Carotid Arterial Compliance during Different Intensities of Submaximal Endurance Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Characteristics
2.2. Experimental Design
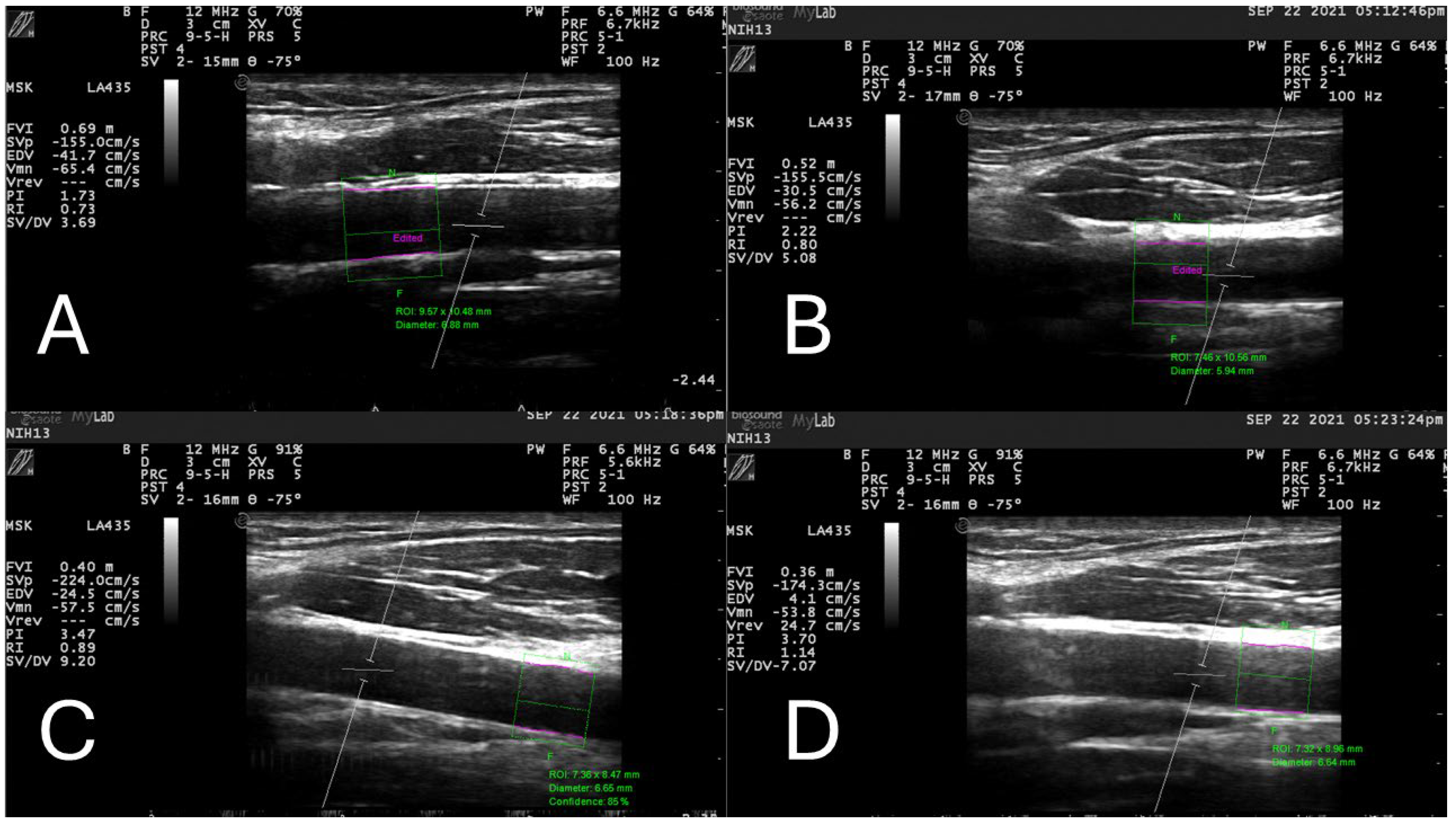
2.3. Common Carotid Arterial Compliance and Beta Stiffness
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urbich, M.; Globe, G.; Pantiri, K.; Heisen, M.; Bennison, C.; Wirtz, H.S.; Di Tanna, G.L. A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014–2020). Pharmacoeconomics 2020, 38, 1219–1236. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [PubMed]
- Gurovich, A.N.; Rodriguez, L.; Morales-Acuña, F. There are no differences in brachial artery endothelial shear stress and blood flow patterns between males and females during exercise. Clin. Physiol. Funct. Imaging 2021, 41, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tempel, D.; van Haperen, R.; van der Baan, A.; Grosveld, F.; Daemen, M.J.; Krams, R.; de Crom, R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 2006, 113, 2744–2753. [Google Scholar] [CrossRef]
- Davies, P.F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. 2009, 6, 16–26. [Google Scholar] [CrossRef]
- Chatzizisis, Y.S.; Jonas, M.; Coskun, A.U.; Beigel, R.; Stone, B.V.; Maynard, C.; Gerrity, R.G.; Daley, W.; Rogers, C.; Edelman, E.R.; et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: An intravascular ultrasound and histopathology natural history study. Circulation 2008, 117, 993–1002. [Google Scholar] [CrossRef]
- Gurovich, A.N.; Rodriguez, L.; Gomez, M.; Caraveo, P.; Ochoa, L.; Morales-Acuña, F. Imaging ultrasound assessment of exercise-induced endothelial shear stress of the brachial and carotid arteries. Cardiopulm. Phys. Ther. J. 2021, 32, 30–36. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Chatzizisis, Y.S.; Baker, A.B.; Edelman, E.R.; Stone, P.H.; Feldman, C.L. The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Curr. Opin. Cardiol. 2009, 24, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, D.; Xiao, Z. Pathophysiology and treatment of stroke: Present status and future perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.-Y. Pathophysiology of ischemic stroke. In Translational Research in Stroke; Springer: Berlin/Heidelberg, Germany, 2017; pp. 51–75. [Google Scholar]
- Shen, B.-Y.; Liu, H.-B.; Cao, L.; Qin, K.-R. Acute effects of different intensities of cycling acute exercise on carotid arterial apparent elasticity and hemodynamic variables. BioMed Res. Int. 2020, 2020, 9027560. [Google Scholar] [CrossRef]
- Liu, H.-B.; Yuan, W.-X.; Wang, Q.-Y.; Wang, Y.-X.; Cao, H.-W.; Xu, J.; Qin, K.-R. Carotid arterial stiffness and hemodynamic responses to acute cycling intervention at different times during 12-week supervised exercise training period. BioMed Res. Int. 2018, 2018, 2907548. [Google Scholar] [CrossRef]
- Tanaka, H.; Dinenno, F.A.; Monahan, K.D.; Clevenger, C.M.; DeSouza, C.A.; Seals, D.R. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000, 102, 1270–1275. [Google Scholar] [CrossRef]
- DeVan, A.E.; Anton, M.M.; Cook, J.N.; Neidre, D.B.; Cortez-Cooper, M.Y.; Tanaka, H. Acute effects of resistance exercise on arterial compliance. J. Appl. Physiol. 2005, 98, 2287–2291. [Google Scholar] [CrossRef]
- Beck, D.T.; Martin, J.S.; Casey, D.P.; Braith, R.W. Exercise training improves endothelial function in resistance arteries of young prehypertensives. J. Hum. Hypertens. 2014, 28, 303–309. [Google Scholar] [CrossRef]
- Ogoh, S. Acute and Chronic Effect of Physiological Factors on Arterial Stiffness. J. Clin. Med. 2023, 12, 3044. [Google Scholar] [CrossRef]
- Liu, H.; Shen, B.; Li, Z.; Xue, C.; Zhao, H.; Pan, X.; Xu, D. Effects of accumulated exercise on the stiffness and hemodynamics of the common carotid artery. Front. Physiol. 2024, 15, 1348811. [Google Scholar] [CrossRef]
- Adkisson, E.J.; Casey, D.P.; Beck, D.T.; Gurovich, A.N.; Martin, J.S.; Braith, R.W. Central, peripheral and resistance arterial reactivity: Fluctuates during the phases of the menstrual cycle. Exp. Biol. Med. 2010, 235, 111–118. [Google Scholar] [CrossRef]
- Rascon, J.; Trujillo, E.; Morales-Acuña, F.; Gurovich, A.N. Differences between males and females in determining exercise intensity. Int. J. Exerc. Sci. 2020, 13, 1305. [Google Scholar]
- Cortez-Cooper, M.Y.; Anton, M.M.; Devan, A.E.; Neidre, D.B.; Cook, J.N.; Tanaka, H. The effects of strength training on central arterial compliance in middle-aged and older adults. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 149–155. [Google Scholar] [CrossRef]
- Hirai, T.; Sasayama, S.; Kawasaki, T.; Yagi, S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation 1989, 80, 78–86. [Google Scholar] [CrossRef]
- RStudio. RStudio: Integrated Development for R. Available online: http://www.rstudio.com/ (accessed on 13 September 2023).
- Costa, E.C.; Boreskie, K.F.; Scott Kehler, D.; Kent, D.E.; Hay, J.L.; Arora, R.C.; Browne, R.A.V.; Duhamel, T.A. Immediate post-exercise blood pressure and arterial compliance in middle-aged and older normotensive females: A cross-sectional study. Sci. Rep. 2020, 10, 9205. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Kent, D.E.; Boreskie, K.F.; Hay, J.L.; Kehler, D.S.; Edye-Mazowita, A.; Nugent, K.; Papadopoulos, J.; Stammers, A.N.; Oldfield, C.; et al. Acute Effect of High-Intensity Interval Versus Moderate-Intensity Continuous Exercise on Blood Pressure and Arterial Compliance in Middle-Aged and Older Hypertensive Women with Increased Arterial Stiffness. J. Strength Cond. Res. 2020, 34, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamaguchi, H.; Amaoka, H.; Takahara, T.; Kunisa, S.; Tamai, N.; Maejima, N.; Watanabe, N.; Kobayashi, Y.; Tanaka, H. Equol-producing status affects exercise training-induced improvement in arterial compliance in postmenopausal women. J. Appl. Physiol. 2021, 130, 827–835. [Google Scholar] [CrossRef]
- Tomoto, T.; Le, T.; Tarumi, T.; Dieppa, M.; Bell, K.; Madden, C.; Zhang, R.; Ding, K. Carotid Arterial Compliance and Aerobic Exercise Training in Chronic Traumatic Brain Injury: A Pilot Study. J. Head Trauma Rehabil. 2022, 37, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Nickel, K.J.; Acree, L.S.; Gardner, A.W. Effects of a Single Bout of Exercise on Arterial Compliance in Older Adults. Angiology 2011, 62, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Jamnick, N.A.; Pettitt, R.W.; Granata, C.; Pyne, D.B.; Bishop, D.J. An Examination and Critique of Current Methods to Determine Exercise Intensity. Sports Med. 2020, 50, 1729–1756. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of Increased Blood Flow (Hyperemia) to Muscles During Exercise: A Hierarchy of Competing Physiological Needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef]
- Fadel, P.J.; Wang, Z.; Watanabe, H.; Arbique, D.; Vongpatanasin, W.; Thomas, G.D. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J. Physiol. 2004, 561, 893–901. [Google Scholar] [CrossRef]
- Vongpatanasin, W.; Wang, Z.; Arbique, D.; Arbique, G.; Adams-Huet, B.; Mitchell, J.H.; Victor, R.G.; Thomas, G.D. Functional sympatholysis is impaired in hypertensive humans. J. Physiol. 2011, 589, 1209–1220. [Google Scholar] [CrossRef]
- Pierce, D.R.; Doma, K.; Leicht, A.S. Acute Effects of Exercise Mode on Arterial Stiffness and Wave Reflection in Healthy Young Adults: A Systematic Review and Meta-Analysis. Front. Physiol. 2018, 9, 73. [Google Scholar] [CrossRef]
- Saz-Lara, A.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Notario-Pacheco, B.; Ruiz-Grao, M.C.; Martínez-Vizcaíno, V. The Acute Effect of Exercise on Arterial Stiffness in Healthy Subjects: A Meta-Analysis. J. Clin. Med. 2021, 10, 291. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 20) | Female (n = 10) | Male (n = 10) | p | |
|---|---|---|---|---|
| Age (years) Mean (SD) | 26.9 (3.4) | 26.3 (2.4) | 27.4 (4.2) | 0.48 |
| Height (m) Mean (SD) | 1.68 (0.10) | 1.62 (0.06) | 1.73 (0.10) | 0.01 |
| Weight (kg) Mean (SD) | 68.9 (14.3) | 62.2 (10.0) | 75.7 (15.1) | 0.03 |
| BMI (kg/m2) Mean (SD) | 24.5 (4.0) | 23.6 (3.7) | 25.3 (4.3) | 0.38 |
| Resting systolic BP (mmHg) Mean (SD) | 113 (9) | 109 (9) | 117 (8) | 0.03 |
| Resting diastolic BP (mmHg) Mean (SD) | 73 (14) | 71 (11) | 76 (17) | 0.41 |
| VO2max (mL/kg/min) Mean (SD) | 27.6 (7.8) | 28.1 (8.0) | 26.9 (8.0) | 0.76 |
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n = 10) | Low (n = 10) | Moderate (n = 10) | High (n = 10) | Baseline (n = 10) | Low (n = 10) | Moderate (n = 10) | High (n = 10) | |
| Workload (watts) Mean (SD) | 0.0 (0.0) | 53.5 (17.0) | 98.0 (34.7) | 124.0 (38.4) *** | 0.0 (0.0) | 75.5 (39.0) | 119.5 (42.7) | 146.3 (42.8) *** |
| VO2 (ml/kg/min) Mean (SD) | 4.16 (4.12) | 15.83 (5.76) | 20.76 (6.07) | 26.93 (8.65) *** | 3.57 (2.38) | 12.85 (5.83) | 20.27 (6.15) | 23.62 (7.41) *** |
| Lactate (mmol/L) Mean (SD) | 1.00 (0.39) | 1.95 (0.87) | 3.14 (0.89) | 5.15 (1.91) *** | 0.90 (0.44) | 1.65 (0.53) | 3.37 (1.40) | 5.37 (2.26) *** |
| Heart rate (bpm) Mean (SD) | 77 (18) | 119 (15) | 143 (24) | 156 (11) * | 77 (16) | 104 (25) | 132 (26) | 144 (33) ** |
| RPE Mean (SD) | 6.1 (0.3) | 9.0 (2.1) | 12.2 (1.4) | 14.9 (3.7) *** | 5.4 (1.9) | 7.5 (1.2) | 10.9 (1.8) | 13.9 (1.9) *** |
| Car. Sys. pressure (mm Hg) Mean (SD) | 114 (14) | 141 (20) | 145 (18) | 153 (15) ** | 119 (11) | 138 (16) | 151 (23) | 159 (22) *** |
| Car. Dias. pressure (mm Hg) Mean (SD) | 77 (10) † | 83 (11) | 88 (11) | 88 (11) | 80 (6) †† | 83 (3) | 88 (9) | 88 (7) |
| Diameter dystolic (mm) Mean (SD) | 6.19 (1.15) | 5.90 (0.91) | 6.07 (1.02) | 6.05 (1.06) | 6.62 (0.92) | 6.56 (0.62) | 6.70 (0.80) | 6.74 (0.62) |
| Diameter diastolic (mm) Mean (SD) | 5.85 (1.05) ‡ | 5.48 (0.82) | 5.56 (0.76) | 5.69 (1.08) | 6.21 (0.95) | 6.20 (0.95) | 6.36 (0.76) | 6.25 (0.63) |
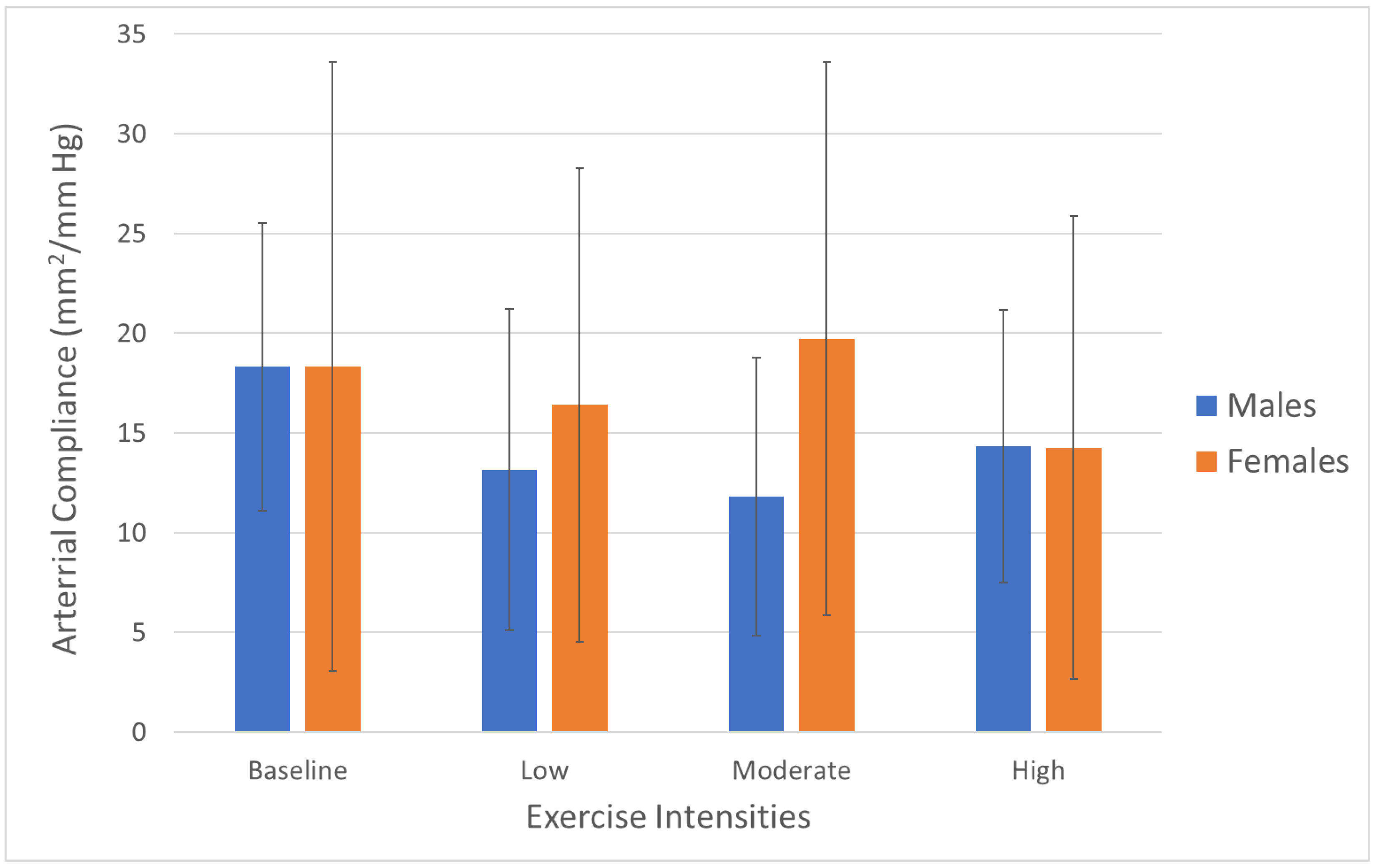
| Compliance (mm2/mm Hg) Mean (SD) | 18.34 (15.27) | 16.41 (11.88) | 19.72 (13.87) | 14.26 (11.59) | 18.31 (7.22) | 13.16 (8.07) | 11.79 (6.96) | 14.34 (6.84) |
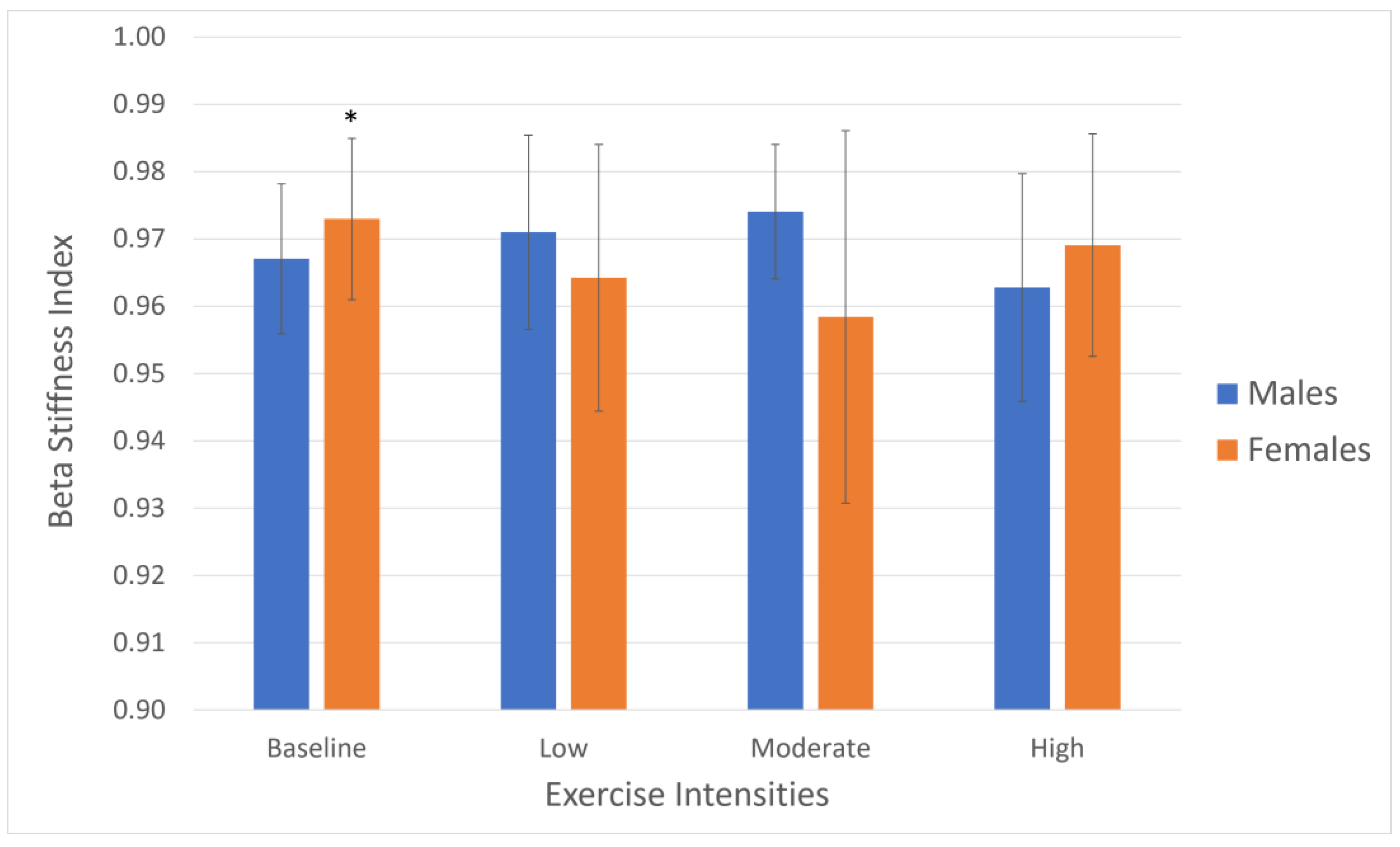
| Beta stiffness (U) Mean (SD) | 0.972 (0.012) ‡‡ | 0.964 (0.020) | 0.958 (0.028) | 0.969 (0.017) | 0.967 (0.011) | 0.971 (0.014) | 0.974 (0.010) | 0.963 (0.017) |
| Baseline (n = 20) | Low (n = 20) | Moderate (n = 20) | High (n = 20) | |
|---|---|---|---|---|
| Workload (watts) | 0.0 | 64.5 | 108.8 | 135.1 |
| Mean (SD) | (0.0) | (31.4) | (39.4) | (41.2) *** |
| VO2 (mL/kg/min) | 3.83 | 14.17 | 20.49 | 25.09 |
| Mean (SD) | (3.18) | (5.83) | (5.94) | (7.92) *** |
| Lactate (mmol/L) | 0.95 | 1.80 | 3.26 | 5.26 |
| Mean (SD) | (0.41) | (0.72) | (1.15) | (2.04) *** |
| Heart rate (bpm) | 77 | 109 | 136 | 149 |
| Mean (SD) | (16) | (22) | (25) | (27) *** |
| RPE | 5.8 | 8.3 | 11.6 | 14.4 |
| Mean (SD) | (1.4) | (1.8) | (1.7) | (2.9) *** |
| Carotid systolic pressure (mm Hg) | 117 | 139 | 148 | 156 |
| Mean (SD) | (13) | (18) | (20) | (18) *** |
| Carotid diastolic pressure (mm Hg) | 78 | 83 | 88 | 88 |
| Mean (SD) | (8) * | (8) | (10) | (9) |
| Diameter systolic (mm) | 6.41 | 6.23 | 6.39 | 6.39 |
| Mean (SD) | (1.04) | (0.83) | (0.95) | (0.92) ** |
| Diameter diastolic (mm) | 6.03 | 5.84 | 5.96 | 5.97 |
| Mean (SD) | (0.99) | (0.82) | (0.84) | (0.91) |
| Compliance (mm2/mm Hg) | 18.33 | 14.78 | 15.76 | 14.30 |
| Mean (SD) | (11.62) | (10.03) | (11.43) | (9.26) |
| Beta stiffness (U) | 0.970 | 0.968 | 0.966 | 0.966 |
| Mean (SD) | (0.012) | (0.017) | (0.022) | (0.017) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurovich, A.N.; Montalvo, S.; Hassan, P.F.; Gomez, M. Carotid Arterial Compliance during Different Intensities of Submaximal Endurance Exercise. J. Clin. Med. 2024, 13, 3316. https://doi.org/10.3390/jcm13113316
Gurovich AN, Montalvo S, Hassan PF, Gomez M. Carotid Arterial Compliance during Different Intensities of Submaximal Endurance Exercise. Journal of Clinical Medicine. 2024; 13(11):3316. https://doi.org/10.3390/jcm13113316
Chicago/Turabian StyleGurovich, Alvaro N., Samuel Montalvo, Progga F. Hassan, and Manuel Gomez. 2024. "Carotid Arterial Compliance during Different Intensities of Submaximal Endurance Exercise" Journal of Clinical Medicine 13, no. 11: 3316. https://doi.org/10.3390/jcm13113316
APA StyleGurovich, A. N., Montalvo, S., Hassan, P. F., & Gomez, M. (2024). Carotid Arterial Compliance during Different Intensities of Submaximal Endurance Exercise. Journal of Clinical Medicine, 13(11), 3316. https://doi.org/10.3390/jcm13113316








