Care Bundles to Improve Hemoperfusion Performance in Patients with Severe COVID-19: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion
2.3. Definitions of Severe COVID-19 Pneumonia
2.4. Data Collection
2.5. Study Outcomes
2.6. Standard of Care for Severe COVID-19 Patients
2.7. Hemoperfusion Setting and Prescription
2.8. Care Bundles for Hemoperfusion
2.9. Statistical Analysis
3. Results
3.1. Demographics, Clinical Features, Treatment, and Outcomes
3.2. HP-Related Information and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Novel Coronavirus (2019-nCoV): Situation Report, 1; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 21 January 2021).
- Hu, M.; Zhou, Q.; Zheng, R.; Li, X.; Ling, J.; Chen, Y.; Jia, J.; Xie, C. Application of high-flow nasal cannula in hypoxemic patients with COVID-19: A retrospective cohort study. BMC Pulm. Med. 2020, 20, 324. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Richards-Belle, A.; Orzechowska, I.; Gould, D.W.; Thomas, K.; Doidge, J.C.; Mouncey, P.R.; Christian, M.D.; Shankar-Hari, M.; Harrison, D.A.; Rowan, K.M.; et al. COVID-19 in critical care: Epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020, 46, 2035–2047. [Google Scholar] [CrossRef] [PubMed]
- Kusirisin, P.; Noppakun, K.; Trongtrakul, K.; Vongsanim, S.; Suteeka, Y.; Ophascharoensuk, V.; Pongsuwan, K.; Narongkiatikhun, P.; Theerakittikul, T.; Apaijai, N.; et al. Efficacy of the Cytokine Adsorption Therapy in Patients with Severe COVID-19-Associated Pneumonia: Lesson Learned from a Prospective Observational Study. Blood Purif. 2023, 53, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.G.; Hiremath, S.; McIntyre, L.; Wald, R.; Hundemer, G.L.; Joannidis, M. Haemoperfusion should only be used for COVID-19 in the context of randomized trials. Nat. Rev. Nephrol. 2020, 16, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Ranjbar, K.; Moghadami, M.; Mirahmadizadeh, A.; Fallahi, M.J.; Khaloo, V.; Shahriarirad, R.; Erfani, A.; Khodamoradi, Z.; Gholampoor Saadi, M.H. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: A triple-blinded randomized controlled trial. BMC Infect. Dis. 2021, 21, 337. [Google Scholar] [CrossRef]
- Zeraatkar, D.; Cusano, E.; Martinez, J.P.D.; Qasim, A.; Mangala, S.; Kum, E.; Bartoszko, J.J.; Devji, T.; Agoritsas, T.; Guyatt, G.; et al. Use of tocilizumab and sarilumab alone or in combination with corticosteroids for COVID-19: Systematic review and network meta-analysis. BMJ Med. 2022, 1, e000036. [Google Scholar] [CrossRef] [PubMed]
- Cherian, J.J.; Eerike, M.; Bagepally, B.S.; Das, S.; Panda, S. Efficacy and safety of baricitinib and tocilizumab in hospitalized patients with COVID-19: A comparison using systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1004308. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili Vardanjani, A.; Ronco, C.; Rafiei, H.; Golitaleb, M.; Pishvaei, M.H.; Mohammadi, M. Early Hemoperfusion for Cytokine Removal May Contribute to Prevention of Intubation in Patients Infected with COVID-19. Blood Purif. 2021, 50, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R. Hemoperfusion: Technical aspects and state of the art. Crit. Care 2022, 26, 135. [Google Scholar] [CrossRef] [PubMed]
- Surasit, K.; Srisawat, N. The Efficacy of Early Additional Hemoperfusion Therapy for Severe COVID-19 Patients: A Prospective Cohort Study. Blood Purif. 2022, 51, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Taba, S.M.M.; Hasibi Taheri, S.; Loghman, A.H.; Shayestehpour, M. The effect of hemoperfusion on the outcome, clinical and laboratory findings of patients with severe COVID-19: A retrospective study. New Microbes New Infect. 2021, 44, 100937. [Google Scholar] [CrossRef] [PubMed]
- Alavi Darazam, I.; Kazempour, M.; Pourhoseingholi, M.A.; Hatami, F.; Rabiei, M.M.; Javandoust Gharehbagh, F.; Amirdosara, M.; Hajiesmaeili, M.; Shabani, M.; Shokouhi, S.; et al. Efficacy of Hemoperfusion in Severe and Critical Cases of COVID-19. Blood Purif. 2023, 52, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Shadvar, K.; Tagizadiyeh, A.; Gamari, A.A.; Soleimanpour, H.; Mahmoodpoor, A. Hemoperfusion as a Potential Treatment for Critically Ill COVID-19 Patients with Cytokine Storm. Blood Purif. 2020, 50, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Asgharpour, M.; Mehdinezhad, H.; Bayani, M.; Zavareh, M.S.H.; Hamidi, S.H.; Akbari, R.; Ghadimi, R.; Bijani, A.; Mouodi, S. Effectiveness of extracorporeal blood purification (hemoadsorption) in patients with severe coronavirus disease 2019 (COVID-19). BMC Nephrol. 2020, 21, 356. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.Y.; Li, L.; Zhao, X.; Ding, F.; Hou, X.; Peng, Z. Hemoperfusion with CytoSorb(R) in Critically Ill COVID-19 Patients. Blood Purif. 2022, 51, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Rampino, T.; Gregorini, M.; Perotti, L.; Ferrari, F.; Pattonieri, E.F.; Grignano, M.A.; Valente, M.; Garrone, A.; Islam, T.; Libetta, C.; et al. Hemoperfusion with CytoSorb as Adjuvant Therapy in Critically Ill Patients with SARS-CoV-2 Pneumonia. Blood Purif. 2021, 50, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Supady, A.; Weber, E.; Rieder, M.; Lother, A.; Niklaus, T.; Zahn, T.; Frech, F.; Müller, S.; Kuhl, M.; Benk, C.; et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): A single centre, open-label, randomised, controlled trial. Lancet Respir. Med. 2021, 9, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Ugurov, P.; Popevski, D.; Gramosli, T.; Neziri, D.; Vuckova, D.; Gjorgon, M.; Stoicovski, E.; Marinkovic, S.; Veljanovska-Kiridjievska, L.; Ignevska, K.; et al. Early Initiation of Extracorporeal Blood Purification Using the AN69ST (oXiris(®)) Hemofilter as a Treatment Modality for COVID-19 Patients: A Single-Centre Case Series. Braz. J. Cardiovasc. Surg. 2022, 37, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Rosalia, R.A.; Ugurov, P.; Neziri, D.; Despotovska, S.; Kostoska, E.; Veljanovska-Kiridjievska, L.; Kuzmanov, D.; Trifunovski, A.; Popevski, D.; Villa, G.; et al. Extracorporeal Blood Purification in Moderate and Severe COVID-19 Patients: A Prospective Cohort Study. Blood Purif. 2022, 51, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, A.; Singh, B.; Vela, M.; Chaudhary, S.; Monaghan, M.; Wilson, G.A.; Dillon, J.J.; Cartin-Ceba, R.; Lieske, J.C.; Gajic, O.; et al. Incidence of Adverse Events during Continuous Renal Replacement Therapy. Blood Purif. 2015, 39, 333–339. [Google Scholar] [CrossRef]
- Morsch, C.M.F.; Haas, J.S.; Plotnick, R.; Cavalcanti, T.C.; Cardoso, P.C.; Pilger, T.; da Silveira, J.T.; Thomé, F.S. Hypothermia related to continuous renal replacement therapy: Incidence and associated factors. Rev. Bras. Ter. Intensiv. 2021, 33, 111–118. [Google Scholar] [CrossRef]
| Adverse Events | Definition | Care Bundles |
|---|---|---|
| Shivering | The presence of body shaking or teeth chattering | Give paracetamol, opioids, or sedative Check for membrane allergy Provide warm blanket if hypothermia |
| Cardiac arrhythmia | New onset of atrial fibrillation/flutter, premature ventilation, among others | Ask about chest pain Check volume status Check electrolytes Reduce blood flow rate Give anti-arrhythmic drugs |
| Hypotension | Mean arterial pressure ≤ 65 mmHg | Decrease preset temp to 36.5 °C Check patient volume status Fluid challenge, if indicated Reduce blood flow rate |
| Hypertension | Blood pressure > 140/90 mmHg | Manage pain/agitation Give anti-hypertensive drugs |
| Hypothermia | Body temperature < 36.0 °C | Check patient temp before HP Increase HP preset temp Provide warm blanket |
| Cartridge clotting | High membrane pressure without the possibility of returning blood to the patient, with evidence of clot at the cartridge | Check vascular access flow before initiating HP Illuminate clot with spotlight Check access and return pressure alarm Forceful return of blood to patient, if possible |
| Circuit shattering | Circuit shaking during HP | Check vascular access Check patient volume status Fluid challenge |
| Variables | All Cases (n = 27) | Phase I (n = 9) | Phase II (n = 18) | p-Value |
|---|---|---|---|---|
| Age (yrs) | 61 (52, 67) | 63 (53, 67) | 58 (52, 67) | 0.81 |
| Female, n (%) | 10 (37) | 5 (56) | 5 (28) | 0.22 |
| Body weight (kg) | 70 (56, 83) | 64 (56, 80) | 75 (56, 83) | 0.57 |
| Height (cm) | 160 (155, 170) | 165 (156, 166) | 159 (155, 170) | 0.62 |
| Body mass index (kg/m2) | 25.4 (21.4, 31.1) | 23.5 (21.4, 28.1) | 27.2 (22.9, 31.1) | 0.46 |
| Diabetes mellitus | 10 (37) | 3 (33) | 7 (39) | 0.56 |
| Hypertension | 16 (59) | 7 (78) | 9 (50) | 0.17 |
| Dyslipidemia | 4 (15) | 2 (22) | 2 (11) | 0.41 |
| Chronic obstructive pulmonary disease | 4 (15) | 1 (11) | 3 (17) | 0.59 |
| Others | 9 (33) | 3 (33) | 6 (33) | 0.66 |
| Body temperature (°C) | 36.4 (36.0, 37.0) | 36.0 (35.6, 36.4) | 36.7 (36.4, 37.2) | <0.001 |
| Heart rate (beats/min) | 76 (62, 88) | 62 (52, 76) | 80 (72, 88) | 0.044 |
| Mean arterial pressure (mmHg) | 89 (80, 103) | 91 (83, 104) | 88 (77, 102) | 0.32 |
| Respiratory rate (breaths/min) | 24 (22, 28) | 28 (24, 28) | 24 (21, 28) | 0.50 |
| Pulse oximetry/fractional inspire oxygen | 188 (115, 235) | 190 (115, 235) | 180 (119, 228) | 0.97 |
| National Early Warning Score 2 | 8 (7, 10) | 10 (7, 10) | 7 (6, 10) | 0.24 |
| Absolute lymphocyte count 103 (/mm3) | 677 (484, 1220) | 694 (500, 1173) | 636 (384, 1220) | 0.60 |
| D-dimer (ng/mL) | 556 (423, 2526) | 740 (481, 1734) | 511 (376, 2526) | 0.67 |
| C-reactive protein (mg/L) | 57 (39, 92) | 88 (53, 134) | 57 (16, 80) | 0.15 |
| Interleukin-6 (pg/mL) * | 75 (29, 109) | 79 (39, 86) | 68 (29, 109) | 0.93 |
| Favipiravir, n (%) | 23 (85) | 7 (78) | 16 (89) | 0.41 |
| Remdesivir, n (%) | 26 (96) | 8 (89) | 18 (100) | 0.33 |
| Systemic corticosteroid (days) | 11 (6, 15) | 6 (5, 8) | 12 (9, 16) | 0.06 |
| Tocilizumab, n (%) | 9 (33) | 1 (11) | 8 (44) | 0.09 |
| Vasopressor, n (%) | 14 (52) | 6 (67) | 8 (44) | 0.25 |
| Prone position, n (%) | 17 (63) | 5 (56) | 12 (67) | 0.44 |
| High-flow nasal cannula, n (%) | 22 (81) | 7 (78) | 15 (83) | 0.55 |
| Non-invasive ventilation, n (%) | 9 (33) | 0 (0, 0) | 9 (50) | 0.10 |
| Mechanical ventilation, n (%) | 22 (81) | 7 (78) | 15 (83) | 0.55 |
| Outcomes | ||||
| Intensive care unit mortality | 8 (30) | 3 (33) | 5 (28) | 0.55 |
| Hospital mortality | 9 (33) | 3 (33) | 6 (33) | 0.66 |
| Intensive care unit length of stay (days) | 12 (7, 19) | 10 (7, 14) | 15 (8, 23) | 0.22 |
| Hospital length of stay (days) | 13 (10, 23) | 12 (9, 16) | 15 (11, 24) | 0.34 |
| HP Characteristics | All Cases (n = 27) | Phase I (n = 9) | Phase II (n = 18) | p-Value |
|---|---|---|---|---|
| 1st HP initiation from admission (days) | 2 (1, 5) | 1 (1, 2) | 3 (2, 6) | 0.08 |
| Total HP sessions (sessions) | 60 | 21 | 39 | n/a |
| Median HP sessions (sessions/case) | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) | 0.78 |
| No. of HP sessions/case, n (%) | 0.72 | |||
| 1 | 6 (22) | 2 (22) | 4 (22) | |
| 2 | 10 (37) | 3 (33) | 7 (39) | |
| 3 | 10 (37) | 3 (33) | 7 (39) | |
| 4 | 1 (4) | 1 (11) | 0 (0) | |
| Total duration of HP operation (min) | 480 (420, 720) | 480 (420, 720) | 480 (480, 720) | 0.91 |
| HP success rate per case, n (%) | 22/27 (81) | 6/9 (67) | 16/18 (89) | 0.19 |
| HP success rate per session, n (%) | 54/60 (90) | 17/21 (81) | 37/39 (95) | 0.11 |
| Total adverse events during HP, n | 49 | 26 | 23 | n/a |
| Median adverse events (events/case) | 1 (0, 3) | 3 (1, 4) | 1 (0, 2) | 0.014 |
| Number of adverse events/cases, n (%) | 0.039 | |||
| 0 | 7 (26) | 0 (0) | 7 (39) | |
| 1–2 | 10 (37) | 3 (33) | 7 (39) | |
| 3–6 | 10 (37) | 6 (67) | 4 (22) | |
| Adverse events, n (%) | ||||
| Shivering | 3 (11) | 2 (22) | 1 (6) | 0.25 |
| Cardiac arrhythmia | 5 (19) | 3 (33) | 2 (11) | 0.19 |
| Hypotension | 7 (26) | 4 (44) | 3 (17) | 0.14 |
| Hypertension | 4 (15) | 2 (22) | 2 (22) | 0.41 |
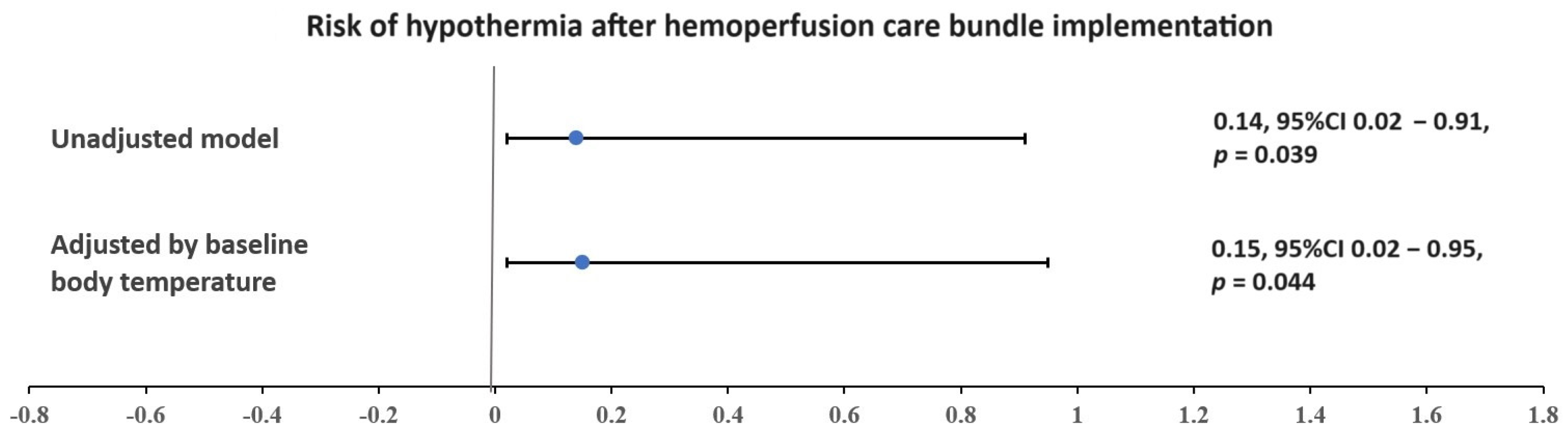
| Hypothermia | 13 (48) | 7 (78) | 6 (33) | 0.037 |
| Cartridge clotting | 5 (19) | 1 (11) | 4 (22) | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueankwan, S.; Trongtrakul, K.; Tajarernmuang, P.; Niyatiwatchanchai, N.; Kusirisin, P.; Narongkiatikhun, P. Care Bundles to Improve Hemoperfusion Performance in Patients with Severe COVID-19: A Retrospective Study. J. Clin. Med. 2024, 13, 3360. https://doi.org/10.3390/jcm13123360
Mueankwan S, Trongtrakul K, Tajarernmuang P, Niyatiwatchanchai N, Kusirisin P, Narongkiatikhun P. Care Bundles to Improve Hemoperfusion Performance in Patients with Severe COVID-19: A Retrospective Study. Journal of Clinical Medicine. 2024; 13(12):3360. https://doi.org/10.3390/jcm13123360
Chicago/Turabian StyleMueankwan, Sirirat, Konlawij Trongtrakul, Pattraporn Tajarernmuang, Nutchanok Niyatiwatchanchai, Prit Kusirisin, and Phoom Narongkiatikhun. 2024. "Care Bundles to Improve Hemoperfusion Performance in Patients with Severe COVID-19: A Retrospective Study" Journal of Clinical Medicine 13, no. 12: 3360. https://doi.org/10.3390/jcm13123360







