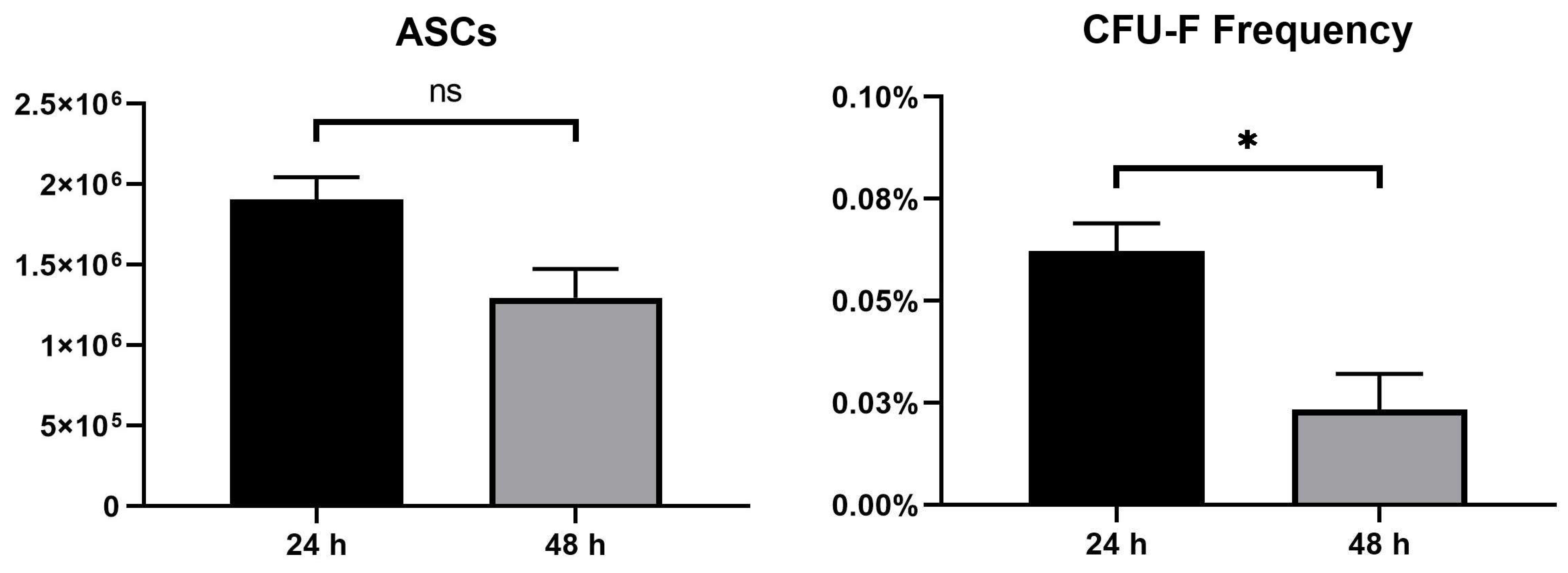
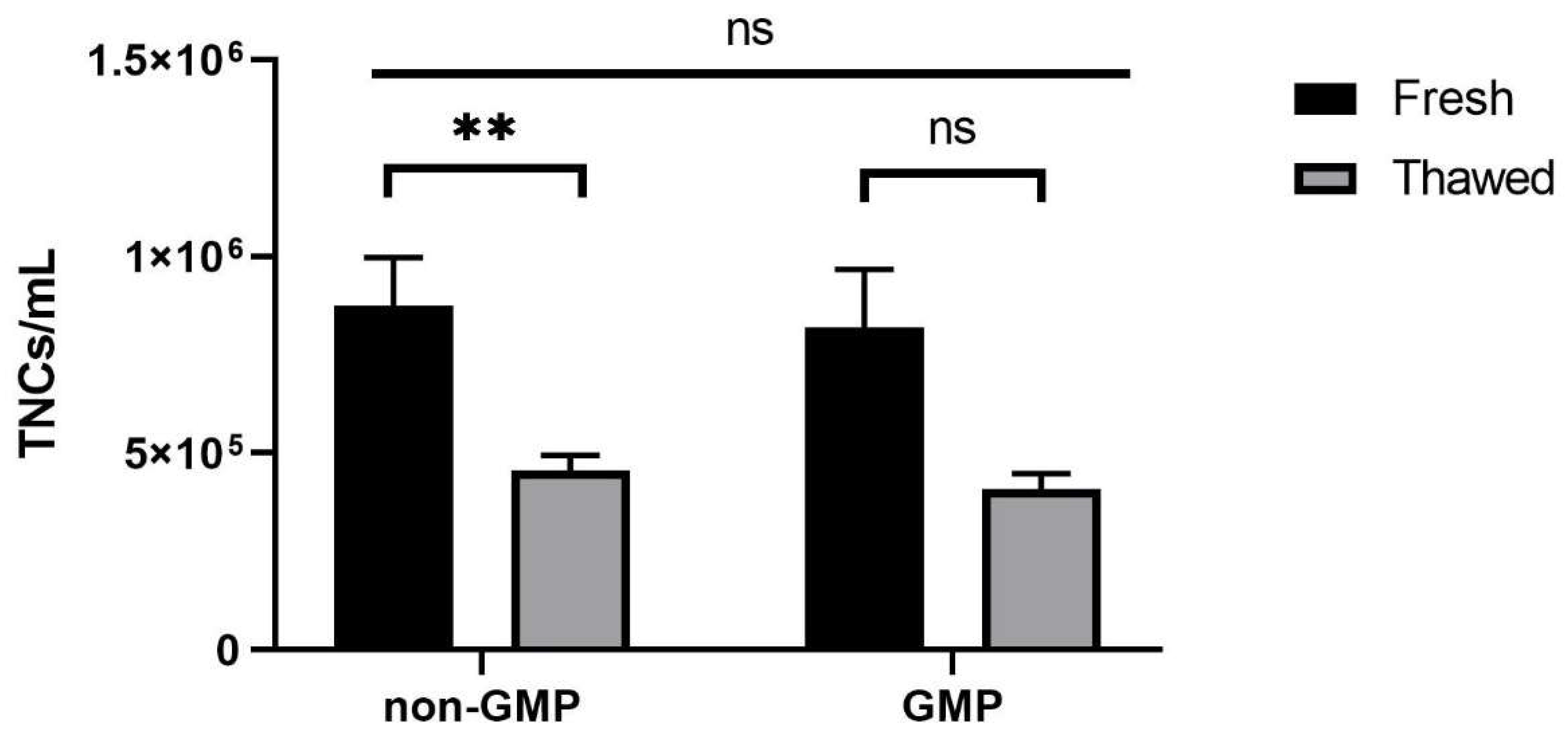
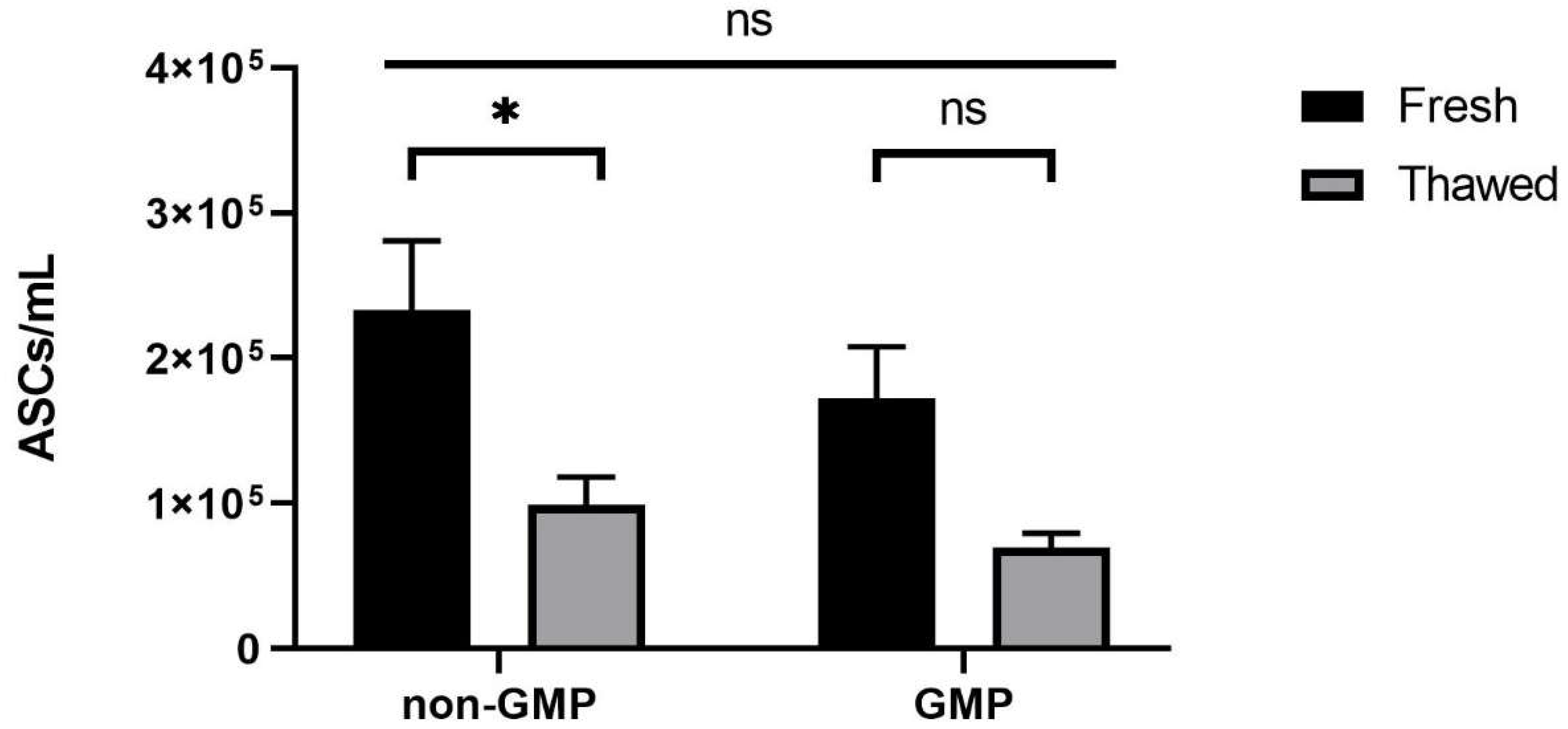
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trujillo, M.E.; Scherer, P.E. Adipose Tissue-Derived Factors: Impact on Health and Disease. Endocr. Rev. 2006, 27, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; I Piatetzky-Shapiro, I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. Development 1966, 16, 381–390. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Badowski, M.S.; Muise, A.; Harris, D.T. Long-Term Biobanking of Intact Tissue from Lipoaspirate. J. Clin. Med. 2019, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Bojic, S.; Murray, A.; Bentley, B.L.; Spindler, R.; Pawlik, P.; Cordeiro, J.L.; Bauer, R.; de Magalhães, J.P. Winter is coming: The future of cryopreservation. BMC Biol. 2021, 19, 56. [Google Scholar] [CrossRef]
- Bakhach, J.; Casoli, V.; Guimberteau, J.-C. The cryopreservation of composite tissues: Principle, literature review and preliminary results of our own experiments. Ann. Chir. Plast. Esthet. 2007, 52, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Rubinsky, B. The Energy Equation for Freezing of Biological Tissue. J. Heat Transf. 1989, 111, 988–997. [Google Scholar] [CrossRef]
- Diller, K.R.; Raymond, J.F. Water transport through a multicellular tissue during freezing: A network thermodynamic modeling analysis. Cryo Lett. 1990, 11, 151–162. [Google Scholar]
- Timasheff, S.N. Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc. Natl. Acad. Sci. USA 2002, 99, 9721–9726. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Carpenter, J.F.; Kita, Y.A.; Crowe, J.H. The basis for toxicity of certain cryoprotectants: A hypothesis. Cryobiology 1990, 27, 401–415. [Google Scholar] [CrossRef]
- Anchordoguy, T.J.; Cecchini, C.A.; Crowe, J.H.; Crowe, L.M. Insights into the cryoprotective mechanism of dimethyl sulfoxide for phospholipid bilayers. Cryobiology 1991, 28, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Shoshani, O.; Ullmann, Y.; Shupak, A.; Ramon, Y.; Gilhar, A.; Kehat, I.; Peled, I.J. The Role of Frozen Storage in Preserving Adipose Tissue Obtained by Suction-Assisted Lipectomy for Repeated Fat Injection Procedures. Dermatol. Surg. 2001, 27, 645–647. [Google Scholar] [CrossRef] [PubMed]
- MacRae, J.W.; Tholpady, S.S.; Ogle, R.C.; Morgan, R.F. Ex Vivo Fat Graft Preservation: Effects and Implications of Cryopreservation. Ann. Plast. Surg. 2004, 52, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Wolter, T.P.; von Heimburg, D.; Stoffels, I.; Groeger, A.; Pallua, N. Cryopreservation of Mature Human Adipocytes. Ann. Plast. Surg. 2005, 55, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Butterwick, K.J.; Nootheti, P.K.; Hsu, J.W.; Goldman, M.P. Autologous Fat Transfer: An In-Depth Look at Varying Concepts and Techniques. Facial Plast. Surg. Clin. N. Am. 2007, 15, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Minonzio, G.; Corazza, M.; Mariotta, L.; Gola, M.; Zanzi, M.; Gandolfi, E.; De Fazio, D.; Soldati, G. Frozen adipose-derived mesenchymal stem cells maintain high capability to grow and differentiate. Cryobiology 2014, 69, 211–216. [Google Scholar] [CrossRef] [PubMed]
- François, P.; Rusconi, G.; Arnaud, L.; Mariotta, L.; Giraudo, L.; Minonzio, G.; Veran, J.; Bertrand, B.; Dumoulin, C.; Grimaud, F.; et al. Inter-center comparison of good manufacturing practices-compliant stromal vascular fraction and proposal for release acceptance criteria: A review of 364 productions. Stem Cell Res. Ther. 2021, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Crowley, C.A.; Smith, W.P.W.; Seah, K.T.M.; Lim, S.-K.; Khan, W.S. Cryopreservation of Human Adipose Tissues and Adipose-Derived Stem Cells with DMSO and/or Trehalose: A Systematic Review Human Adipose Tissues and Adipose-Derived Stem Cells with DMSO and/or Trehalose: A Systematic Review. Cells 2021, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Cryopreservation of whole adipose tissue for future use in regenerative medicine. J. Surg. Res. 2014, 187, 24–35. [Google Scholar] [CrossRef]
- Mashiko, T.; Wu, S.-H.; Kanayama, K.; Asahi, R.; Shirado, T.; Mori, M.; Sunaga, A.; Sarukawa, S.; Uda, H.; Yoshimura, K. Biological Properties and Therapeutic Value of Cryopreserved Fat Tissue. Plast. Reconstr. Surg. 2018, 141, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Alotto, D.; Belisario, D.C.; Casarin, S.; Fumagalli, M.; Cambieri, I.; Piana, R.; Stella, M.; Ferracini, R.; Castagnoli, C. Adipose Derived-Mesenchymal Stem Cells Viability and Differentiating Features for Orthopaedic Reparative Applications: Banking of Adipose Tissue. Stem Cells Int. 2016, 2016, 4968724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-Q.; Tan, P.-C.; Gao, Y.-M.; Zhang, X.-J.; Xie, Y.; Zheng, D.-N.; Zhou, S.-B.; Li, Q.-F. The effect of glycerol as a cryoprotective agent in the cryopreservation of adipose tissue. Stem Cell Res. Ther. 2022, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, F.; Compagnin, C.; Cogliati, E.; Montagner, G.; Dell’antonia, F.; Berna, G.; Vettor, R.; Milan, G.; Trojan, D. Characterization of Human Subcutaneous Adipose Tissue and Validation of the Banking Procedure for Autologous Transplantation. Int. J. Mol. Sci. 2023, 24, 8190. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusconi, G.; Cremona, M.; Gallazzi, M.; Mariotta, L.; Gola, M.; Gandolfi, E.; Malacco, M.; Soldati, G. Good Manufacturing Practice-Compliant Cryopreserved and Thawed Native Adipose Tissue Ready for Fat Grafting. J. Clin. Med. 2024, 13, 3028. https://doi.org/10.3390/jcm13113028
Rusconi G, Cremona M, Gallazzi M, Mariotta L, Gola M, Gandolfi E, Malacco M, Soldati G. Good Manufacturing Practice-Compliant Cryopreserved and Thawed Native Adipose Tissue Ready for Fat Grafting. Journal of Clinical Medicine. 2024; 13(11):3028. https://doi.org/10.3390/jcm13113028
Chicago/Turabian StyleRusconi, Giulio, Martina Cremona, Matteo Gallazzi, Luca Mariotta, Mauro Gola, Eugenio Gandolfi, Matteo Malacco, and Gianni Soldati. 2024. "Good Manufacturing Practice-Compliant Cryopreserved and Thawed Native Adipose Tissue Ready for Fat Grafting" Journal of Clinical Medicine 13, no. 11: 3028. https://doi.org/10.3390/jcm13113028
APA StyleRusconi, G., Cremona, M., Gallazzi, M., Mariotta, L., Gola, M., Gandolfi, E., Malacco, M., & Soldati, G. (2024). Good Manufacturing Practice-Compliant Cryopreserved and Thawed Native Adipose Tissue Ready for Fat Grafting. Journal of Clinical Medicine, 13(11), 3028. https://doi.org/10.3390/jcm13113028





