Exploring Divergent Views: A Comparative Study of Uterus Transplantation Perceptions among Transplant and Obstetrics/Gynecology Providers
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Development
2.2. Survey Distribution
2.3. Data Analysis
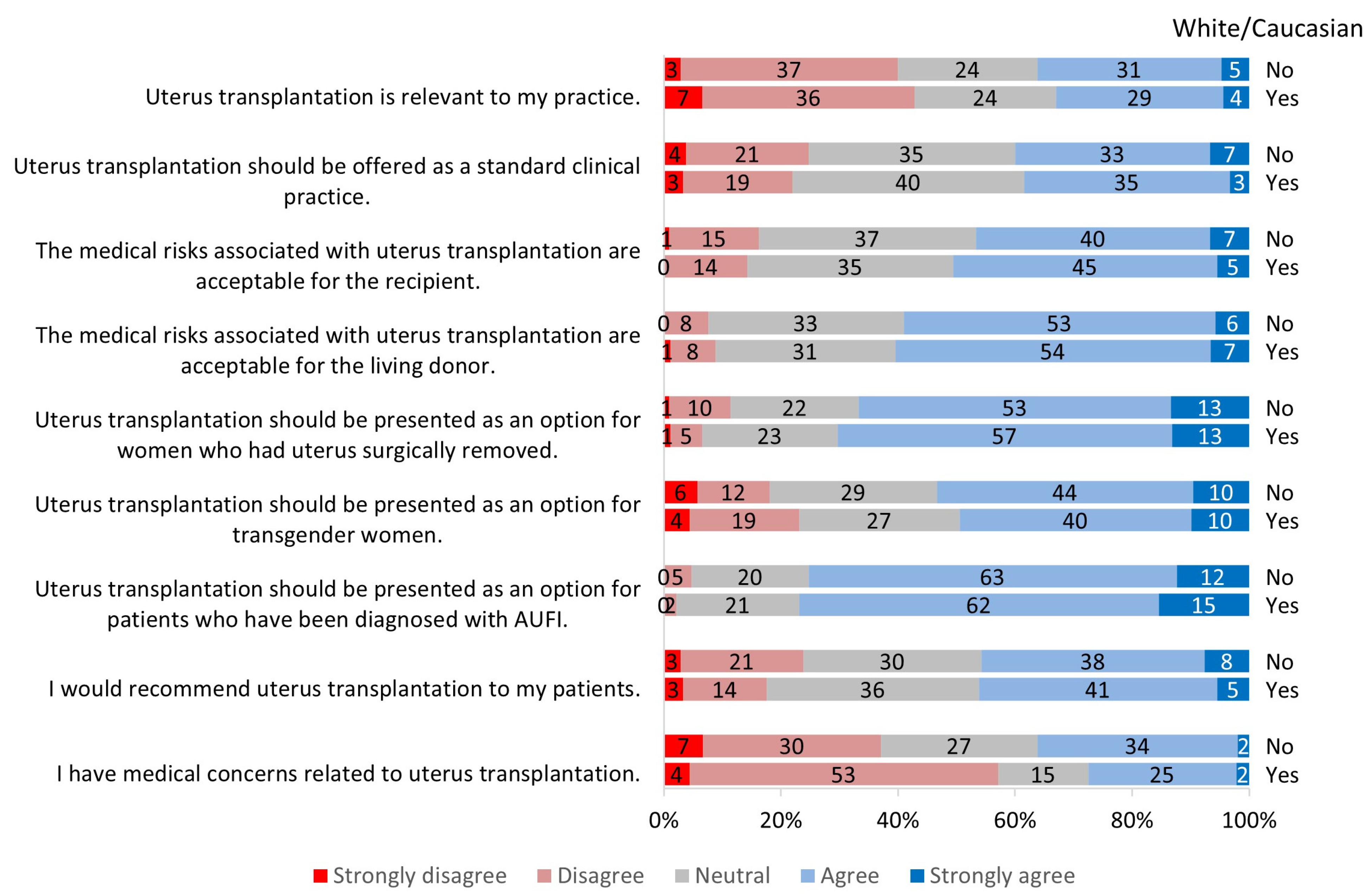
3. Results
3.1. Demographic Data
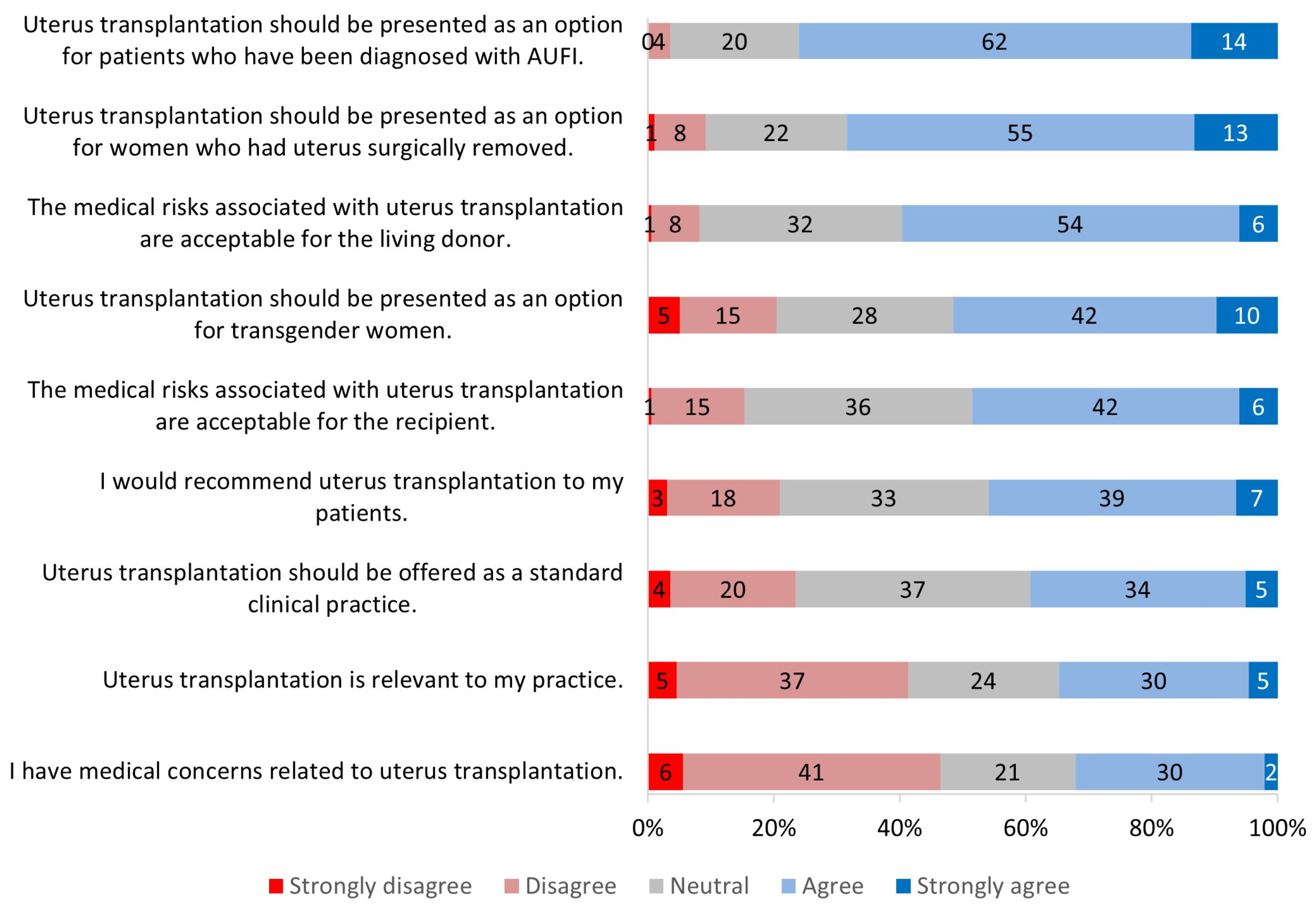
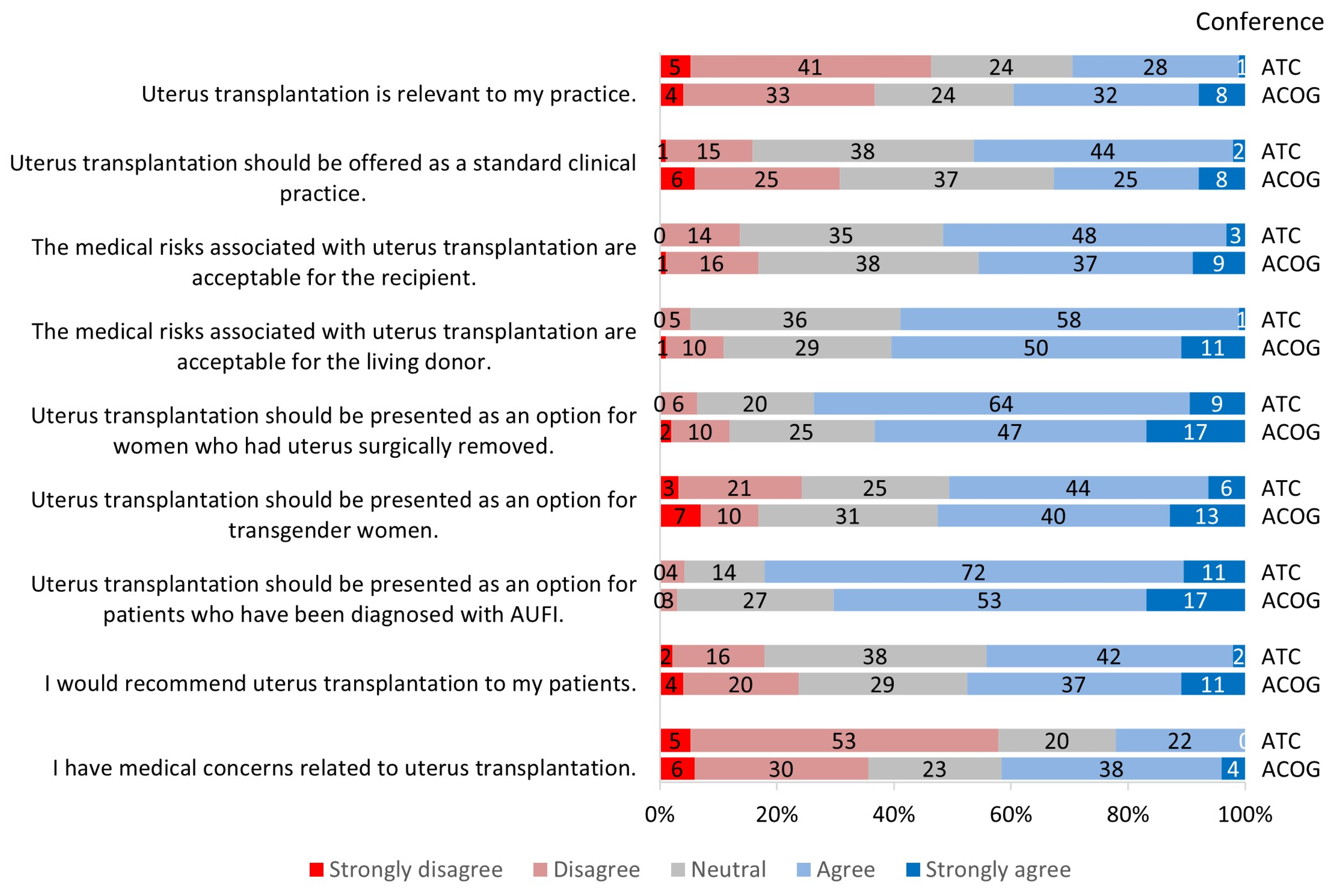
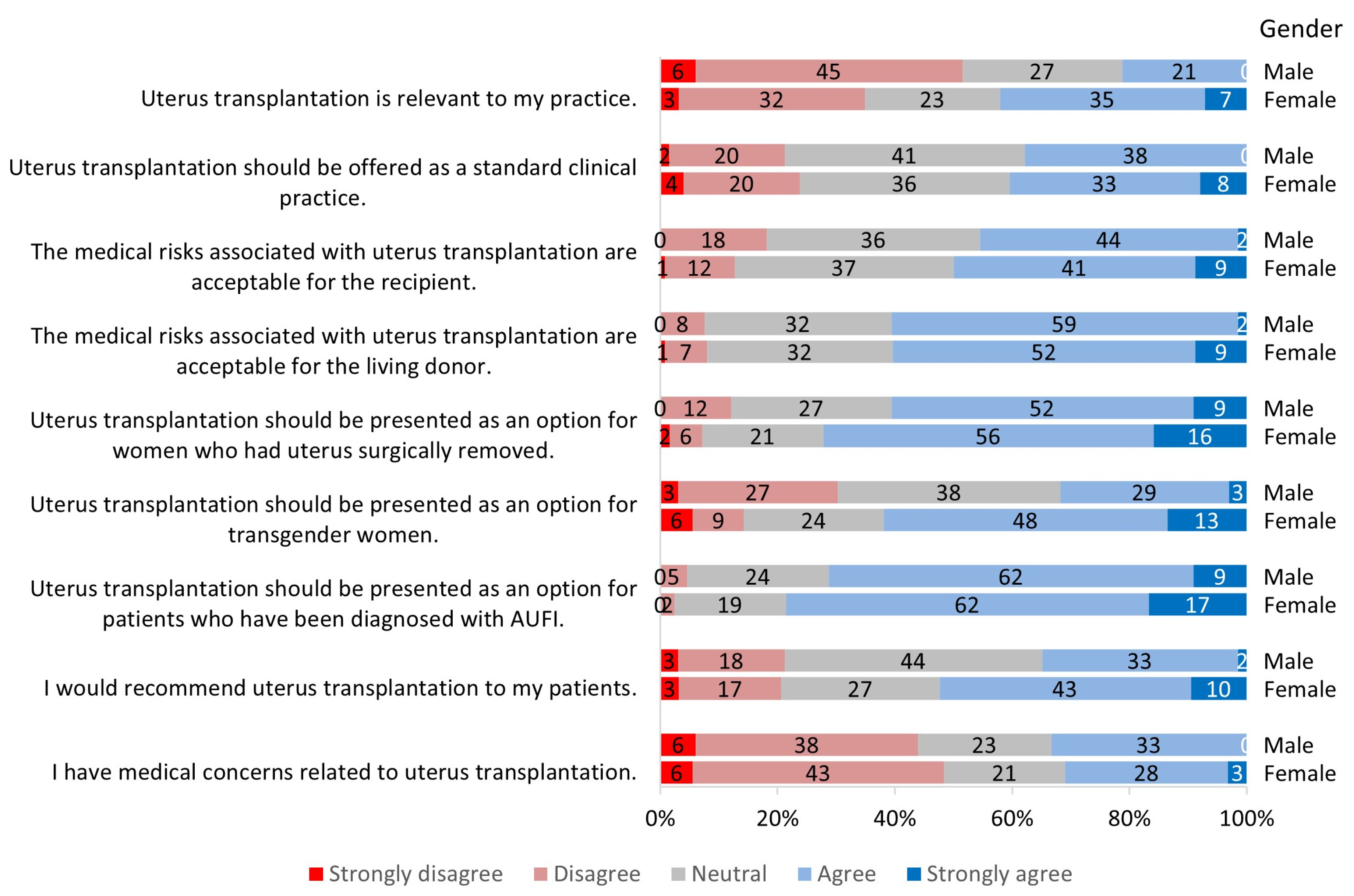
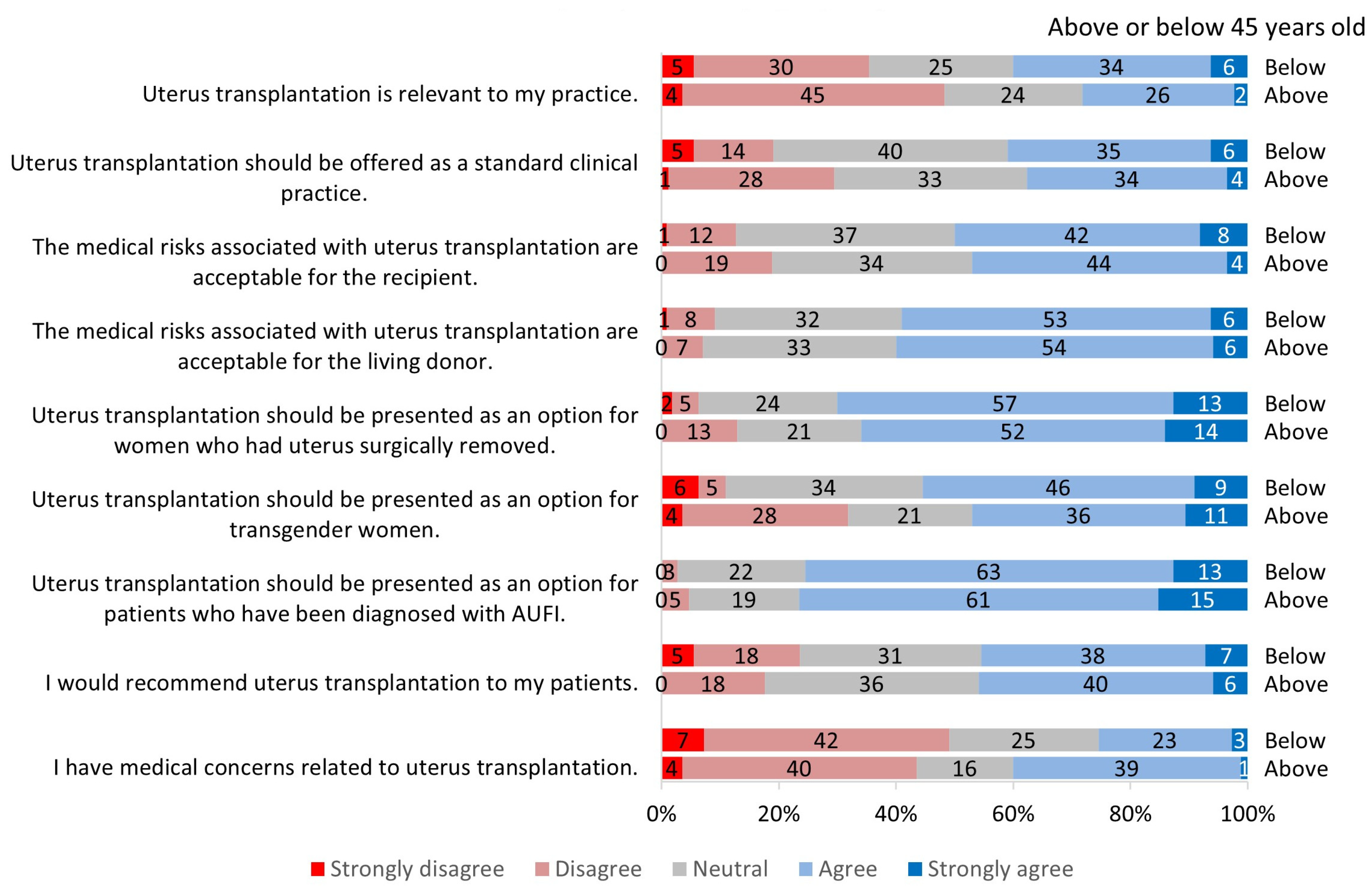
3.2. Perception of Uterus Transplantation
3.2.1. Relevance to Practice
3.2.2. Clinical Candidacy for Uterus Transplantation
3.2.3. Risks Associated with Uterus Transplantation
3.2.4. Future of Uterus Transplantation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Background Information
- What is your specialty?
- ☐
- General obstetrician/gynecologist
- ☐
- Maternal fetal medicine specialist
- ☐
- Reproductive endocrinologist
- ☐
- Gynecological oncologist
- ☐
- Pediatric & adolescent gynecologist
- ☐
- Complex family planning
- ☐
- Transplant surgeon
- ☐
- Transplant physician
- ☐
- Other (please specify): ______________
- 2.
- What is your current professional role?
- ☐
- Resident
- ☐
- Fellow
- ☐
- Attending
- 3.
- Gender:
- ☐
- Male
- ☐
- Female
- ☐
- Not listed (Please specify)
- ☐
- Prefer not to answer
- 4.
- How old are you?
- ☐
- 25–35 years old
- ☐
- 36–45 years old
- ☐
- 46–55 years old
- ☐
- 56–65 years old
- ☐
- 66+ years old
- 5.
- Please identify your race/ethnicity:
- ☐
- Non-Hispanic White/Caucasian
- ☐
- Non-Hispanic Black or African American
- ☐
- Asian
- ☐
- American Indian or Alaska Native
- ☐
- Native Hawaiian or Other Pacific Islander
- ☐
- Middle Eastern or North African
- ☐
- Hispanic, Latino, or Spanish origin
- ☐
- Other (please specify): __________________________
- 6.
- How long have you been practicing as an attending?
- 0–5 years
- 6–10 years
- 11–15 years
- 16–20
- 21–25 years
- 26+ years
- 7.
- What best describes the medical setting you practice in?
- University or teaching hospital or medical center
- Community clinic or hospital
- Single- specialty group private practice
- Staff model HMO
- Fertility clinic
- Military/government
- Multispecialty group or private practice
- Other (please specify)_________________________
| Strongly Disagree | Disagree | Neutral | Agree | Strongly Agree | |
| I have medical concerns related to uterus transplantation | |||||
| I would recommend uterus transplantation to my patients | |||||
| Uterus transplantation should be a viable option for patients who have been diagnosed with congenital AUFI | |||||
| Uterus transplantation should be a viable option for transgender women | |||||
| Uterus transplantation should be a viable option for women who have had their uterus surgically removed | |||||
| The medical risks associated with uterus transplantation are acceptable for the donor | |||||
| The medical risks associated with uterus transplantation are acceptable for the recipient | |||||
| Uterus transplantation should be a standard clinical practice | |||||
| Uterus transplantation is relevant to my practice |
References
- Testa, G.; McKenna, G.J.; Gunby, R.T., Jr.; Anthony, T.; Koon, E.C.; Warren, A.M.; Putman, J.M.; Zhang, L.; dePrisco, G.; Mitchell, J.M.; et al. First live birth after uterus transplantation in the United States. Am. J. Transplant. 2018, 18, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Richards, E.; Reddy, V.; Walter, J.; Olthoff, K.; Quintini, C.; Tzakis, A.; Latif, N.; Porrett, P.; O’Neill, K.; et al. The first 5 years of uterus transplant in the US: A report from the United States Uterus Transplant Consortium. JAMA Surg. 2022, 157, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Saso, S.; Clarke, A.; Bracewell-Milnes, T.; Al-Memar, M.; Hamed, A.H.; Thum, M.Y.; Ghaem-Maghami, S.; Del Priore, G.; Smith, J.R. Survey of perceptions of health care professionals in the United Kingdom toward uterine transplant. Prog. Transplant. 2015, 25, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto, P.; Hariton, E.; Farland, L.V.; Goldman, R.H.; Gargiulo, A.R. Uterine transplantation: A survey of perceptions and attitudes of American reproductive endocrinologists and gynecologic surgeons. J. Minim. Invasive Gynecol. 2018, 25, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Riggan, K.A.; Khan, Z.; Langstraat, C.L.; Allyse, M.A. Provider knowledge and support of uterus transplantation: Surveying multidisciplinary team members. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. American Society for Reproductive Medicine position statement on uterus transplantation: A committee opinion. Fertil. Steril. 2018, 110, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.P.; Rajamanoharan, A.; Vali, S.; Williams, N.J.; Saso, S.; Thum, M.Y.; Ghaem-Maghami, S.; Quiroga, I.; Diaz-Garcia, C.; Thomas, P.; et al. Perceptions and motivations for uterus transplant in transgender women. JAMA Netw Open. 2021, 4, e2034561. [Google Scholar] [CrossRef] [PubMed]
- Irani, R.A.; Coscia, L.A.; Chang, E.; Lappen, J.R.; SMFM Publications Committee. Society for Maternal-Fetal Medicine consult series #66: Prepregnancy evaluation and pregnancy management of patients with solid organ transplants. Am. J. Obstet. Gynecol. 2023, 229, B10–B32. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Del Prete, L.; Eghtesad, B.; Hashimoto, K.; Miller, C.; Tzakis, A.; Quintini, C.; Falcone, T. Immunosuppression in uterus transplantation: From transplant to delivery. Expert Opin. Pharmacother. 2023, 24, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Testa, G.; Putman, J.M.; McKenna, G.J.; Koon, E.C.; York, J.R.; Bayer, J.; Zhang, L.; Rubeo, Z.S.; Gunby, R.T.; et al. Twelve Live Births After Uterus Transplantation in the Dallas UtErus Transplant Study. Obstet Gynecol. 2021, 137, 241–249. [Google Scholar] [CrossRef] [PubMed]
| Conferences | |||
|---|---|---|---|
| ACOG | ATC | ||
| Participants (n) | 102 | 98 | |
| Age | 25–35 years old | 42 (41.2%) | 21 (21.4%) |
| 36–45 years old | 18 (17.6%) | 30 (30.6%) | |
| 46–55 years old | 24 (23.5%) | 34 (34.7%) | |
| 56–65 years old | 16 (15.7%) | 11 (11.2%) | |
| 66+ years old | 2 (2.0%) | 1 (1.0%) | |
| Gender | Female | 81 (79.4%) | 47 (48.0%) |
| Male | 20 (19.6%) | 48 (49.0%) | |
| Not listed (please specify) | 1 (1.0%) | 1 (1.0%) | |
| Prefer not to answer | 0 (0.0%) | 1 (1.0%) | |
| Race/ethnicity | Asian | 20 (19.6%) | 26 (26.5%) |
| Hispanic, Latino, or Spanish origin | 9 (8.8%) | 5 (5.1%) | |
| Middle Eastern or North African | 0 (0.0%) | 3 (3.1%) | |
| Non-Hispanic Black or African American | 30 (29.4%) | 10 (10.2%) | |
| Non-Hispanic White/Caucasian | 41 (40.2%) | 52 (53.1%) | |
| Other | 2 (2.0%) | 2 (2.0%) | |
| Professional role | Attending | 59 (57.8%) | 66 (67.3%) |
| Fellow | 7 (6.9%) | 3 (3.1%) | |
| Resident | 18 (17.6%) | 10 (10.2%) | |
| Other | 17 (16.7%) | 18 (18.4%) | |
| Professional setting | Community clinic or hospital | 23 (22.5%) | 13 (13.3%) |
| Military/government | 5 (4.9%) | 3 (3.1%) | |
| Multispecialty group or private practice | 2 (2.0%) | 0 (0.0%) | |
| Other (please specify) | 7 (6.9%) | 3 (3.1%) | |
| Single-specialty group private practice | 11 (10.8%) | 0 (0.0%) | |
| Staff model HMO | 3 (2.9%) | 0 (0.0%) | |
| University or teaching hospital or medical center | 50 (49.0%) | 78 (79.6%) | |
| Other | 1 (1.0%) | 1 (1.0%) | |
| Medical specialty | Complex family planning | 4 (3.9%) | 0 (0.0%) |
| General obstetrician/gynecologist | 72 (70.6%) | 0 (0.0%) | |
| Gynecological oncologist | 1 (1.0%) | 0 (0.0%) | |
| Maternal-fetal medicine specialist | 10 (9.8%) | 0 (0.0%) | |
| Reproductive endocrinologist | 3 (2.9%) | 0 (0.0%) | |
| Transplant physician | 0 (0.0%) | 26 (26.5%) | |
| Transplant surgeon | 0 (0.0%) | 22 (22.4%) | |
| Other (please specify) | 12 (11.8%) | 50 (51.0%) | |
| Years of practice | <5 years | 42 (41.2%) | 22 (22.4%) |
| 6–10 years | 14 (13.7%) | 12 (12.2%) | |
| 9–15 years | 7 (6.9%) | 17 (17.3%) | |
| 16–20 years | 22 (21.6%) | 16 (16.3%) | |
| 21–25 years | 15 (14.7%) | 16 (16.3%) | |
| >25 years | 2 (2.0%) | 14 (14.3%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vyas, P.; Sader, D.; Testa, G.; Du, J.; Wall, A.; Johannesson, L. Exploring Divergent Views: A Comparative Study of Uterus Transplantation Perceptions among Transplant and Obstetrics/Gynecology Providers. J. Clin. Med. 2024, 13, 3182. https://doi.org/10.3390/jcm13113182
Vyas P, Sader D, Testa G, Du J, Wall A, Johannesson L. Exploring Divergent Views: A Comparative Study of Uterus Transplantation Perceptions among Transplant and Obstetrics/Gynecology Providers. Journal of Clinical Medicine. 2024; 13(11):3182. https://doi.org/10.3390/jcm13113182
Chicago/Turabian StyleVyas, Prema, Danielle Sader, Giuliano Testa, Jinyu Du, Anji Wall, and Liza Johannesson. 2024. "Exploring Divergent Views: A Comparative Study of Uterus Transplantation Perceptions among Transplant and Obstetrics/Gynecology Providers" Journal of Clinical Medicine 13, no. 11: 3182. https://doi.org/10.3390/jcm13113182
APA StyleVyas, P., Sader, D., Testa, G., Du, J., Wall, A., & Johannesson, L. (2024). Exploring Divergent Views: A Comparative Study of Uterus Transplantation Perceptions among Transplant and Obstetrics/Gynecology Providers. Journal of Clinical Medicine, 13(11), 3182. https://doi.org/10.3390/jcm13113182







