Prevalence of Abnormal Cardiovascular Magnetic Resonance Findings in Athletes Recovered from COVID-19 Infection: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection Process and Quality Assessment
2.3. Statistical Analysis
3. Results
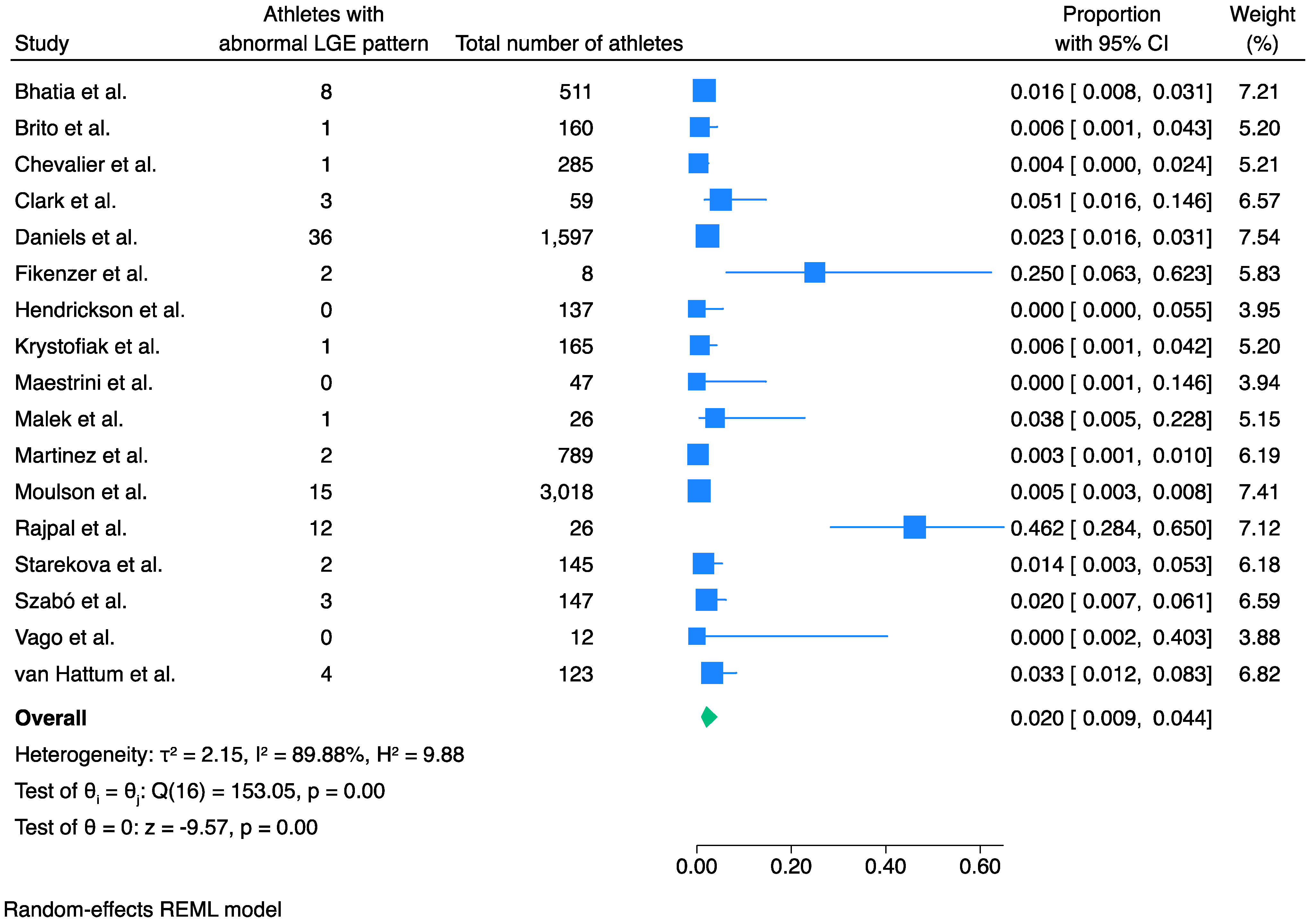
3.1. Prevalence of LGE
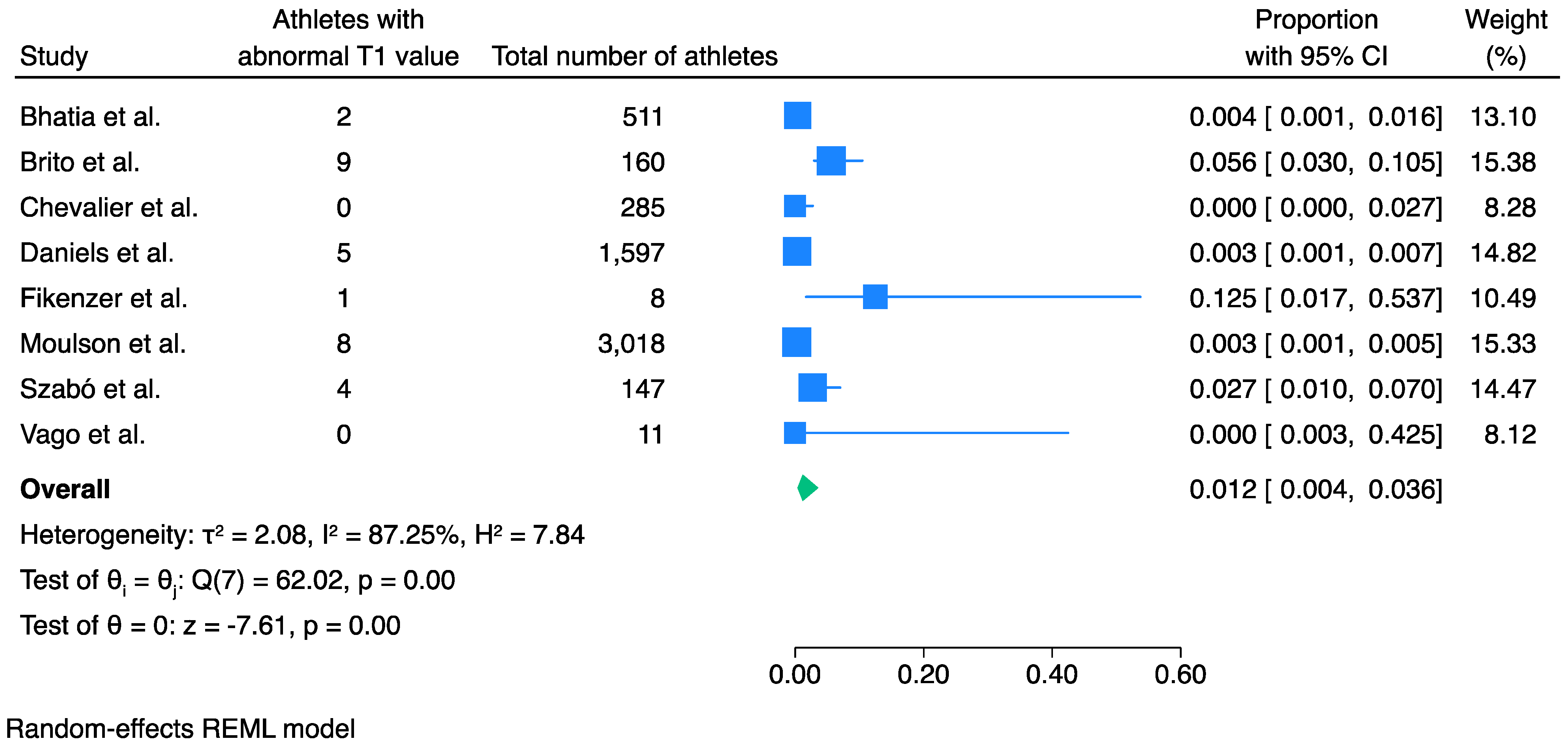
3.2. Prevalence of Abnormal T1 Values
3.3. Prevalence of Abnormal T2 Values
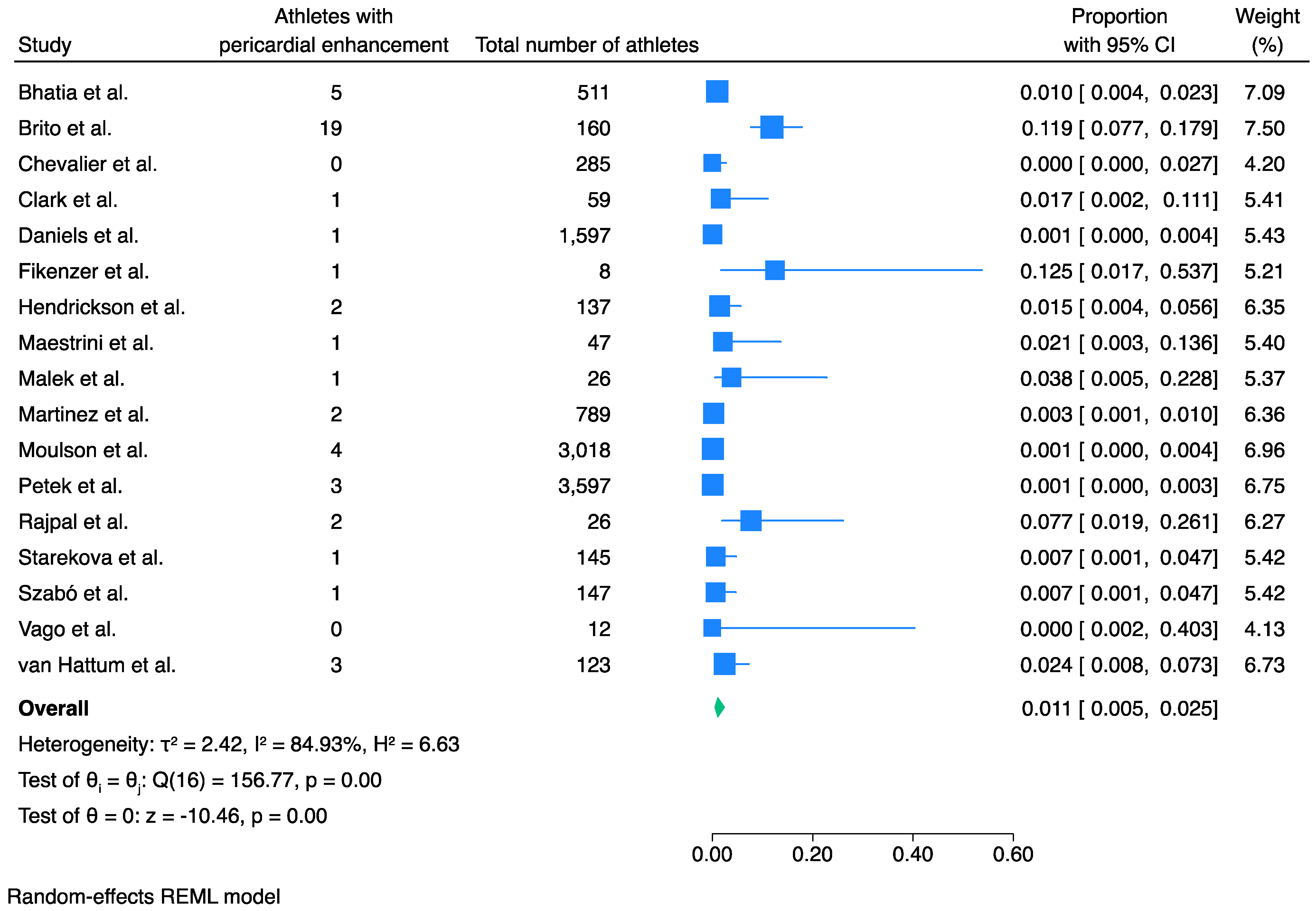
3.4. Prevalence of Pericardial Involvement
3.5. ECG Abnormalities and Elevated Troponin Levels in Athletes Post-COVID-19 Infection, in the Studies Reviewed in This Meta-Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- La, G.A.; Wasfy, M.M.; Brosnan, M.J.; Claessen, G.; Fatkin, D.; Heidbuchel, H.; Baggish, A.L.; Kovacic, J.C. The Athlete’s Heart—Challenges and Controversies. J. Am. Coll. Cardiol. 2022, 80, 1346–1362. [Google Scholar] [CrossRef]
- Prior, D.L.; Gerche, A.L. The Athlete’s Heart. Heart 2012, 98, 947–955. [Google Scholar] [CrossRef]
- Androulakis, E.; Mouselimis, D.; Tsarouchas, A.; Antonopoulos, A.; Bakogiannis, C.; Papagkikas, P.; Vlachopoulos, C. The Role of Cardiovascular Magnetic Resonance Imaging in the Assessment of Myocardial Fibrosis in Young and Veteran Athletes: Insights From a Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 784474. [Google Scholar] [CrossRef]
- Domenech-Ximenos, B.; Sanz-de La Garza, M.; Prat-González, S.; Sepúlveda-Martínez, A.; Crispi, F.; Duran-Fernandez, K.; Perea, R.J.; Bijnens, B.; Sitges, M. Prevalence and Pattern of Cardiovascular Magnetic Resonance Late Gadolinium Enhancement in Highly Trained Endurance Athletes. J. Cardiovasc. Magn. Reson. 2020, 22, 62. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Baggish, A.L.; Hays, A.G.; Jerosch-Herold, M.; Kim, J.; Ordovas, K.G.; Reddy, G.; Shenoy, C.; Weinsaft, J.W.; Woodard, P.K. Utilization of Cardiovascular Magnetic Resonance Imaging for Resumption of Athletic Activities Following COVID-19 Infection: An Expert Consensus Document on Behalf of the American Heart Association Council on Cardiovascular Radiology and Intervention Leadership and Endorsed by the Society for Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2023, 16, e014106. [Google Scholar] [CrossRef]
- Dove, J.; Gage, A.; Kriz, P.; Tabaddor, R.R.; Owens, B.D. COVID-19 and Review of Current Recommendations for Return to Athletic Play. RI Med. J. 2020, 103, 15–20. [Google Scholar]
- Ceglie, N.; Petito, A.; Cibelli, G. Return to Play of Young and Adult Professional Athletes after COVID-19: A Scoping Review. J. Exerc. Sci. Fit. 2024, 22, 208–220. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ Clin. Res. Ed. 2021, 372, n71. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 28 November 2022).
- Bhatia, R.T.; Malhotra, A.; MacLachlan, H.; Gati, S.; Marwaha, S.; Chatrath, N.; Fyyaz, S.; Aleixo, H.; Al-Turaihi, S.; Babu, A.; et al. Prevalence and Diagnostic Significance of De-Novo 12-Lead ECG Changes after COVID-19 Infection in Elite Soccer Players. Heart 2023, 109, 936–943. [Google Scholar] [CrossRef]
- Brito, D.; Meester, S.; Yanamala, N.; Patel, H.B.; Balcik, B.J.; Casaclang-Verzosa, G.; Seetharam, K.; Riveros, D.; Beto, R.J.; Balla, S.; et al. High Prevalence of Pericardial Involvement in College Student Athletes Recovering From COVID-19. Jacc. Cardiovasc. Imaging 2021, 14, 541. [Google Scholar] [CrossRef]
- Chevalier, L.; Cochet, H.; Mahida, S.; Benard, A.; Cariou, T.; Sridi-Cheniti, S.; Benhenda, S.; Doutreleau, S.; Cade, S.; Guerard, S.; et al. Resuming Training in High-Level Athletes After Mild COVID-19 Infection: A Multicenter Prospective Study (ASCCOVID-19). Sports Med. Open 2022, 8, 83. [Google Scholar] [CrossRef]
- Clark, D.E.; Parikh, A.; Dendy, J.M.; Diamond, A.B.; George-Durrett, K.; Fish, F.A.; Slaughter, J.C.; Fitch, W.; Hughes, S.G.; Soslow, J.H. COVID-19 Myocardial Pathology Evaluation in Athletes with Cardiac Magnetic Resonance (COMPETE CMR). Circulation 2021, 143, 609–612. [Google Scholar] [CrossRef]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes with Recent SARS-CoV-2 Infection: Results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef]
- Fikenzer, S.; Kogel, A.; Pietsch, C.; Lavall, D.; Stöbe, S.T.; Rudolph, U.; Laufs, U.; Hepp, P.; Hagendorff, A. SARS-CoV2 Infection: Functional and Morphological Cardiopulmonary Changes in Elite Handball Players. Sci. Rep. 2021, 11, 17798. [Google Scholar] [CrossRef]
- Hendrickson, B.S.; Stephens, R.E.; Chang, J.V.; Amburn, J.M.; Pierotti, L.L.; Johnson, J.L.; Hyden, J.C.; Johnson, J.N.; Philip, R.R. Cardiovascular Evaluation After COVID-19 in 137 Collegiate Athletes: Results of an Algorithm-Guided Screening. Circulation 2021, 143, 1926–1928. [Google Scholar] [CrossRef]
- Krystofiak, J.; Kim, M.; Navia, A.; Lander, J.; Altobelli, A.; Vucic, E.; Womack, J.; Toto, D.; Siddiqui, A.; Bershad, J.; et al. Post–COVID-19 Cardiovascular Evaluation in National Collegiate Athletic Association Division I Athletes. Clin. J. Sport. Med. 2022, 32, 334–337. [Google Scholar] [CrossRef]
- Maestrini, V.; Filomena, D.; Birtolo, L.I.; Serdoz, A.; Fiore, R.; Tatangelo, M.; Lemme, E.; Squeo, M.R.; Mango, R.; Di Gioia, G.; et al. Systematic Cardiovascular Screening in Olympic Athletes before and after SARS-CoV-2 Infection. JCM 2022, 11, 3499. [Google Scholar] [CrossRef]
- Małek, Ł.A.; Marczak, M.; Miłosz-Wieczorek, B.; Konopka, M.; Braksator, W.; Drygas, W.; Krzywański, J. Cardiac Involvement in Consecutive Elite Athletes Recovered from Covid-19: A Magnetic Resonance Study. J. Magn. Reson. Imaging 2021, 53, 1723–1729. [Google Scholar] [CrossRef]
- Martinez, M.W.; Tucker, A.M.; Bloom, O.J.; Green, G.; Difiori, J.P.; Solomon, G.; Phelan, D.; Kim, J.H.; Meeuwisse, W.; Sills, A.K.; et al. Prevalence of Inflammatory Heart Disease among Professional Athletes with Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol. 2021, 6, 745–752. [Google Scholar] [CrossRef]
- Moulson, N.; Petek, B.J.; Drezner, J.A.; Harmon, K.G.; Kliethermes, S.A.; Patel, M.R.; Baggish, A.L.; Asif, I.M.; Borchers, J.; Edenfield, K.M.; et al. SARS-CoV-2 Cardiac Involvement in Young Competitive Athletes. Circulation 2021, 144, 256–266. [Google Scholar] [CrossRef]
- Petek, B.J.; Moulson, N.; Baggish, A.L.; Kliethermes, S.A.; Patel, M.R.; Churchill, T.W.; Harmon, K.G.; Drezner, J.A. Prevalence and Clinical Implications of Persistent or Exertional Cardiopulmonary Symptoms Following SARS-CoV-2 Infection in 3597 Collegiate Athletes: A Study from the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA). Br. J. Sports Med. 2022, 56, 913–918. [Google Scholar] [CrossRef]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection. JAMA Cardiol. 2021, 6, 116–118. [Google Scholar] [CrossRef]
- Starekova, J.; Bluemke, D.A.; Bradham, W.S.; Eckhardt, L.L.; Grist, T.M.; Kusmirek, J.E.; Purtell, C.S.; Schiebler, M.L.; Reeder, S.B. Evaluation for Myocarditis in Competitive Student Athletes Recovering from Coronavirus Disease 2019 with Cardiac Magnetic Resonance Imaging. JAMA Cardiol. 2021, 6, 945–950. [Google Scholar] [CrossRef]
- Szabó, L.; Juhász, V.; Dohy, Z.; Fogarasi, C.; Kovács, A.; Lakatos, B.K.; Kiss, O.; Sydó, N.; Csulak, E.; Suhai, F.I.; et al. Is Cardiac Involvement Prevalent in Highly Trained Athletes after SARS-CoV-2 Infection? A Cardiac Magnetic Resonance Study Using Sex-Matched and Age-Matched Controls. Br. J. Sports Med. 2022, 56, 553–560. [Google Scholar] [CrossRef]
- Vago, H.; Szabo, L.; Dohy, Z.; Merkely, B. Cardiac Magnetic Resonance Findings in Patients Recovered From COVID-19. Cardiovasc. Imaging 2021, 14, 1279–1281. [Google Scholar] [CrossRef]
- Van Hattum, J.C.; Daems, J.J.N.; Verwijs, S.M.; Wismans, L.V.; Van Diepen, M.A.; Groenink, M.; Boekholdt, S.M.; Planken, R.N.; Van Randen, A.; Hirsch, A.; et al. Long-Term Cardiac Follow-up of Athletes Infected with SARS-CoV-2 after Resumption of Elite-Level Sports. Heart 2023, 110, 254–262. [Google Scholar] [CrossRef]
- Modica, G.; Bianco, M.; Sollazzo, F.; Di Murro, E.; Monti, R.; Cammarano, M.; Morra, L.; Nifosì, F.M.; Gervasi, S.F.; Gravina, E.M.; et al. Myocarditis in Athletes Recovering from COVID-19: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4279. [Google Scholar] [CrossRef]
- Van Hattum, J.C.; Spies, J.L.; Verwijs, S.M.; Verwoert, G.C.; Planken, R.N.; Boekholdt, S.M.; Groenink, M.; Malekzadeh, A.; Pinto, Y.M.; Wilde, A.A.M.; et al. Cardiac Abnormalities in Athletes after SARS-CoV-2 Infection: A Systematic Review. BMJ Open Sport Exerc. Med. 2021, 7, e001164. [Google Scholar] [CrossRef]
- Emery, M.S.; Kovacs, R.J. Sudden Cardiac Death in Athletes. JACC Heart Fail. 2018, 6, 30–40. [Google Scholar] [CrossRef]
- Nieß, A.; Bloch, W.; Friedmann-Bette, B.; Grim, C.; Halle, M.; Hirschmüller, A.; Kopp, C.; Meyer, T.; Niebauer, J.; Reinsberger, C.; et al. Position Stand: Return to Sport in the Current Coronavirus Pandemic (SARS-CoV-2/COVID-19). Dtsch. Z. Für Sportmed. 2020, 71, E1–E4. [Google Scholar] [CrossRef]
- Małek, Ł.A.; Bucciarelli-Ducci, C. Myocardial Fibrosis in Athletes—Current Perspective. Clin. Cardiol. 2020, 43, 882–888. [Google Scholar] [CrossRef]

| Study | Study Design | Study Period | Study Population | Age | Tests Athletes Underwent | Number of Athletes That Had CMR | Time Interval from Infection/to CMR |
|---|---|---|---|---|---|---|---|
| Bhatia et al. [10] | Prospective observational study | March 2020–May 2022 | 511 soccer players (494 with no de novo ECG changes and 17 with de novo ECG changes) | 21 years old (median) | Clinical assessment, ECG, echocardiogram, CMR | 30 athletes underwent mandatory CMR, 17 athletes had CMR after abnormal ECG | 15 days (median) |
| Brito et al. [11] | Cross-sectional observational study | June–August 2020 | 160 athletes from West Virginia University | 19 years old (median) | Clinical assessment, questionnaire, ECG and echocardiogram (54), blood tests (Troponin, CRP, ESR, BNP) during individualised clinical assessments | 48 athletes had CMR: symptomatic (mild or moderate illness) and asymptomatic with ECG/echocardiographic abnormalities) | 27 days (median time interval from initial tests performed to the imaging assessment) |
| Chevalier et al. [12] | Prospective cohort study | June–December 2020 | 285 athletes (rugby players and student athletes) | 25.8 years old (mean age of rugby players) and 20.1 years old (mean age of student athletes) | Clinical assessment, questionnaire, ECG and blood sampling (CRP, troponin I, D-Dimer, SARS-CoV-2 serology), Echocardiogram (including stress), Troponin, CMR | 102 symptomatic and asymptomatic athletes that agreed to proceed with CMR assessment (CMR was offered to all) | 51 ± 37 days |
| Clark et al. [13] | Retrospective case control study | March–December 2020 | 59 COVID-19-positive athletes, 60 athletic controls, and 27 healthy controls were included | 20 years old (covid athletes) and 25 years old (athletic controls) | Clinical examination, ECG, troponin I, echocardiogram and CMR | The whole study population (symptomatic and asymptomatic subjects had CMR) | 21.5 days (median) |
| Daniels et al. [14] | Retrospective observational study (Big Ten COVID-19 Registry from 13 Big Ten Universities in the USA across 17 sport disciplines) | March–December 2020 | 1597 athletes | Not provided | COVID-19-positive athletes underwent cardiac evaluation prior to CMR | All study participants had a CMR test (there were different diagnostic strategies across universities but, ultimately, only those who had CMR were included in the study) | 22.5 days (median) |
| Fikenzer et al. [15] | Prospective cohort study | 2020 (months not defined) | 8 COVID-19-positive athletes and 4 non-infected athletes (controls) | 27 years old (mean) | Clinical assessment, questionnaire, ECG, echo, CMR | All participants had CMR | 19 ± 7 days |
| Hendrickson et al. [16] | Retrospective observational study | July–October 2020 | 137 collegiate athletes | 20 years old (median) | Clinical assessment, ECG, Troponin | Anyone with abnormal test or clinical concern (n = 5) | 16 days (median) |
| Krystofiak et al. [17] | Retrospective case series | August–December 2020 | 165 athletes | 20 years old (median) | Trop, ecg, echo, CMR | All participants had CMR (regardless of symptoms) | 25 days (median) |
| Maestrini et al. [18] | Prospective cohort study | November 2020 | 47 Italian Olympic athletes | 26 years old (mean) | 12 lead ECG, CPET, blood tests, 24-h ECG, spirometry, CMR | All participants had CMR (regardless of symptoms) | Median duration of the infection was 14 days, median time between the first negative covid test (NPS) and the RTP evaluation was 9 days |
| Małek et al. [19] | Retrospective cohort study | August–October 2020 | 26 Olympians | 24 years old (median) | Clinical assessment, ECG, blood tests, CMR | All participants had CMR (regardless of symptoms) | 32 days (median) |
| Martinez et al. [20] | Cross-sectional study | May–October 2020 | 789 professional athletes | 25 years old (mean) | Clinical assessment, ECG, blood tests, echocardiogram | 27 athletes with abnormal initial screening | 19 days (mean) |
| Moulson et al. [21] | Prospective observational cohort study | September–December 2020 | 3018 athletes | 20 years old (mean) | Clinical assessment, ECG, troponin, echocardiogram, CMR | 317 athletes (primary screening with CMR performed in 198 athletes, but only 119 athletes had CMR as initial screening was abnormal) | 33 days (median) |
| Petek et al. [22] | Prospective observational cohort study | September 2020–May 2021 | 3597 athletes with confirmed COVID and persistent (>3 weeks) or exertional symptoms | 20 years old (mean) | Clinical assessment, ECG, troponin, echocardiogram, CMR | 44 athletes with persistent symptoms, 137 with exertional symptoms | 44 days (median) |
| Rajpal et al. [23] | Prospective cohort study | June–August 2020 | 26 athletes | 19.5 years old (mean) | ECG, troponin, echocardiogram, CMR | All participants had CMR | CMR was performed after recommended quarantine (11–53 days) |
| Starekova et al. [24] | Retrospective observational study | January–November 2020 | 145 athletes | 19.6 years old (mean) | Clinical assessment, ECG, troponin, echocardiogram, CMR | All participants had CMR | 15 days (median) |
| Szabó et al. [25] | Observational case control study | July 2020–February 2021 | 147 athletes | 23 years old (median) | Clinical assessment, questionnaire, ECG, troponin, echocardiogram, CMR | All participants had CMR [asymptomatic (n = 19) or with mild (n = 80), moderate (n = 43) or persistent (>4 weeks) (n = 5) symptoms] | 32 days (median) |
| Vago et al. [26] | Prospective observational study | Not provided | 12 athletes | 23 years old (median) | Blood tests (CRP, NTproBNP, high sensitivity Troponin T), CMR | All participants had CMR | 17 days for 10 female athletes, and 67 and 90 days in 2 male athletes, respectively. |
| Van Hattum et al. [27] | Prospective longitudinal study | May 2019–November 2022 | 123 COVID-19-positive athletes and 136 athletes (controls) | 25 years old (mean) | Demographics, ECG, high sensitivity Troponin T, NTproBNP, CKMB, CMR | All participants had CMR (regardless of symptoms) | 3.9 ± 2.9 months |
| Study | Number of Athletes with Elevated Troponin | Number of Athletes with One or More ECG Abnormalities | Number of Athletes That Had CMR | Number of Athletes with Pathological LGE Pattern | Number of Athletes with Abnormal T1 Values | Number of Athletes with Abnormal T2 Values | Number of Athletes with Pericardial Enhancement or Effusion |
|---|---|---|---|---|---|---|---|
| Bhatia et al. [10] | n/a | 17 (3%) | 47 (9.2%) | 8 (17%) | 2 (4%) | 1 (2%) | 5 (10%) |
| Brito et al. [11] | 1 (3%) | 1 (3%) (abnormal sinus tachycardia with ST segment and T wave changes) | 48 (30%) | 1 (2%) | 9 (19%) | 0 | 19 (39%) |
| Chevalier et al. [12] | 8 (3%) | 6 (2%) | 102 (35.8%) | 1 (1%) | 0 | 0 | 0 |
| Clark et al. [13] | 0 | 0 | 59 (100%) | 3 (5%) | Mild segmental increases in T1, T2, or extracellular volume were found in 39% of COVID-19-positive athletes, 13% of athletic controls, and 8% of healthy controls. Two asymptomatic COVID-19-positive athletes (3%) met criteria for myocarditis; one athlete had pericarditis. These athletes had normal electrocardiograms, troponin I, and echocardiograms with strain. | 1 (2%) | |
| Daniels et al. [14] | 4 [14.3% of athletes with probable myocarditis (n = 28)] | 1 [3.5% of athletes with probable myocarditis (n = 28)] | 1597 (100%) | 36 (2%) | 5 (0.3%) | 31 (2%) | 1 0.1%) |
| Fikenzer et al. [15] | n/a | 0 | 8 (100%) | 2 (25%) | 1 (12.5%) | 0 | 1 (12.5%) |
| Hendrickson et al. [16] | 4 (3%) | 0 | 5 (3.6%) | 0 | 0 | 0 | 2 (1.5%) |
| Krystofiak et al. [17] | 0 | 0 | 165 (100%) | 1 (0.6%) | 1 (0.6%) | 0 | Not provided |
| Maestrini et al. [18] | 1 (2.1%) | 0 newly detected ECG abnormalities. 3 athletes had new PVCs during CPET. | 47 (100%) | 0 | 1 (2.1%) | 1 (2.1%) | 1 (2.1%) |
| Małek et al. [19] | 4 (15%) | 0 | 26 (100%) | 1 (3.8%) | 0 | 1 (3.8%) | 1 (3.8%) |
| Martinez et al. [20] | 6 (0.7%) | 10 (1.3%) | 27 (3.4%) | 2 (0.25%) | Not provided | Not provided | 2 (0.25%) |
| Moulson et al. [21] | 24 (0.9%) | 21 (0.7%) | 317 (10.5%) | 15 (4.7%) | 8 (2.5%) | 7 (2.2%) | 4 (1.3%) |
| Petek et al. [22] | 0 | 1 (0.8%) | 181 (5%) | Five of forty-four (11.4%) athletes who underwent a CMR for exertional cardiopulmonary symptoms on return to exercise had probable or definite SARS-CoV-2 cardiac involvement, including 3 cases of pericardial involvement, 1 definite case of myopericardial involvement and 1 probable case of myopericardial involvement. | 3 (6.8%) | ||
| Rajpal et al. [23] | 0 | 0 | 26 (100%) | 12 (46%) | 0 | 4 (15%) | 2 (7.7%) |
| Starekova et al. [24] | 1 (0.7%) | 1 (0.7%) | 145 (100%) | 2 (1.4%) | 0 | 1 (0.7%) | 1 (0.7%) |
| Szabó et al. [25] | 6 (4.5%) | 4 (2.7%) | 147 (100%) | 3 (2%) | 4 (2.7%) | 3 (2%) | 1 (0.7%) |
| Vago et al. [26] | 0 | n/a | 12 (100%) | 0 | 0 | 0 | 0 |
| Van Hattum et al. [27] | 0 | 2 (1.6%) | 123 (100%) | 4 (3.3%) | 0 | 0 | 3 (2.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsampasian, V.; Androulakis, E.; Catumbela, R.; Gati, S.; Papadakis, M.; Vassiliou, V.S. Prevalence of Abnormal Cardiovascular Magnetic Resonance Findings in Athletes Recovered from COVID-19 Infection: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3290. https://doi.org/10.3390/jcm13113290
Tsampasian V, Androulakis E, Catumbela R, Gati S, Papadakis M, Vassiliou VS. Prevalence of Abnormal Cardiovascular Magnetic Resonance Findings in Athletes Recovered from COVID-19 Infection: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(11):3290. https://doi.org/10.3390/jcm13113290
Chicago/Turabian StyleTsampasian, Vasiliki, Emmanuel Androulakis, Ricardo Catumbela, Sabiha Gati, Michael Papadakis, and Vassilios S. Vassiliou. 2024. "Prevalence of Abnormal Cardiovascular Magnetic Resonance Findings in Athletes Recovered from COVID-19 Infection: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 11: 3290. https://doi.org/10.3390/jcm13113290
APA StyleTsampasian, V., Androulakis, E., Catumbela, R., Gati, S., Papadakis, M., & Vassiliou, V. S. (2024). Prevalence of Abnormal Cardiovascular Magnetic Resonance Findings in Athletes Recovered from COVID-19 Infection: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(11), 3290. https://doi.org/10.3390/jcm13113290







