Alternative Access for TAVR: Choosing the Right Pathway
Abstract
:1. Introduction
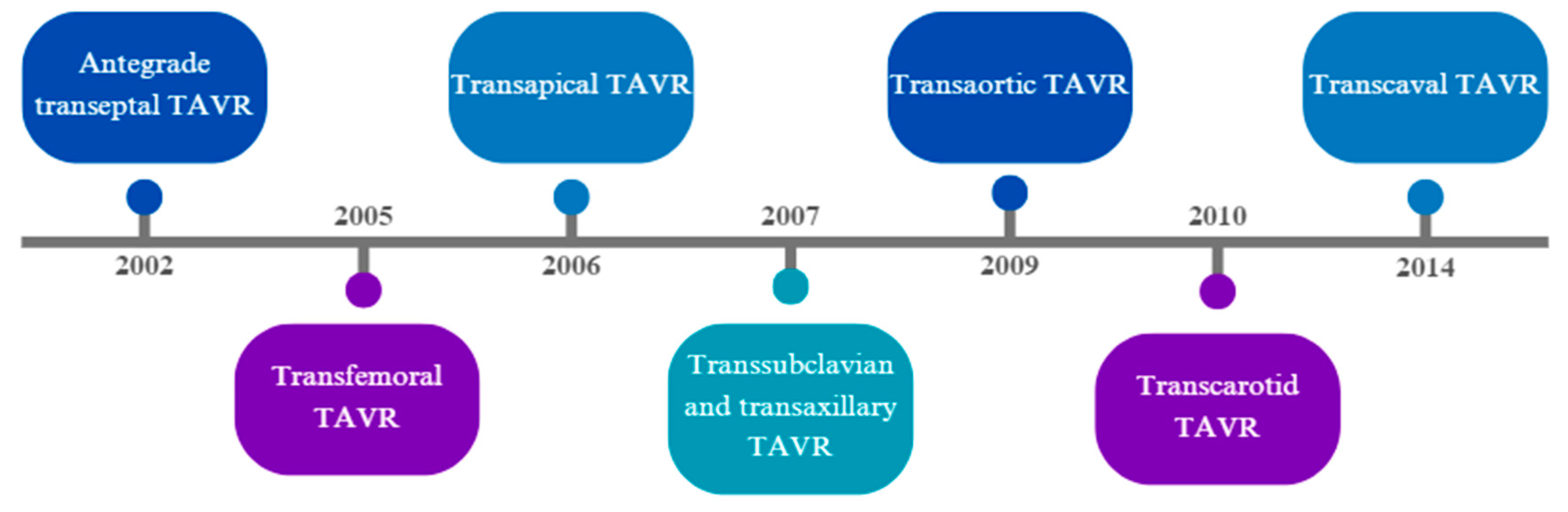
1.1. Evolution of Alternative Accesses for TAVR
1.2. Transfemoral Access and Access Site Modification
2. Intrathoracic Alternative Accesses
2.1. Transapical Access
2.1.1. Procedural Technique
2.1.2. Outcomes
2.2. Patient Selection
2.3. Transaortic Access
2.3.1. Procedural Technique
2.3.2. Outcomes
2.3.3. Patient Selection
3. Extrathoracic Alternative Accesses
3.1. Transaxillary and Transsubclavian Access
3.1.1. Procedural Technique
3.1.2. Outcomes
3.1.3. Patient Selection
3.2. Transcarotid Access
3.2.1. Procedural Technique
3.2.2. Outcomes
3.2.3. Patient Selection
3.3. Transcaval Access
3.3.1. Procedural Technique
3.3.2. Outcomes
3.3.3. Patient Selection
3.4. Choosing the Right Access
Author Contributions
Funding
Conflicts of Interest
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, E72–E227. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, M.; Leone, P.P.; Tanner, R.; Dhulipala, V.; Camaj, A.; Makhija, R.R.K.; Hooda, A.; Kini, A.S.; Sharma, S.K.; Khera, S. Transcatheter Aortic Valve Replacement: Current Status and Future Indications. J. Clin. Med. 2024, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.; Gai, J.; Torguson, R.; Okubagzi, P.G.; Shults, C.; Ben-Dor, I.; Satler, L.F.; Waksman, R. Predicted magnitude of alternate access in the contemporary transcatheter aortic valve replacement era. Catheter. Cardiovasc. Interv. 2018, 92, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Beurtheret, S.; Karam, N.; Resseguier, N.; Houel, R.; Modine, T.; Folliguet, T.; Chamandi, C.; Com, O.; Gelisse, R.; Bille, J.; et al. Femoral Versus Nonfemoral Peripheral Access for Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 2728–2739. [Google Scholar] [CrossRef] [PubMed]
- FFanaroff, A.C.; Manandhar, P.; Holmes, D.R.; Cohen, D.J.; Harrison, J.K.; Hughes, G.C.; Thourani, V.H.; Mack, M.J.; Sherwood, M.W.; Jones, W.S.; et al. Peripheral artery disease and transcatheter aortic valve replacement outcomes: A report from the STS/TVT Registry. Circ. Cardiovasc. Interv. 2017, 10, e005456. [Google Scholar] [CrossRef] [PubMed]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B.; et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, D.; A Condado, J.; Besso, J.; Vélez, M.; Burger, B.; Bibbo, S.; Cedeno, D.; Acquatella, H.; Mejia, C.; Induni, E.; et al. First Human Case of Retrograde Transcatheter Implantation of an Aortic Valve Prosthesis. Tex. Heart Inst. J. 2005, 32, 393. [Google Scholar] [PubMed]
- Lichtenstein, S.V.; Cheung, A.; Ye, J.; Thompson, C.R.; Carere, R.G.; Pasupati, S.; Webb, J.G. Transapical transcatheter aortic valve implantation in humans: Initial clinical experience. Circulation 2006, 114, 591–596. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Tron, C.; Bauer, F.; Agatiello, C.; Sebagh, L.; Bash, A.; Nusimovici, D.; Litzler, P.; Bessou, J.-P.; et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J. Am. Coll. Cardiol. 2004, 43, 698–703. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Tron, C.; Bauer, F.; Agatiello, C.; Nercolini, D.; Tapiero, S.; Litzler, P.-Y.; Bessou, J.-P.; Babaliaros, V. Treatment of calcific aortic stenosis with the percutaneous heart valve: Mid-term follow-up from the initial feasibility studies: The French experience. J. Am. Coll. Cardiol. 2006, 47, 1214–1223. [Google Scholar] [CrossRef]
- Hou, L.; Patel, P.; Rutkin, B.; Boutis, L.S.; Lee, A.; Singh, A. BAIL-OUT ANTEGRADE TRANSSEPTAL TRANSCATHETER AORTIC VALVEREPLACEMENT IN STENOTIC DEGENERATIVE BIOPROSTHETIC AORTIC VALVE. J. Am. Coll. Cardiol. 2022, 79, 2178. [Google Scholar] [CrossRef]
- Cohen, M.G.; Singh, V.; Martinez, C.A.; O'Neill, B.P.; Alfonso, C.E.; Martinezclark, P.O.; Heldman, A.W.; O'Neill, W.W. Transseptal antegrade transcatheter aortic valve replacement for patients with no other access approach—A contemporary experience. Catheter. Cardiovasc. Interv. 2013, 82, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Misumida, N.; Anderson, J.H.; Greason, K.L.; Rihal, C.S. Antegrade transseptal transcatheter aortic valve replacement: Back to the future? Catheter. Cardiovasc. Interv. 2020, 96, E552–E556. [Google Scholar] [CrossRef] [PubMed]
- Asgar, A.W.; Mullen, M.J.; Delahunty, N.; Davies, S.W.; Dalby, M.; Petrou, M.; Kelleher, A.; Moat, N. Transcatheter aortic valve intervention through the axillary artery for the treatment of severe aortic stenosis. J. Thorac. Cardiovasc. Surg. 2009, 137, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Ruge, H.; Lange, R.; Bleiziffer, S.; Hutter, A.; Mazzitelli, D.; Will, A.; Schreiber, C.; Laborde, J.-C.; Bauernschmitt, R. First successful aortic valve implantation with the CoreValve ReValving System via right subclavian artery access: A case report. Heart Surg. Forum. 2008, 11, E323–E324. [Google Scholar] [CrossRef] [PubMed]
- Bauernschmitt, R.; Schreiber, C.; Bleiziffer, S.; Ruge, H.; Mazzitelli, D.; Hutter, A.; Tassani, P.; Lange, R. Transcatheter aortic valve implantation through the ascending aorta: An alternative option for no-access patients. Heart Surg. Forum. 2009, 12, E63–E64. [Google Scholar] [CrossRef] [PubMed]
- Modine, T.; Lemesle, G.; Azzaoui, R.; Sudre, A. Aortic valve implantation with the CoreValve ReValving System via left carotid artery access: First case report. J. Thorac. Cardiovasc. Surg. 2010, 140, 928–929. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.B.; O'Neill, W.W.; Paone, G.; Guerrero, M.E.; Wyman, J.F.; Cooper, R.L.; Lederman, R.J. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: Initial human experience. J. Am. Coll. Cardiol. 2014, 63 25 Pt A, 2795–2804. [Google Scholar] [CrossRef]
- Auffret, V.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Iung, B.; Koning, R.; Motreff, P.; Leprince, P.; Verhoye, J.P.; Manigold, T.; et al. Temporal Trends in Transcatheter Aortic Valve Replacement in France: FRANCE 2 to FRANCE TAVI. J. Am. Coll. Cardiol. 2017, 70, 42–55. [Google Scholar] [CrossRef]
- Kaneko, T.; Hirji, S.A.; Yazdchi, F.; Sun, Y.-P.; Nyman, C.; Shook, D.; Cohen, D.J.; Stebbins, A.; Zeitouni, M.; Vemulapalli, S.; et al. Association Between Peripheral Versus Central Access for Alternative Access Transcatheter Aortic Valve Replacement and Mortality and Stroke: A Report from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circ. Cardiovasc. Interv. 2022, 15, E011756. [Google Scholar] [CrossRef]
- Beohar, N.; Kirtane, A.J.; Blackstone, E.; Waksman, R.; Holmes, D.; Minha, S.; Alli, O.; Suri, R.M.; Svensson, L.G.; Leon, M.; et al. Trends in Complications and Outcomes of Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement: Experience from the PARTNER Continued Access Registry. JACC Cardiovasc. Interv. 2016, 9, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.N.; Rao, S.V. Percutaneous or surgical access for transfemoral transcatheter aortic valve implantation. J. Thorac. Dis. 2018, 10 (Suppl. 30), S3595. [Google Scholar] [CrossRef] [PubMed]
- Holper, E.M.; Kim, R.J.; Mack, M.; Brown, D.; Brinkman, W.; Herbert, M.; Stewart, W.; Vance, K.; Bowers, B.; Dewey, T. Randomized trial of surgical cutdown versus percutaneous access in transfemoral TAVR. Catheter. Cardiovasc. Interv. 2014, 83, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Enriquez, M.; Andrea, R.; Brugaletta, S.; Jiménez-Quevedo, P.; Hernández-García, J.M.; Trillo, R.; Larman, M.; Fernández-Avilés, F.; Vázquez-González, N.; Iñiguez, A.; et al. Puncture Versus Surgical Cutdown Complications of Transfemoral Aortic Valve Implantation (from the Spanish TAVI Registry). Am. J. Cardiol. 2016, 118, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Chakravarty, T.; Jilaihawi, H.; Doctor, N.; Dohad, S.; Fontana, G.; Cheng, W.; Makkar, R.R. Complete percutaneous approach for arterial access in transfemoral transcatheter aortic valve replacement: A comparison with surgical cut-down and closure. Catheter. Cardiovasc. Interv. 2014, 84, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Goldsweig, A.M.; Faheem, O.; Cleman, M.W.; Forrest, J.K. A balloon-expandable sheath facilitates transfemoral TAVR in patients with peripheral vascular disease and tortuosity. Ther. Adv. Cardiovasc. Dis. 2015, 9, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Soukas, P.A.; Shammas, N.; Chamberlain, J.; Pop, A.; Adams, G.; de Freitas, D.; Valle, J.; Woo, E.; Bernardo, N.L. Intravascular Lithotripsy for Treatment of Calcified, Stenotic Iliac Arteries: A Cohort Analysis from the Disrupt PAD III Study. Cardiovasc. Revascularization Med. 2020, 21, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Nardi, G.; De Backer, O.; Saia, F.; Søndergaard, L.; Ristalli, F.; Meucci, F.; Stolcova, M.; Mattesini, A.; Demola, P.; Wang, X.; et al. Peripheral intravascular lithotripsy for transcatheter aortic valve implantation: A multicentre observational study. EuroIntervention 2022, 17, E1397–E1406. [Google Scholar] [CrossRef]
- Di Mario, C.; Goodwin, M.; Ristalli, F.; Ravani, M.; Meucci, F.; Stolcova, M.; Sardella, G.; Salvi, N.; Bedogni, F.; Berti, S.; et al. A Prospective Registry of Intravascular Lithotripsy-Enabled Vascular Access for Transfemoral Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 502–504. [Google Scholar] [CrossRef]
- Kaluski, E.; Khan, S.U.; Singh, M.; Reitknecht, F.; Sattur, S.; Rogers, G.; Sporn, D. Iliofemoral peripheral orbital atherectomy for optimizing TAVR access: An innovative strategy in the absence of alternative access options. Cardiovasc. Revascularization Med. 2018, 19, 71–76. [Google Scholar] [CrossRef]
- Ramlawi, B.; Anaya-Ayala, J.E.; Reardon, M.J. Transcatheter Aortic Valve Replacement (TAVR): Access Planning and Strategies. Methodist. Debakey Cardiovasc. J. 2012, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Kindzelski, B.; Mick, S.L.; Krishnaswamy, A.; Kapadia, S.R.; Attia, T.; Hodges, K.; Siddiqi, S.; Lowry, A.M.; Blackstone, E.H.; Popovic, Z.; et al. Evolution of Alternative-access Transcatheter Aortic Valve Replacement. Ann. Thorac. Surg. 2021, 112, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Overtchouk, P.; Modine, T. Alternate Access for TAVI: Stay Clear of the Chest. Interv. Cardiol. 2018, 13, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Panchal, H.B.; Ladia, V.; Amin, P.; Patel, P.; Veeranki, S.P.; Albalbissi, K.; Paul, T. A meta-analysis of mortality and major adverse cardiovascular and cerebrovascular events in patients undergoing transfemoral versus transapical transcatheter aortic valve implantation using edwards valve for severe aortic stenosis. Am. J. Cardiol. 2014, 114, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, E.H.; Suri, R.M.; Rajeswaran, J.; Babaliaros, V.; Douglas, P.S.; Fearon, W.F.; Miller, D.C.; Hahn, R.T.; Kapadia, S.; Kirtane, A.J.; et al. Propensity-matched comparisons of clinical outcomes after transapical or transfemoral transcatheter aortic valve replacement: A placement of aortic transcatheter valves (PARTNER)-I trial substudy. Circulation 2015, 131, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.; Klaaborg, K.; Nissen, H.; Terp, K.; Mortensen, P.; Kjeldsen, B.; Jakobsen, C.-J.; Andersen, H.; Egeblad, H.; Krusell, L.; et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: The STACCATO trial. EuroIntervention. 2012, 8, 383–389. [Google Scholar] [CrossRef]
- Sohal, S.; Mehta, H.; Kurpad, K.; Mathai, S.V.; Tayal, R.; Visveswaran, G.K.; Wasty, N.; Waxman, S.; Cohen, M. Declining Trend of Transapical Access for Transcatheter Aortic Valve Replacement in Patients with Aortic Stenosis. J. Interv. Cardiol. 2022, 2022, 5688026. [Google Scholar] [CrossRef]
- Mach, M.; Koschutnik, M.; Wilbring, M.; Winkler, B.; Reinweber, M.; Alexiou, K.; Kappert, U.; Adlbrecht, C.; Delle-Karth, G.; Grabenwöger, M.; et al. Impact of COPD on Outcome in Patients Undergoing Transfemoral versus Transapical TAVI. Thorac. Cardiovasc. Surg. 2019, 67, 251–256. [Google Scholar] [CrossRef]
- Arai, T.; Romano, M.; Lefèvre, T.; Hovasse, T.; Farge, A.; Le Houerou, D.; Hayashida, K.; Watanabe, Y.; Garot, P.; Benamer, H.; et al. Direct Comparison of Feasibility and Safety of Transfemoral Versus Transaortic Versus Transapical Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2016, 9, 2320–2325. [Google Scholar] [CrossRef]
- O’hair, D.P.; Bajwa, T.K.; Popma, J.J.; Watson, D.R.; Yakubov, S.J.; Adams, D.H.; Sharma, S.; Robinson, N.; Petrossian, G.; Caskey, M.; et al. Direct Aortic Access for Transcatheter Aortic Valve Replacement Using a Self-Expanding Device. Ann. Thorac. Surg. 2018, 105, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Dunne, B.; Tan, D.; Chu, D.; Yau, V.; Xiao, J.; Ho, K.M.; Yong, G.; Larbalestier, R. Transapical Versus Transaortic Transcatheter Aortic Valve Implantation: A Systematic Review. Ann. Thorac. Surg. 2015, 100, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Ranka, S.; Lahan, S.; Chhatriwalla, A.K.; Allen, K.B.; Chiang, M.; O'Neill, B.; Verma, S.; Wang, D.D.; Lee, J.; Frisoli, T.; et al. Network Meta-Analysis Comparing the Short- and Long-Term Outcomes of Alternative Access for Transcatheter Aortic Valve Replacement. Cardiovasc. Revasc Med. 2022, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Biasco, L.; Ferrari, E.; Pedrazzini, G.; Faletra, F.; Moccetti, T.; Petracca, F.; Moccetti, M. Access Sites for TAVI: Patient Selection Criteria, Technical Aspects, and Outcomes. Tech. Asp. Outcomes Front. Cardiovasc. Med. 2018, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Seto, A.H.; Estep, J.D.; Tayal, R.; Tsai, S.; Messenger, J.C.; Alraies, M.C.; Schneider, D.B.; Klein, A.J.; Duwayri, Y.; McCabe, J.M.; et al. SCAI Position Statement on Best Practices for Percutaneous Axillary Arterial Access and Training. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100041. [Google Scholar] [CrossRef]
- Eng, M.H.; Qintar, M.; Apostolou, D.; O’Neill, W.W. Alternative Access for Transcatheter Aortic Valve Replacement: A Comprehensive Review. Interv. Cardiol. Clin. 2021, 10, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Petronio, A.S.; De Carlo, M.; Bedogni, F.; Maisano, F.; Ettori, F.; Klugmann, S.; Poli, A.; Marzocchi, A.; Santoro, G.; Napodano, M.; et al. 2-Year Results of CoreValve Implantation Through the Subclavian Access: A Propensity-Matched Comparison with the Femoral Access. J. Am. Coll. Cardiol. 2012, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Price, J.; Bob-Manuel, T.; Tafur, J.; Joury, A.; Aymond, J.; Duran, A.; Almusawi, H.; Cloninger, A.; Parrino, P.; Ramee, S. Transaxillary TAVR Leads to Shorter Ventilator Duration and Hospital Length of Stay Compared to Transapical TAVR. Curr. Probl. Cardiol. 2021, 46, 100624. [Google Scholar] [CrossRef]
- Schäfer, U.; Ho, Y.; Frerker, C.; Schewel, D.; Sanchez-Quintana, D.; Schofer, J.; Bijuklic, K.; Meincke, F.; Thielsen, T.; Kreidel, F.; et al. Direct Percutaneous Access Technique for Transaxillary Transcatheter Aortic Valve Implantation: “The Hamburg Sankt Georg Approach”. JACC Cardiovasc. Interv. 2012, 5, 477–486. [Google Scholar] [CrossRef]
- Elison, D.; Vincent, L.; Chung, C.; Aldea, G.; McCabe, J. Percutaneous Carotid Transcatheter Aortic Valve Implantation. JACC Case Rep. 2023, 26, 102069. [Google Scholar] [CrossRef]
- Debry, N.; Delhaye, C.; Azmoun, A.; Ramadan, R.; Fradi, S.; Brenot, P.; Sudre, A.; Moussa, M.D.; Tchetche, D.; Ghostine, S.; et al. Transcarotid Transcatheter Aortic Valve Replacement: General or Local Anesthesia. JACC Cardiovasc. Interv. 2016, 9, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Kirker, E.B.; Hodson, R.W.; Spinelli, K.J.; Korngold, E.C. The Carotid Artery as a Preferred Alternative Access Route for Transcatheter Aortic Valve Replacement. Ann. Thorac. Surg. 2017, 104, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Chamandi, C.; Abi-Akar, R.; Rodés-Cabau, J.; Blanchard, D.; Dumont, E.; Spaulding, C.; Doyle, D.; Pagny, J.-Y.; DeLarochellière, R.; Lafont, A.; et al. Transcarotid compared with other alternative access routes for transcatheter aortic valve replacement. Circ. Cardiovasc. Interv. 2018, 11, e006388. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.M.; Kumar, V.; Chiu, S.T.; Korngold, E.; Hodson, R.W.; Spinelli, K.J.; Kirker, E.B. Comparable Outcomes for Transcarotid and Transfemoral Transcatheter Aortic Valve Replacement at a High Volume US Center. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Overtchouk, P.; Folliguet, T.; Pinaud, F.; Fouquet, O.; Pernot, M.; Bonnet, G.; Hubert, M.; Lapeze, J.; Claudel, J.P.; Ghostine, S.; et al. Transcarotid Approach for Transcatheter Aortic Valve Replacement with the Sapien 3 Prosthesis: A Multicenter French Registry. Cardiovasc. Interv. 2019, 12, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, G.; Leroux, L.; Peltan, J.; Labrousse, L.; Ternacle, J.; Lafitte, S.; Modine, T. Transcarotid Access for TAVR Advancements, feasibility, and clinical outcomes. Card. Interv. 2023, 17, 51–53. [Google Scholar]
- Lederman, R.J.; Greenbaum, A.B.; Khan, J.M.; Bruce, C.G.; Babaliaros, V.C.; Rogers, T. Transcaval Access and Closure Best Practices. JACC Cardiovasc. Interv. 2023, 16, 371–395. [Google Scholar] [CrossRef]
- Greenbaum, A.B.; Babaliaros, V.C.; Chen, M.Y.; Stine, A.M.; Rogers, T.; O’neill, W.W.; Paone, G.; Thourani, V.H.; Muhammad, K.I.; Leonardi, R.A.; et al. Transcaval Access and Closure for Transcatheter Aortic Valve Replacement: A Prospective Investigation. J. Am. Coll. Cardiol. 2017, 69, 511–521. [Google Scholar] [CrossRef]
- Lederman, R.J.; Babaliaros, V.C.; Rogers, T.; Stine, A.M.; Chen, M.Y.; Muhammad, K.I.; Leonardi, R.A.; Paone, G.; Khan, J.M.; Leshnower, B.G.; et al. The Fate of Transcaval Access Tracts: 12-Month Results of the Prospective NHLBI Transcaval Transcatheter Aortic Valve Replacement Study. JACC Cardiovasc. Interv. 2019, 12, 448–456. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; Tijssen, J. Alternative Access for TAVR: See the Forest for the Trees. JACC Cardiovasc. Interv. 2022, 15, 976–978. [Google Scholar] [CrossRef]
- Dahle, T.G.; Kaneko, T.; McCabe, J.M. Outcomes Following Subclavian and Axillary Artery Access for Transcatheter Aortic Valve Replacement: Society of the Thoracic Surgeons/American College of Cardiology TVT Registry Report. JACC Cardiovasc. Interv. 2019, 12, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Lederman, R.J.; Greenbaum, A.B.; Rogers, T.; Khan, J.M.; Fusari, M.; Chen, M.Y. Anatomic Suitability for Transcaval Access Based on Computed Tomography. JACC Cardiovasc. Interv. 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed]


| Type of Access | Benefits | Risks | Pre-Procedural Planning | Closure Techniques | |
|---|---|---|---|---|---|
| Intrathoracic | Transapical | Useful alternative when all other accesses are precluded | Considered the most invasive approach Requires thoracotomy and general anesthesia Unable to accommodate self-expanding valves Can be associated with damage to mitral valve apparatus and formation of left ventricular aneurysm | Imaging to rule out abnormalities in the regional thoracic wall or apical myocardium | Primary surgical closure |
| Transaortic | Useful alternative when extrathoracic accesses are precluded Improved outcomes, when compared to transapical access (see text) | Requires partial sternotomy or mini thoracotomy Requires general anesthesia High risk of bleeding complications | Pre-TAVR imaging used to rule out:
| Primary surgical closure | |
| Extrathoracic | Transaxillary/ transsubclavian | Conscious sedation can be used (with percutaneous approach) Improved outcomes, when compared to intrathoracic accesses (see text) | General anesthesia is required (with surgical cutdown approach) Axillary/subclavian arteries are more prone to dissection (see text) | Pre-TAVR imaging used to confirm:
| Perclose sutures (with percutaneous approach) Primary surgical closure (with surgical cutdown approach) |
| Transcarotid | Better device control and positioning (direct and short distance to aortic valve) | General anesthesia is required (majority of cases done with surgical cutdown approach) | Pre-TAVR imaging must include carotid and vertebral ultrasound and CT/MRI of brain, used to:
| Primary surgical closure of carotid artery | |
| Transcaval | Conscious sedation is typically used | Steep learning curve Risk of retroperitoneal bleed Risk of creation of AV fistula Requires occluder device to be placed in aortic wall | Pre-TAVR imaging used to ensure:
| Occluder device placed in the aortic wall Venous hemostasis is achieved | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutz, K.; Asturias, K.M.; Garg, J.; Poudyal, A.; Lantz, G.; Golwala, H.; Doberne, J.; Politano, A.; Song, H.K.; Zahr, F. Alternative Access for TAVR: Choosing the Right Pathway. J. Clin. Med. 2024, 13, 3386. https://doi.org/10.3390/jcm13123386
Lutz K, Asturias KM, Garg J, Poudyal A, Lantz G, Golwala H, Doberne J, Politano A, Song HK, Zahr F. Alternative Access for TAVR: Choosing the Right Pathway. Journal of Clinical Medicine. 2024; 13(12):3386. https://doi.org/10.3390/jcm13123386
Chicago/Turabian StyleLutz, Katherine, Karla M. Asturias, Jasmine Garg, Abhushan Poudyal, Gurion Lantz, Harsh Golwala, Julie Doberne, Amani Politano, Howard K. Song, and Firas Zahr. 2024. "Alternative Access for TAVR: Choosing the Right Pathway" Journal of Clinical Medicine 13, no. 12: 3386. https://doi.org/10.3390/jcm13123386
APA StyleLutz, K., Asturias, K. M., Garg, J., Poudyal, A., Lantz, G., Golwala, H., Doberne, J., Politano, A., Song, H. K., & Zahr, F. (2024). Alternative Access for TAVR: Choosing the Right Pathway. Journal of Clinical Medicine, 13(12), 3386. https://doi.org/10.3390/jcm13123386






