Eccentric Muscle Strengthening Using Maximal Contractions Is Deleterious in Knee Osteoarthritis: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Interventions
2.4. Outcomes
2.5. Statistics
2.6. Ethics
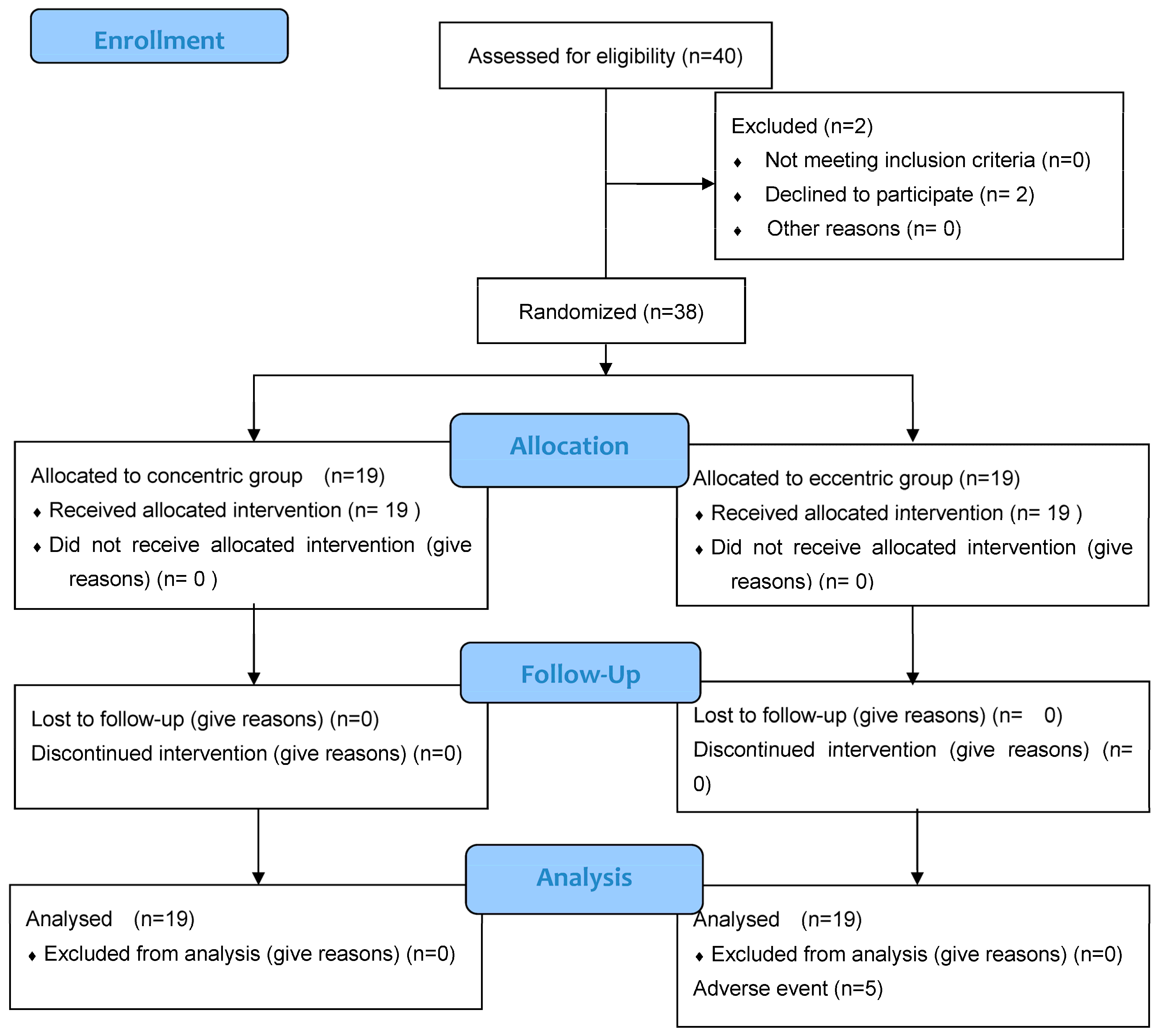
3. Results
3.1. Sample
3.2. Adverse Events and Adherence
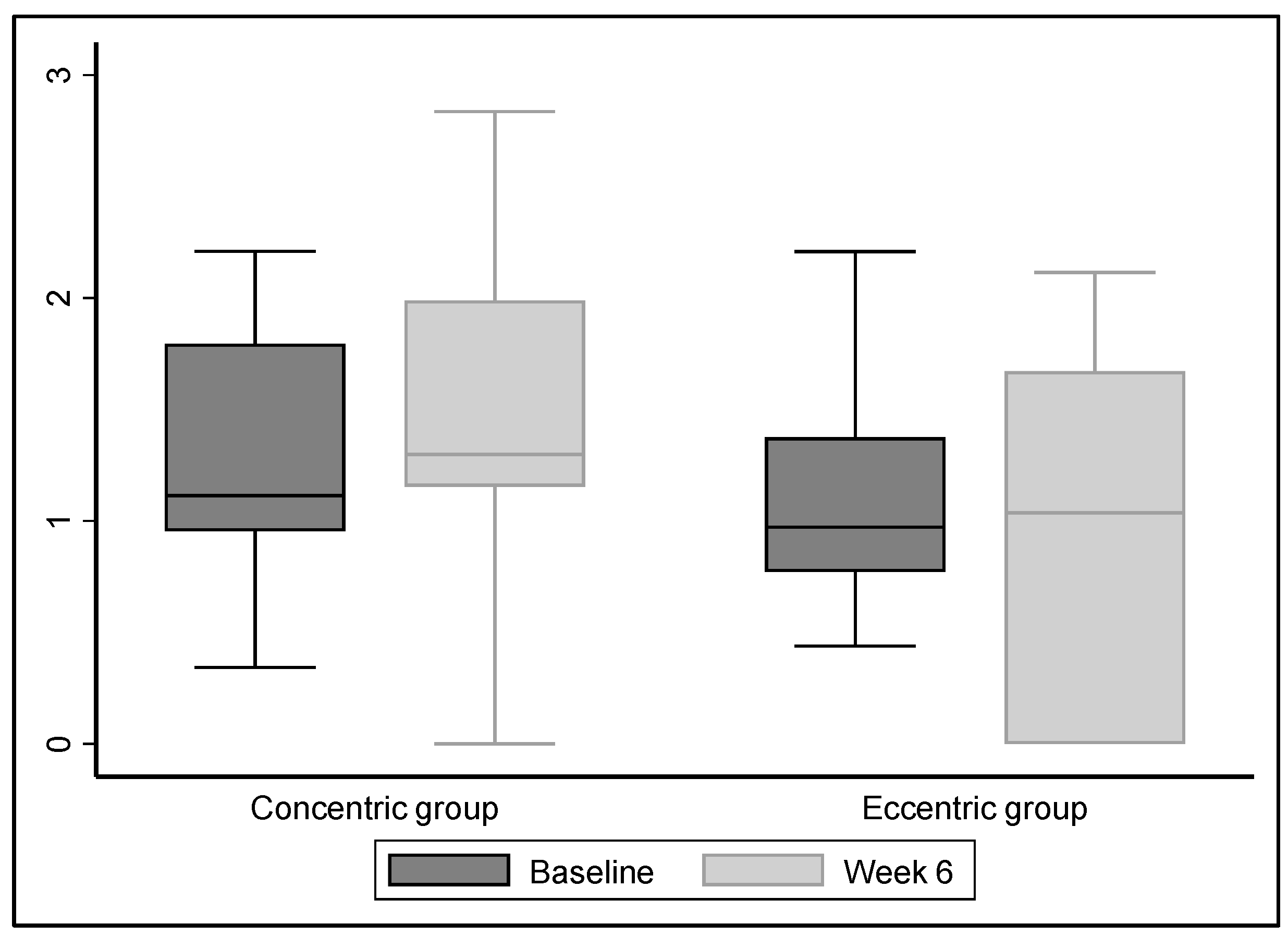
3.3. Primary Outcome
3.4. Secondary Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section/Topic Item | Checklist Item No. | CONSORT Item | Extension for NPT Trials |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomized trial in the title | p. 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | pp. 2–3 | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | p. 4 |
| 2b | Specific objectives or hypotheses | p. 5 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | p. 5 When applicable, how care providers were allocated to each trial group NA |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | NA | |
| Participants | 4a | Eligibility criteria for participants | p. 5 |
| 4b | Settings and locations where the data were collected | p. 5 | |
| Interventions † | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | Precise details of both the experimental treatment and comparator p 6 + protocol publication doi:10.1186/1745-6215-15-106 |
| 5a | Description of the different components of the interventions and, when applicable, description of the procedure for tailoring the interventions to individual participants p 6 + protocol publication doi: 10.1186/s12906-018-2339-x. | ||
| 5b | Details of whether and how the interventions were standardized p. 7 | ||
| 5c. | Details of whether and how adherence of care providers to the protocol was assessed or enhanced p. 7 | ||
| 5d | Details of whether and how adherence of participants to interventions was assessed or enhanced p. 7 adherence to physical exercise | ||
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | p. 7 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | NA | |
| Sample size | 7a | How sample size was determined | pp. 7–8 When applicable, details of whether and how the clustering by care providers or centers was addressed p. 7 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | p. 9 | |
| Randomization: | |||
| - Sequence generation | 8a | Method used to generate the random allocation sequence | p. 7 |
| 8b | Type of randomization; details of any restriction (such as blocking and block size) | p. 7 | |
| - Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | p. 9 |
| - Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | p. 9 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | p. 7 If done, who was blinded after assignment to interventions (e.g., participants, care providers, those administering co-interventions, those assessing outcomes) and how |
| 11b | If relevant, description of the similarity of interventions | p. 7 | |
| 11c | p. 7 | ||
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | p. 8 When applicable, details of whether and how the clustering by care providers or centers was addressed P 8 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | NA | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome | p. 9 The number of care providers or centers performing the intervention in each group and the number of patients treated by each care provider or in each center p. 17 and diagram flow |
| 13b | For each group, losses and exclusions after randomization, together with reasons | p. 9 and diagram flow | |
| 13c | For each group, the delay between randomization and the initiation of the intervention NA immediate allocation due to time period | ||
| new | Details of the experimental treatment and comparator as they were implemented NA | ||
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | p. 9 |
| 14b | Why the trial ended or was stopped | p. 10 | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | p. 18 When applicable, a description of care providers (case volume, qualification, expertise, etc.) and centers (volume) in each group NA |
| Numbers analyzed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | p. 9+ flow chart p. 17 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | ||
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | NA |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | p. 9 |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | pp. 10–11 In addition, take into account the choice of the comparator, lack of or partial blinding, and unequal expertise of care providers or centers in each group NA |
| Generalizability | 21 | Generalizability (external validity, applicability) of the trial findings | Generalizability (external validity) of the trial findings according to the intervention, comparators, patients, and care providers and centers involved in the trial p. 11 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | p. 12 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | p. 5 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | Protocol published doi:10.1186/1745-6215-15-106 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | p. 21 |
Appendix B

| Item Number | Item | Where Located ** | |
|---|---|---|---|
| Primary Paper (Page or Appendix Number) | Other † (Details) | ||
| BRIEF NAME | |||
| 1. | Provide the name or a phrase that describes the intervention. | Line 93 | ______________ |
| WHY | |||
| 2. | Describe any rationale, theory, or goal of the elements essential to the intervention. | Line 42 | _____________ |
| WHAT | |||
| 3. | Materials: Describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (e.g., online appendix, URL). | Line 36 | _____________ |
| 4. | Procedures: Describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities. | Line 96 | _____________ |
| WHO PROVIDED | |||
| 5. | For each category of intervention provider (e.g., psychologist, nursing assistant), describe their expertise, background and any specific training given. | Line 94 | _____________ |
| HOW | |||
| 6. | Describe the modes of delivery (e.g., face-to-face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group. | Line 94 | _____________ |
| WHERE | |||
| 7. | Describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features. | Line 94 | _____________ |
| WHEN and HOW MUCH | |||
| 8. | Describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity or dose. | Line 96 | _____________ |
| TAILORING | |||
| 9. | If the intervention was planned to be personalized, titrated or adapted, then describe what, why, when, and how. | NA | _____________ |
| MODIFICATIONS | |||
| 10.‡ | If the intervention was modified during the course of this study, describe the changes (what, why, when, and how). | NA | _____________ |
| HOW WELL | |||
| 11. | Planned: If intervention adherence or fidelity was assessed, describe how and by whom, and if any strategies were used to maintain or improve fidelity, describe them. | Line 122 | _____________ |
| 12.‡ | Actual: If intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned. | Line 122 | _____________ |
References
- Le Pen, C.; Reygrobellet, C.; Gérentes, I. Financial cost of osteoarthritis in France. Jt. Bone Spine 2005, 72, 567–570. [Google Scholar] [CrossRef]
- Guillemin, F.; Rat, A.; Mazieres, B.; Pouchot, J.; Fautrel, B.; Euller-Ziegler, L.; Fardellone, P.; Morvan, J.; Roux, C.; Verrouil, E.; et al. Prevalence of symptomatic hip and knee osteoarthritis: A two-phase population-based survey. Osteoarthr. Cartil. 2011, 19, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Fautrel, B.; Hilliquin, P.; Rozenberg, S.; Allaert, F.-A.; Coste, P.; Leclerc, A.; Rossignol, M. Impact of osteoarthritis: Results of a nationwide survey of 10,000 patients consulting for OA. Jt. Bone Spine 2004, 72, 235–240. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.V.; Newham, D.J. The influence of arthrogenous muscle inhibition on quadriceps réhabilitation of patients with early unilatéral osteoarthritic knees. Br. J. Rheumatol. 1993, 32, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Slemenda, C.; Heilman, D.K.; Brandt, K.D.; Katz, B.P.; Mazzuca, S.A.; Braunstein, E.M.; Byrd, D. Reduced quadriceps strength relative to body weight: A risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998, 41, 1951–1959. [Google Scholar] [CrossRef]
- O’reilly, S.C.; Jones, A.; Muir, K.R.; Doherty, M. Quadriceps weakness in knee osteoarthritis: The effect on pain and disability. Ann. Rheum. Dis. 1998, 57, 588–594. [Google Scholar] [CrossRef]
- Critères de Suivi en Rééducation et d’orientation en Ambulatoire ou en Soins de Suite ou de Réadaptation après Ligamentoplastie du Croisé Antérieur du Genou. Recommandation de Bonne Pratique. Haute Autorité de Santé. Avril 2008, Paris. (has-sante.fr). Available online: https://www.has-sante.fr/jcms/c_639105/fr/criteres-de-suivi-en-reeducation-et-d-orientation-en-ambulatoire-ou-en-soins-de-suite-ou-de-readaptation-apres-ligamentoplastie-du-croise-anterieur-du-genou (accessed on 1 December 2011).
- Huang, M.-H.; Lin, Y.-S.; Yang, R.-C.; Lee, C.-L. A comparison of various therapeutic exercises on the functional status of patients with knee osteoarthritis. Semin. Arthritis Rheum. 2003, 32, 398–406. [Google Scholar] [CrossRef]
- Tüzün, E.H.; Aytar, A.; Eker, L.; Daşkapan, A. Effectiveness of two physical therapy programmes in the treatment of knee osteoarthritis. Pain Clin. 2004, 16, 379–387. [Google Scholar] [CrossRef]
- Gür, H.; Çakın, N.; Akova, B.; Okay, E.; Küçükoğlu, S. Concentric versus combined concentric-eccentric isokinetic training: Effects on functional capacity and symptoms in patients with osteoarthrosis of the knee. Arch. Phys. Med. Rehabil. 2002, 83, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.T.; Stern, A.G.; Kinossian, B.; Cook, K.D.; Schumacher, H. Osteoarthritis of the knee: Isokinetic quadriceps exercise versus an educational intervention. Arch. Phys. Med. Rehabil. 1999, 80, 1293–1299. [Google Scholar] [CrossRef]
- Eyigor, S. A comparison of muscle training methods in patients with knee osteoarthritis. Clin. Rheumatol. 2004, 23, 109–115. [Google Scholar] [CrossRef]
- Elliott, J. Assessing muscle strength isokinetically. JAMA 1978, 240, 2408. [Google Scholar] [CrossRef] [PubMed]
- Anaes, Service d’évaluation des Technologies, Service Évaluation Économique: Les Appareils d’isocinétisme en Évaluation et en Rééducation Musculaire. Février 2001. (has-sante.fr). Available online: https://www.em-consulte.com/article/86609/l-evaluation-isocinetique-du-genou (accessed on 1 December 2011).
- Higbie, E.J.; Cureton, K.J.; Warren, G.L.; Prior, B.M. Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J. Appl. Physiol. 1996, 81, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Reich, T.E.; Urquhart, M.; Lindstedt, S.L.; Kilgas, M.A.; Elmer, S.J.; Marcus, R.; Dibble, L.; Frajacomo, F.; Vogt, M.; Hoppeler, H.H.; et al. Chronic eccentric exercise: Improvements in muscle strength can occur with little demand for oxygen. Am. J. Physiol. Integr. Comp. Physiol. 1999, 276, R611–R615. [Google Scholar] [CrossRef]
- Stanish, W.D.; Rubinovich, R.M.; Curwin, S. Eccentric Exercise in Chronic Tendinitis. Clin. Orthop. Relat. Res. 1986, 208, 65–68. [Google Scholar] [CrossRef]
- Vallejo, A.F.; Schroeder, E.T.; Zheng, L.; Jensky, N.E.; Sattler, F.R. Cardiopulmonary responses to eccentric and concentric resistance exercise in older adults. Age Ageing 2006, 35, 291–297. [Google Scholar] [CrossRef]
- Coudeyre, E.; Jegu, A.; Giustanini, M.; Marrel, J.; Edouard, P.; Pereira, B. Isokinetic muscle strengthening for knee osteoarthritis: A systematic review of randomized controlled trials with meta-analysis. Ann. Phys. Rehabil. Med. 2016, 59, 207–215. [Google Scholar] [CrossRef]
- Jegu, A.-G.; Pereira, B.; Andant, N.; Coudeyre, E. Effect of eccentric isokinetic strengthening in the rehabilitation of patients with knee osteoarthritis: Isogo, a randomized trial. Trials 2014, 15, 106. [Google Scholar] [CrossRef]
- Rodineau, J. Evaluation clinique des lésions musculaires récentes et essai de classification. Sport. Med. 1997, 73, 287–290. [Google Scholar]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537 Pt 2, 333–345. [Google Scholar] [CrossRef]
- McHugh, M.P. Recent advances in the understanding of the repeated bout effect: The protective effect against muscle damage from a single bout of eccentric exercise. Scand. J. Med. Sci. Sports 2003, 13, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; O‘Brien, K.; Kirk, G.; Murray, R.; McKinnon, P.; Shadgan, B.; Reid, W.D. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: A systematic review with meta-analysis. Br. J. Sports Med. 2008, 43, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.K.; Vanwanseele, B.; Singh, M.A.F. Strength training for treatment of osteoarthritis of the knee: A systematic review. Arthritis Care Res. 2008, 59, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Van Baar, M.E.; Dekker, J.; Oostendorp, R.A.; Bijl, D.; Voorn, T.; Lemmens, J.A.; Bijlsma, J.W. The effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: A randomized clinical trial. J. Rheumatol. 1998, 25, 2432–2439. [Google Scholar]
- Rochcongar, P. Évaluation isocinétique des extenseurs et fléchisseurs du genou en médecine du sport: Revue de la littérature. Ann. De Réadaptation Et De Médecine Phys. 2004, 47, 274–281. [Google Scholar] [CrossRef]
| Concentric Group (n = 19) | Eccentric Group (n = 19) | Total (N = 38) | |
|---|---|---|---|
| Sex (male) | 13 (68.4) | 14 (73.7) | 27 (71.1) |
| Age (years) | 55.9 ± 7.3 | 59.7 ± 7.6 | 57.8 ± 7.6 |
| BMI (kg/m2) | 25.2 ± 3.9 | 26.3 ± 3.9 | 25.8 ± 3.9 |
| Kellgren–Lauwrence score = 1 | 6 (31.6) | 5 (26.3) | 11 (29.0) |
| Kellgren–Lauwrence score = 2 + 3 | 11 (57.9) | 13 (68.4) | 24 (63.2) |
| Knee flexion (injured) Knee extension (injured) | 136.6 ± 6.5 −2.5 ± 3.5 | 132.6 ± 7.4 −3.8 ± 4.4 | 134.6 ± 7.1 −3.2 ± 4.0 |
| Knee flexion (healthy) Knee extension (healthy) | 140.8 ± 7.2 −0.5 ± 1.4 | 139.2 ± 5.2 −0.6 ± 2.3 | 140.0 ± 6.2 −0.6 ± 1.9 |
| VAS pain (OA) (/100) | 25.3 ± 17.1 | 35.5 ± 26.6 | 30.5 ± 22.8 |
| VAS pain (non-OA) (/100) | 12.9 ± 9.6 | 11.7 ± 19.2 | 12.3 ± 15.1 |
| PT OA knee (Nm/kg): | |||
| Quadriceps concentric | 1.29 ± 0.47 | 1.07 ± 0.44 | 1.18 ± 0.46 |
| Hamstring concentric | 0.84 ± 0.32 | 0.76 ± 0.29 | 0.80 ± 0.31 |
| Quadriceps excentric | 2.04 ± 0.66 | 1.81 ± 0.70 | 1.92 ± 0.69 |
| Hamstring excentric | 1.38 ± 0.49 | 1.29 ± 0.61 | 1.33 ± 0.54 |
| PT non-OA knee (Nm/kg): | |||
| Quadriceps concentric | 1.37± 0.44 | 1.22 ± 0.43 | 1.29 ± 0.44 |
| Hamstring concentric | 0.89 ± 0.25 | 0.82 ± 0.28 | 0.85 ± 0.26 |
| Quadriceps excentric | 2.22 ± 0.76 | 2.03 ± 0.77 | 2.12 ± 0.76 |
| Hamstring excentric | 1.29 ± 0.35 | 1.25 ± 0.50 | 1.27 ± 0.42 |
| WOMAC A pain (/20) | 5.2 ± 2.6 | 6.8 ± 3.6 | 6.0 ± 3.2 |
| WOMAC B disability (/68) | 13.7 ± 8.7 | 22.7 ± 13.1 | 18.2 ± 11.9 |
| WOMAC C stiffness (/8) | 2.7 ± 1.5 | 3.8 ± 1.9 | 3.3 ± 1.8 |
| 10 m walk speed (m/s) | 1.97 ± 0.19 | 1.90 ± 0.28 | 1.93 ± 0.24 |
| 200 m walk speed (s) | 112.9 ± 17.3 | 119.7 ± 15.8 | 116.3 ± 16.7 |
| Patient | Randomization | Events |
|---|---|---|
| #5 | concentric group | PREMATE STOP |
| #25 | concentric group | PREMATE STOP |
| #36 | concentric group | INJURY M6 |
| #8 | eccentric group | PREMATE STOP |
| #10 | eccentric group | INJURY W6 |
| #23 | eccentric group | INJURY W6 |
| #26 | eccentric group | INJURY W6 |
| #31 | eccentric group | INJURY W6 |
| #35 | eccentric group | INJURY W6 |
| Concentric Group | Eccentric Group | p-Value | |
|---|---|---|---|
| Concentric OA knee | |||
| Quadriceps | |||
| Baseline | 1.29 ± 0.47 | 1.07 ± 0.44 | |
| At W6 | 1.41 ± 0.77 | 0.94 ± 0.70 | |
| Change at W6 (primary endpoint) | 28 [−17 to 55] | 10 [−100 to 52] | 0.34 |
| At M6 | 1.26 ± 0.99 | 1.03 ± 0.83 | |
| Change at M6 | 23 [−99 to 74] | 16 [−100 to 74] | 0.74 |
| Hamstring | |||
| Baseline | 0.84 ± 0.32 | 0.76 ± 0.29 | |
| At W6 | 0.83 ± 0.42 | 0.59 ± 0.39 | |
| Change at W6 | 14 [−12 to 44] | −1 [−100 to 24] | 0.21 |
| At M6 | 0.62 ± 0.48 | 0.59 ± 0.48 | |
| Change at M6 | −13 [−99 to 36] | −3 [−100 to 29] | 0.96 |
| Ratio Hamstring/Quadriceps | |||
| Change at W6 | −10 [−26 to 3] | −13 [−100 to 0] | 0.42 |
| Change at M6 | −39 [−99 to −16] * | −47 [−100 to −11] * | 0.96 |
| Eccentric OA knee | |||
| Quadriceps | |||
| Baseline | 2.04 ± 0.66 | 1.81 ± 0.70 | |
| At W6 | 1.91 ± 1.04 | 1.41 ± 1.02 | |
| Change at W6 | 0 [−24 to 30] | 0 [−100 to 22] | 0.65 |
| At M6 | 1.44 ± 1.11 | 1.43 ± 1.14 | |
| Change at M6 | −12 [−99 to 6] * | −1 [−100 to 38] | 0.59 |
| Hamstring | |||
| Baseline | 1.38 ± 0.49 | 1.29 ± 0.61 | |
| At W6 | 1.12 ± 0.66 | 0.86 ± 0.68 | |
| Change at W6 | −17 [−45 to 0] | −13 [−100 to 13] * | 0.99 |
| At M6 | 0.74 ± 0.64 | 0.78 ± 0.68 | |
| Change at M6 | −40 [−99 to 16] * | −40 [−100 to 20] * | 0.85 |
| Ratio Hamstring/Quadriceps | |||
| Change at W6 | −8 [−39 to 2] | −20 [−100 to 0] | 0.39 |
| Change at M6 | −35 [−99 to 12] * | −32 [−100 to 0] * | 0.83 |
| Concentric–eccentric OA knee | |||
| Quadriceps | |||
| Change at W6 | 28 [−17 to 55] | 0 [−100 to 22] | 0.04 |
| Change at M6 | 23 [−99 to 74] | −1 [−100 to 38] | 0.22 |
| Hamstring | |||
| Change at W6 | 14 [−12 to 44] | −13 [−100 to 13] * | 0.04 |
| Change at M6 | −13 [−99 to 36] | −40 [−100 to 20] * | 0.42 |
| Ratio Hamstring/Quadriceps | |||
| Change at W6 | −10 [−26 to 3] | −20 [−100 to 0] | 0.24 |
| Change at M6 | −39 [−99 to −16] * | −32 [−100 to 0] * | 0.94 |
| Concentric Group | Eccentric Group | p-Value | |
|---|---|---|---|
| Flexion OA knee (°) | |||
| Baseline | 136.6 ± 6.5 | 132.6 ± 7.4 | |
| At W6 | 122.8 ± 43.8 | 99.0 ± 61.2 | |
| Change at W6 | 0 [−4 to 0.7] | 0 [−100 to 2] * | 0.79 |
| At M6 | 108.9 ± 58.0 | 98.2 ± 60.7 | |
| Change at M6 | 0 [−4 to 1] | 0 [−100 to 2] * | 0.54 |
| Extension OA knee (°) | |||
| Baseline | −2.5 ± 3.5 | −3.8 ± 4.3 | |
| At W6 | −2.3 ± 3.3 | −2.3 ± 3.7 | |
| Change at W6 | 0 [−71 to 0] | −42 [−100 to −13] * | 0.34 |
| At M6 | −2.0 ± 2.8 | −2.4 ± 3.8 | |
| Change at M6 | −21 [−85 to 0] | −75 [−100 to −33] | 0.28 |
| VAS OA knee (/100) | |||
| Baseline | 25.2 ± 17.1 | 35.5 ± 26.6 | |
| At W6 | 17.3 ± 14.5 | 21.8 ± 22.1 | |
| Change at W6 | −17 [−75 to 0] | −41 [−100 to −17] * | 0.31 |
| At M6 | 18.0 ± 16.1 | 17.5 ± 20.1 | |
| Change at M6 | −40 [−100 to 0] | −76 [−100 to −33] * | 0.44 |
| WOMAC A pain (/20) | |||
| Baseline | 5.2 ± 2.6 | 6.8 ± 3.6 | |
| At W6 | 4.2 ± 2.7 | 4.1 ± 4.0 | |
| Change at W6 | −10 [−50 to 0] | −29 [−100 to 0] * | 0.30 |
| At M6 | 3.9 ± 2.8 | 4.5 ± 4.3 | |
| Change at M6 | −12 [−50 to 0] * | −29 [−100 to 0] * | 0.43 |
| WOMAC B disability (/68) | |||
| Baseline | 13.7 ± 8.7 | 22.7 ± 13.1 | |
| At W6 | 12.3 ± 8.8 | 14.1 ± 15.2 | |
| Change at W6 | −8 [−29 to 6] | −38 [−100 to 0] * | 0.12 |
| At M6 | 13.5 ± 11.0 | 14.8 ± 15.1 | |
| Change at M6 | 0 [−22 to 25] | −20 [−100 to 0] * | 0.11 |
| WOMAC C stiffness (/8) | |||
| Baseline | 2.7 ± 1.5 | 3.8 ± 1.9 | |
| At W6 | 2.3 ± 1.3 | 2.3 ± 2.4 | |
| Change at W6 | −20 [−33 to 0] | −25 [−100 to 0] * | 0.35 |
| At M6 | 2.1 ± 1.4 | 2.5 ± 2.1 | |
| Change at M6 | −50 [−75 to 0] | −20 [−100 to 0] * | 0.87 |
| 10 m walk speed (m/s) | |||
| Baseline | 2.0 ± 0.2 | 1.9 ± 0.3 | |
| At W6 | 1.8 ± 0.7 | 1.4 ± 0.9 | |
| Change at W6 | 0 [−7 to 5] | −2 [−100 to 4] * | 0.32 |
| At M6 | 1.6 ± 0.8 | 1.4 ± 0.9 | |
| Change at M6 | 0 [−16 to 3] | −6 [−100 to 2] * | 0.54 |
| 200 m walk speed (s) | |||
| Baseline | 112.9 ± 17.3 | 119.7 ± 15.7 | |
| At W6 | 98.4 ± 37.4 | 81.9 ± 51.0 | |
| Change at W6 | −3 [−8 to 0] | −5 [−100 to −1] * | 0.56 |
| At M6 | 87.3 ± 47.7 | 86.0 ± 53.4 | |
| Change at M6 | −6 [−13 to −1] * | 0 [−100 to 7] * | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coudeyre, E.; Pereira, B.; Lechauve, J.-B.; Girold, S.; Richard, R.; Dobija, L.; Lanhers, C. Eccentric Muscle Strengthening Using Maximal Contractions Is Deleterious in Knee Osteoarthritis: A Randomized Clinical Trial. J. Clin. Med. 2024, 13, 3318. https://doi.org/10.3390/jcm13113318
Coudeyre E, Pereira B, Lechauve J-B, Girold S, Richard R, Dobija L, Lanhers C. Eccentric Muscle Strengthening Using Maximal Contractions Is Deleterious in Knee Osteoarthritis: A Randomized Clinical Trial. Journal of Clinical Medicine. 2024; 13(11):3318. https://doi.org/10.3390/jcm13113318
Chicago/Turabian StyleCoudeyre, Emmanuel, Bruno Pereira, Jean-Baptiste Lechauve, Sebastien Girold, Ruddy Richard, Lech Dobija, and Charlotte Lanhers. 2024. "Eccentric Muscle Strengthening Using Maximal Contractions Is Deleterious in Knee Osteoarthritis: A Randomized Clinical Trial" Journal of Clinical Medicine 13, no. 11: 3318. https://doi.org/10.3390/jcm13113318
APA StyleCoudeyre, E., Pereira, B., Lechauve, J.-B., Girold, S., Richard, R., Dobija, L., & Lanhers, C. (2024). Eccentric Muscle Strengthening Using Maximal Contractions Is Deleterious in Knee Osteoarthritis: A Randomized Clinical Trial. Journal of Clinical Medicine, 13(11), 3318. https://doi.org/10.3390/jcm13113318






