Ultrasound in Microsurgery: Current Applications and New Frontiers
Abstract
:1. Introduction
2. Materials and Methods
3. Discussion
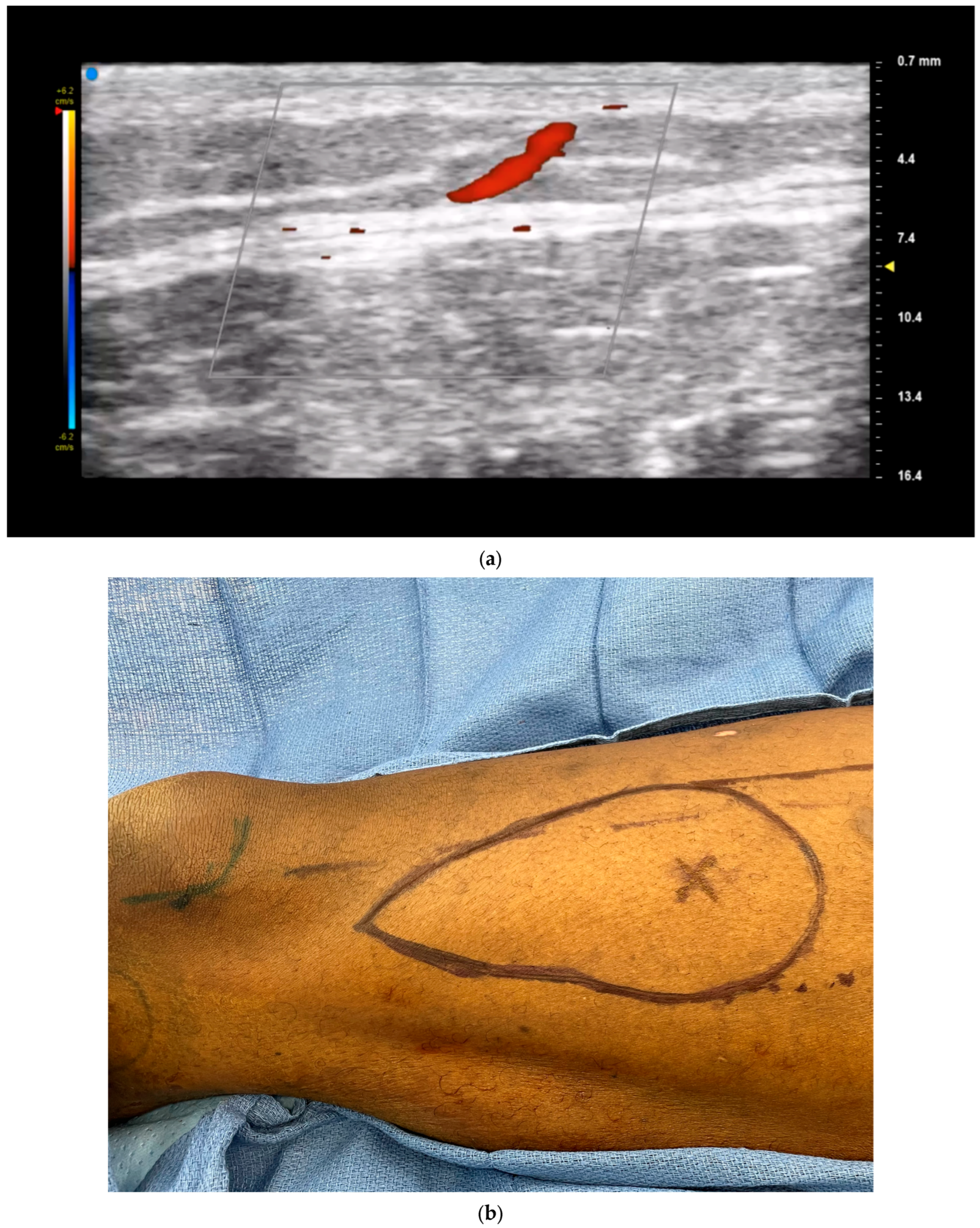
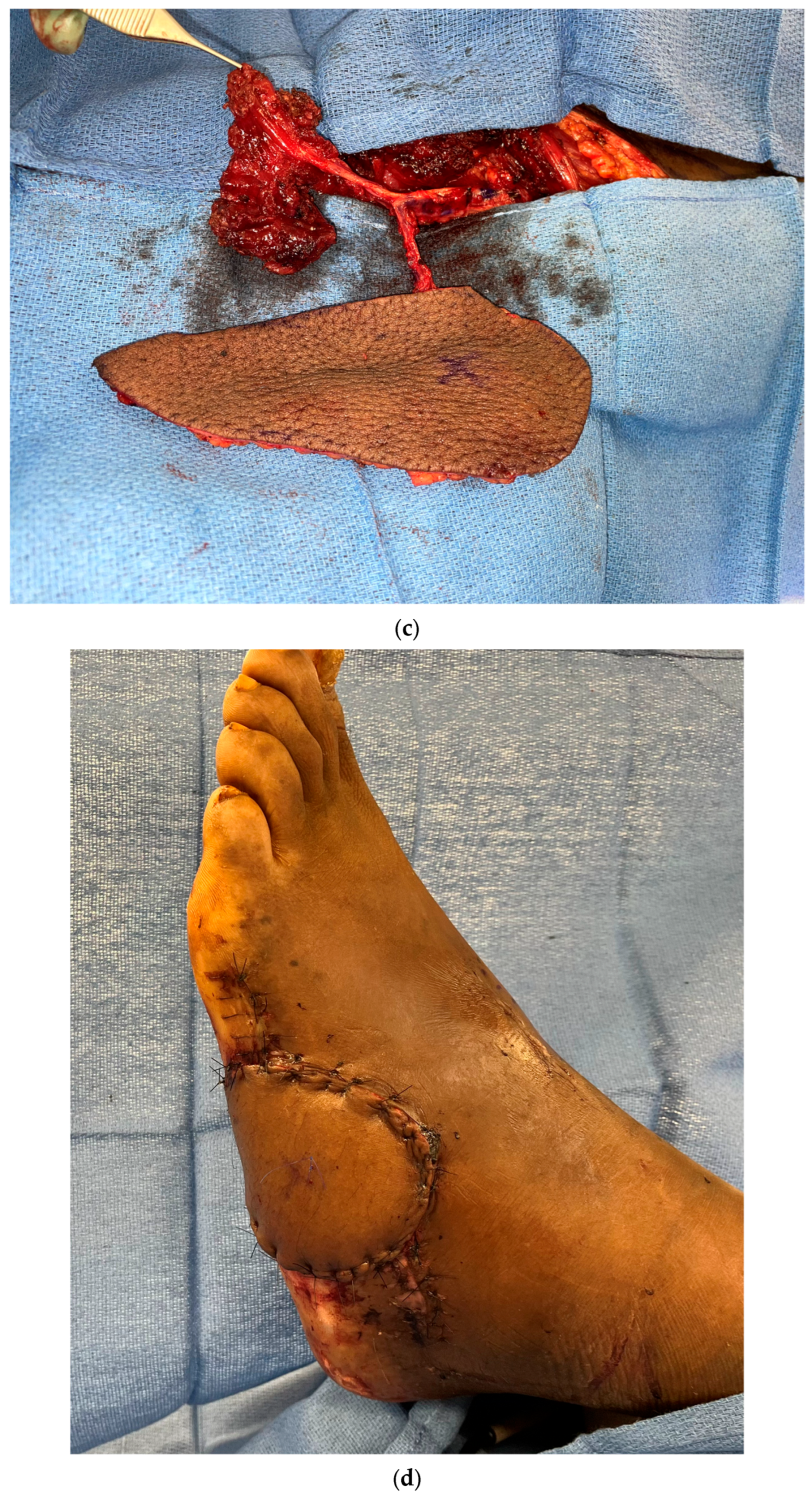
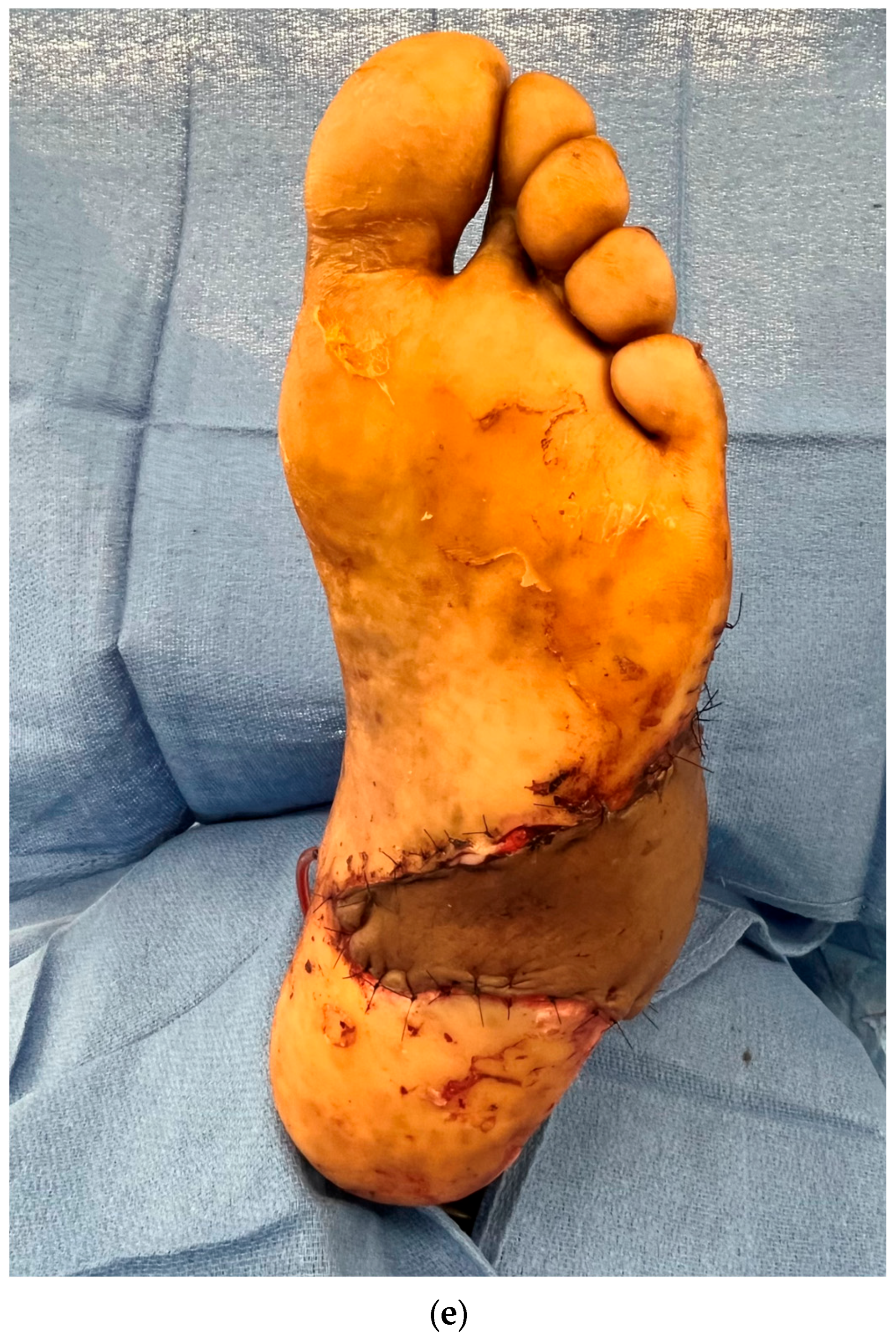
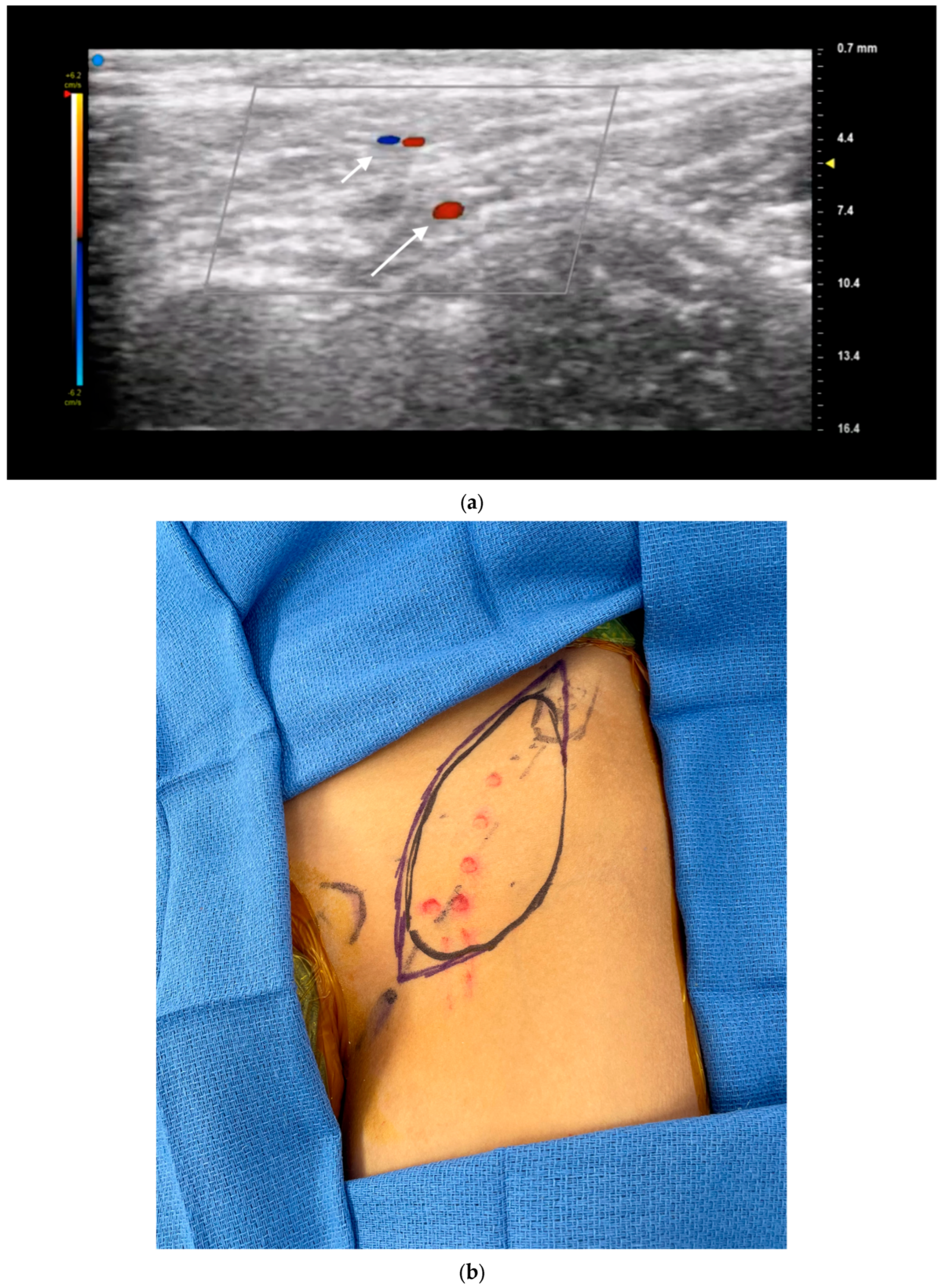
3.1. Perforator Flap Planning and Design
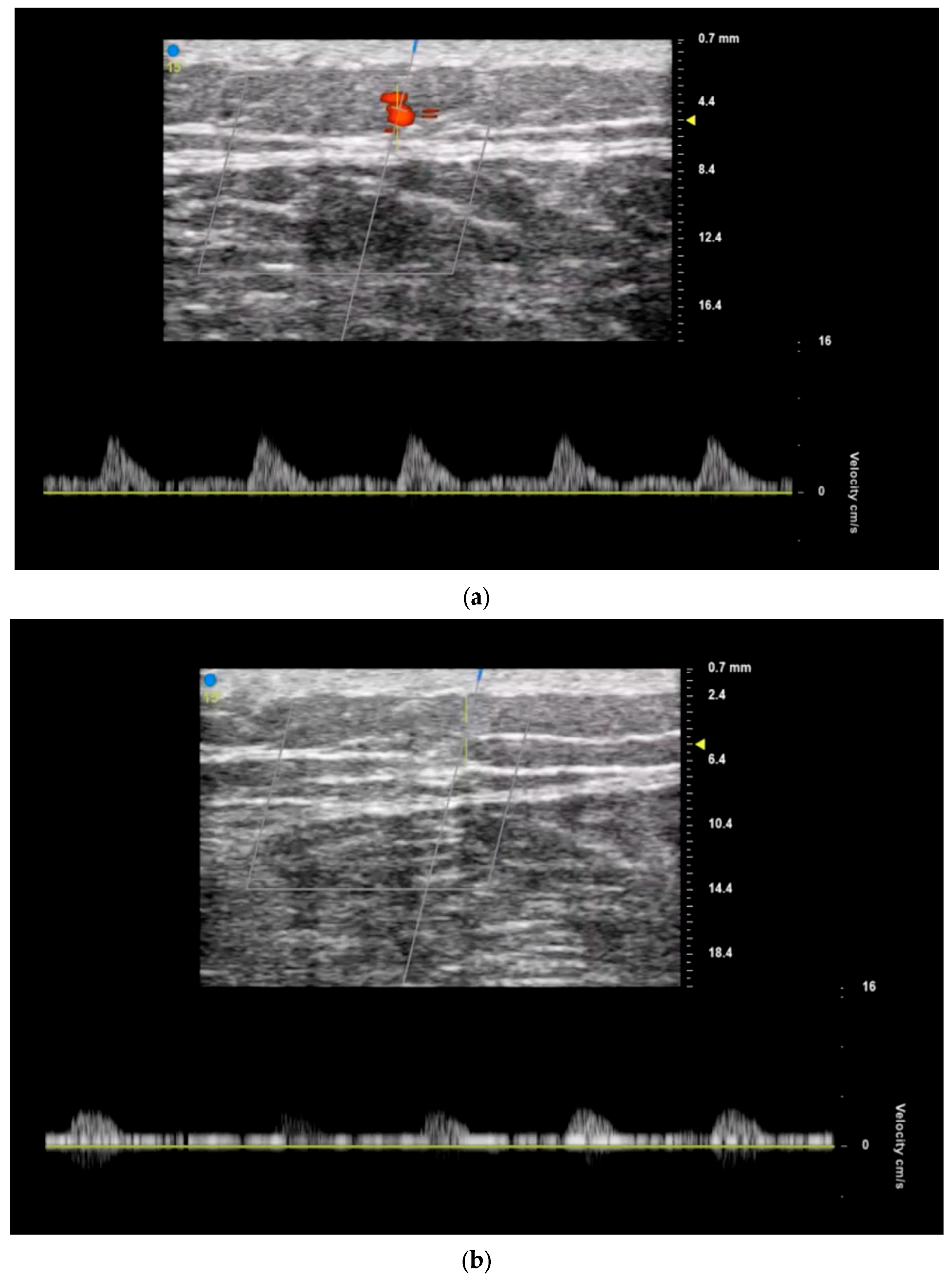
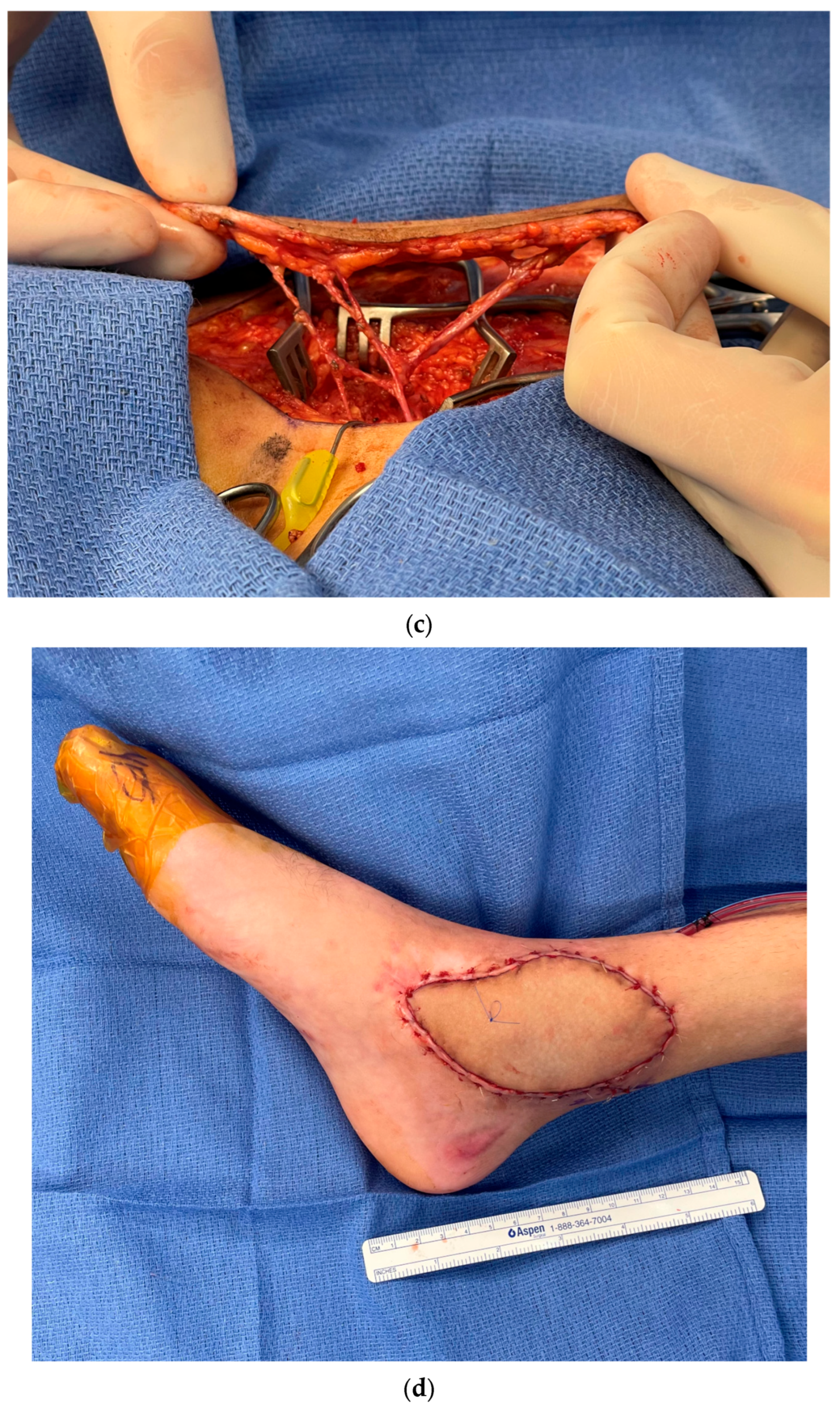
3.2. Recipient Vessel Selection
3.3. Postoperative Monitoring
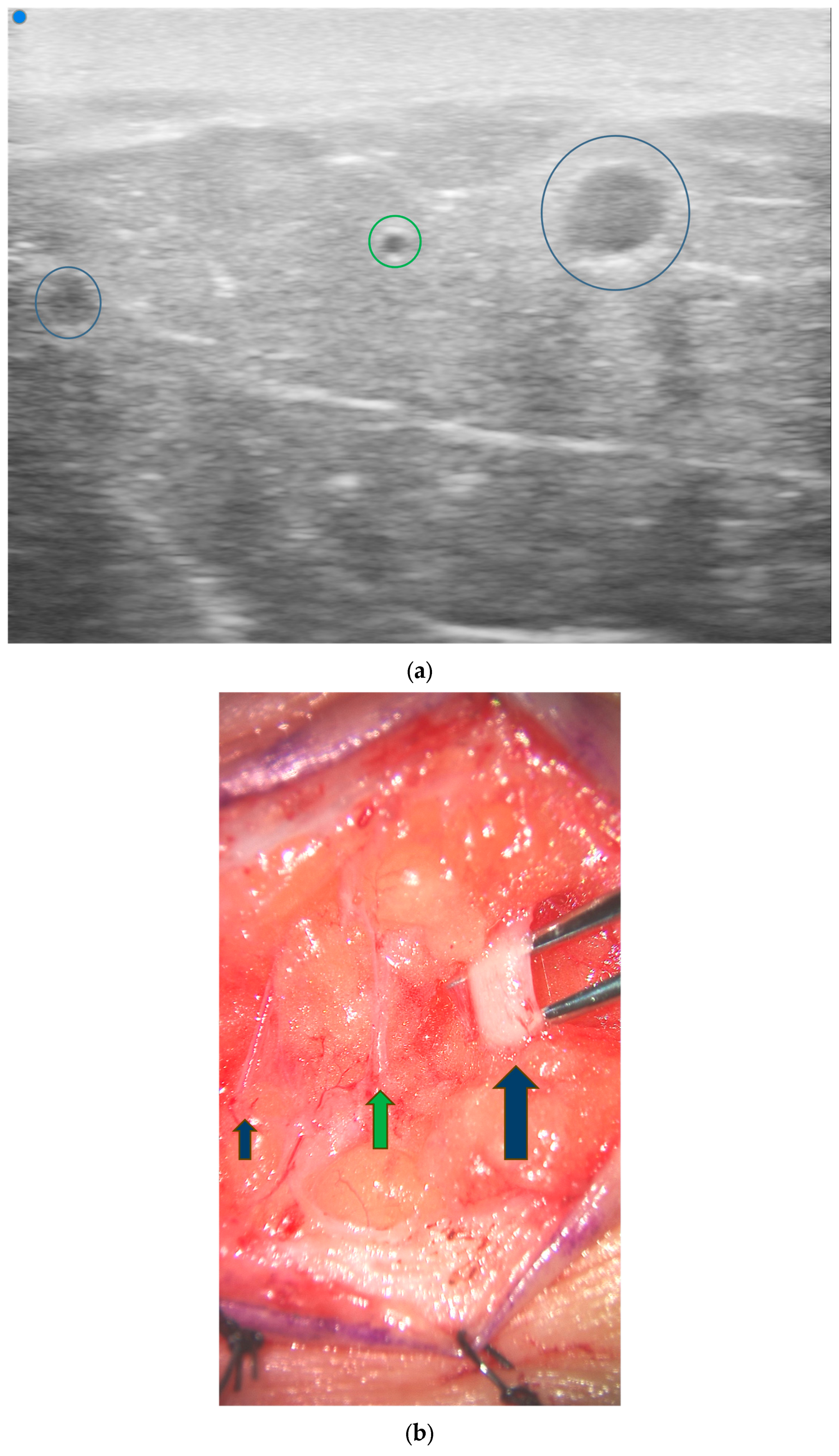
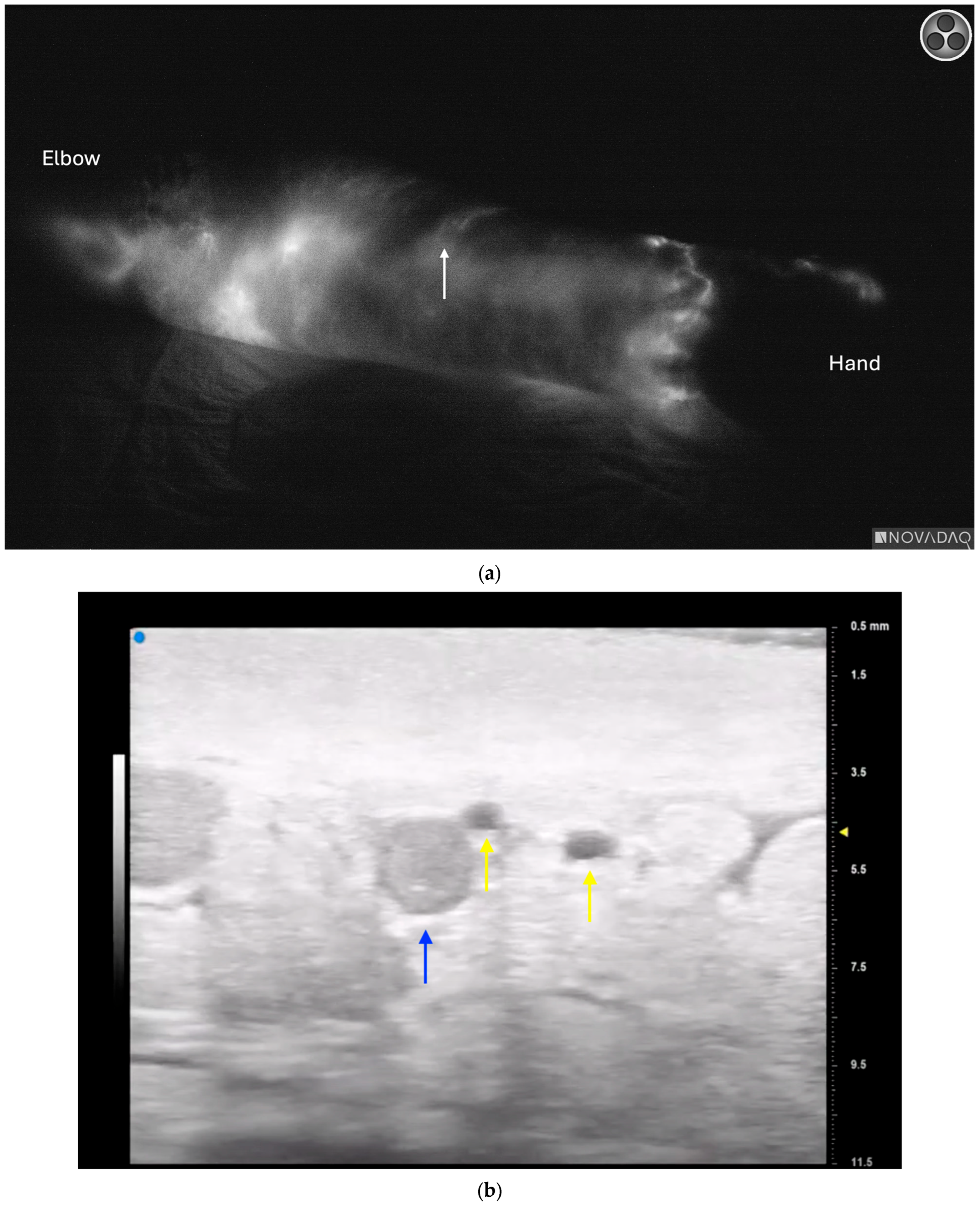
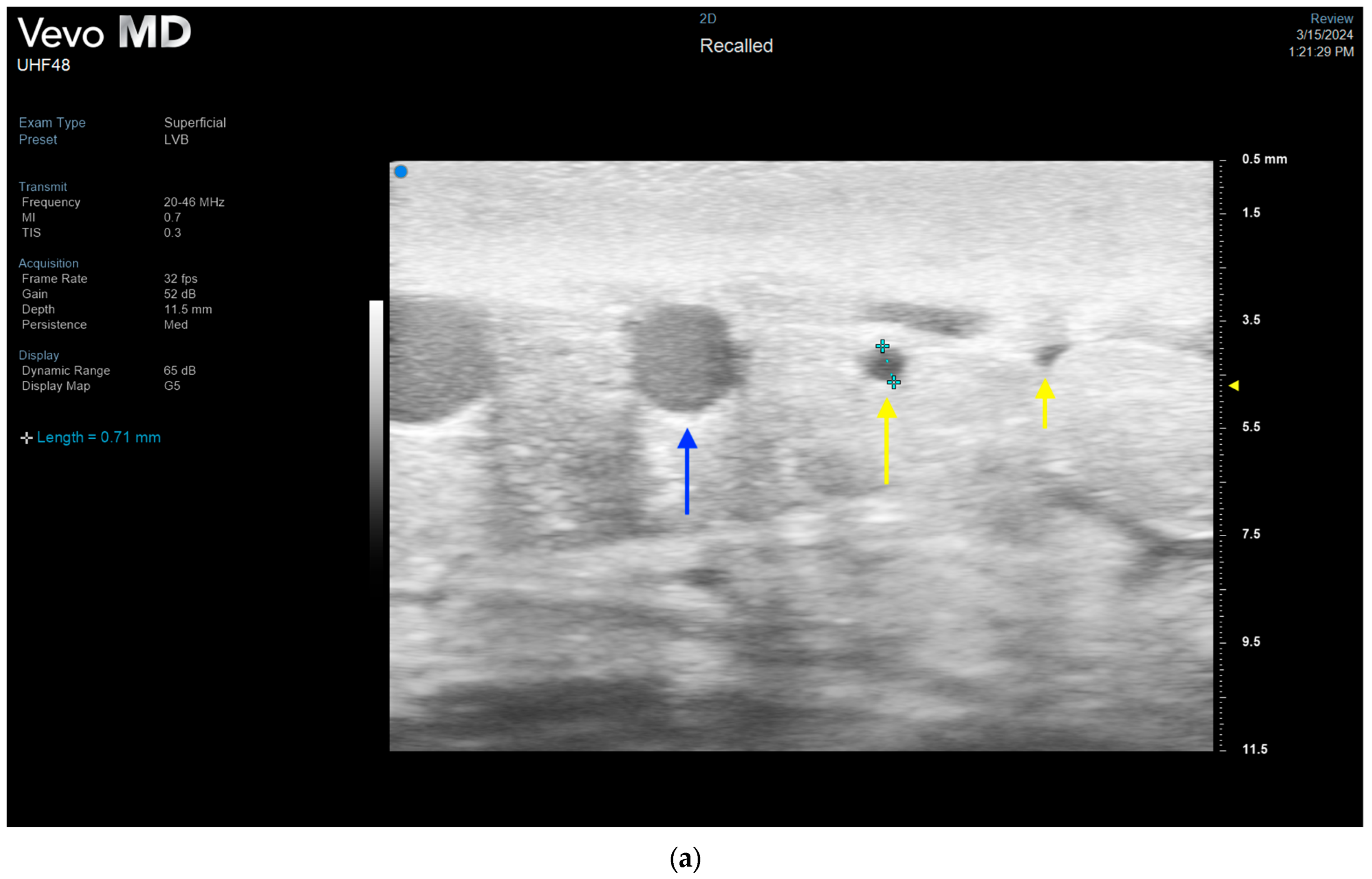
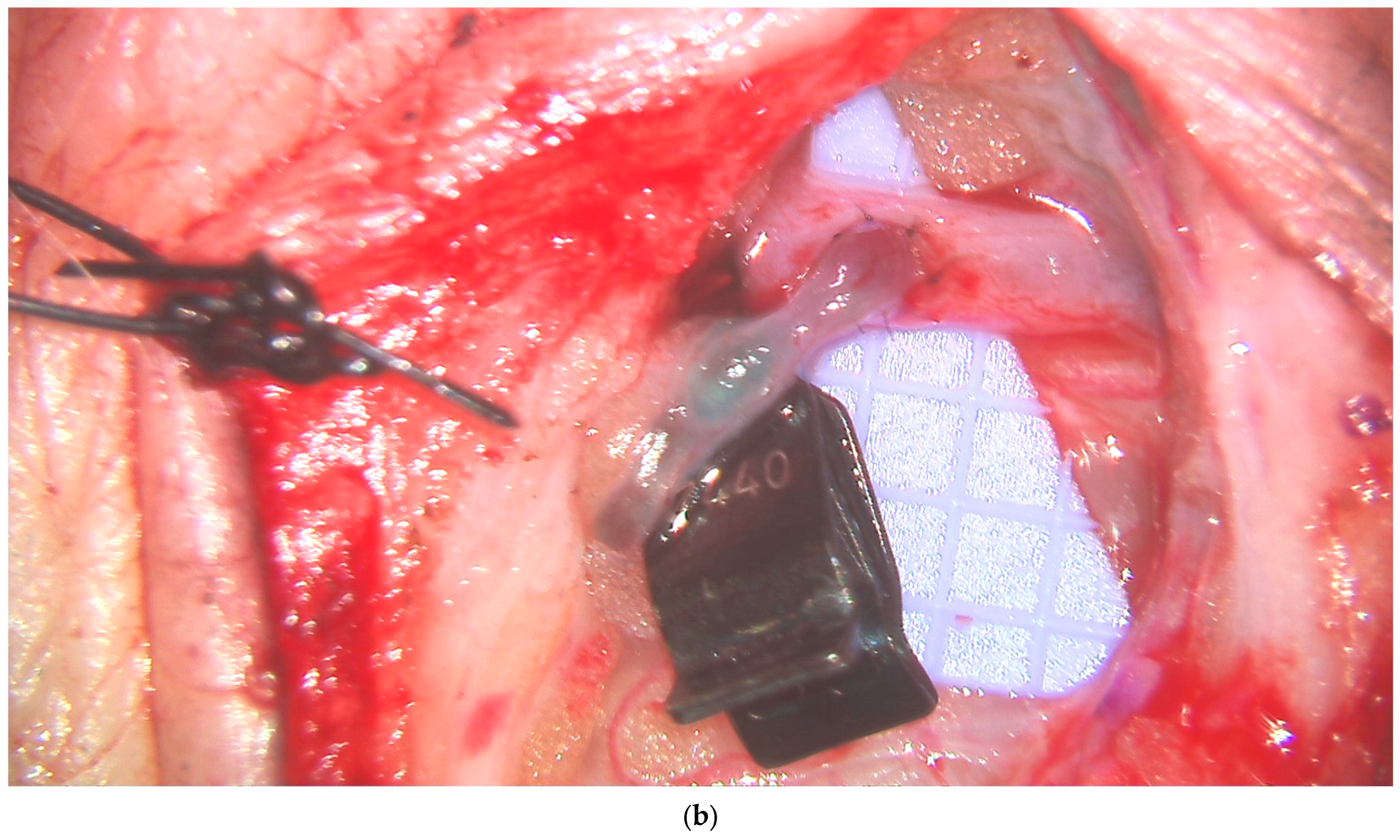
3.4. Lymphatic Surgery
3.5. Future Directions and Additional Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sosin, M.; Ceradini, D.J.; Hazen, A.; Levine, J.P.; Staffenberg, D.A.; Saadeh, P.B.; Flores, R.L.; Brecht, L.E.D.; Bernstein, G.L.M.; Rodriguez, E.D.M. Total Face, Eyelids, Ears, Scalp, and Skeletal Subunit Transplant Cadaver Simulation: The Culmination of Aesthetic, Craniofacial, and Microsurgery Principles. Plast. Reconstr. Surg. 2016, 137, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Bojovic, B.; Dorafshar, A.H.; Brown, E.N.; Christy, M.R.; Borsuk, D.E.; Hui-Chou, H.G.; Shaffer, C.K.; Kelley, T.N.; Sauerborn, P.J.; Kennedy, K.; et al. Total Face, Double Jaw, and Tongue Transplant Research Procurement. Plast. Reconstr. Surg. 2012, 130, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Day, K.M.M.; Kelley, P.K.M.; Harshbarger, R.J.M.; Dorafshar, A.H.M.; Kumar, A.R.M.; Steinbacher, D.M.M.; Patel, P.M.; Combs, P.D.M.; Levine, J.P.M. Advanced Three-Dimensional Technologies in Craniofacial Reconstruction. Plast. Reconstr. Surg. 2021, 148, 94E–108E. [Google Scholar] [CrossRef]
- Lee, Z.-H.; Alfonso, A.R.B.; Ramly, E.P.; Kantar, R.S.; Yu, J.W.D.; Daar, D.M.; Hirsch, D.L.M.; Jacobson, A.; Levine, J.P. The Latest Evolution in Virtual Surgical Planning: Customized Reconstruction Plates in Free Fibula Flap Mandibular Reconstruction. Plast. Reconstr. Surg. 2020, 146, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.T.; Saint-Cyr, M. Advances in imaging technologies for planning breast reconstruction. Gland. Surg. 2016, 5, 242–254. [Google Scholar] [CrossRef]
- D’angelo, A.; Cina, A.; Macrì, G.; Belli, P.; Mercogliano, S.; Barbieri, P.; Grippo, C.; Franceschini, G.; D’archi, S.; Mason, E.J.; et al. Conventional CT versus Dedicated CT Angiography in DIEP Flap Planning: A Feasibility Study. J. Pers. Med. 2021, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Lu, L.; Lazzeri, D.; Zhang, Y.X.; Wang, D.; Innocenti, M.; Qian, Y.; Agostini, T.; Levin, L.S.; Messmer, C. Contrast-Enhanced ultrasound combined with three-dimensional reconstruction in preoperative perforator flap planning. Plast. Reconstr. Surg. 2013, 131, 80–93. [Google Scholar] [CrossRef]
- Cho, M.-J.; Kwon, J.G.; Pak, C.J.; Suh, H.P.; Hong, J.P. The Role of Duplex Ultrasound in Microsurgical Reconstruction: Review and Technical Considerations. J. Reconstr. Microsurg. 2020, 36, 514–521. [Google Scholar] [CrossRef]
- Cevik, J.; Seth, I.; Hunter-Smith, D.J.; Rozen, W.M. A History of Innovation: Tracing the Evolution of Imaging Modalities for the Preoperative Planning of Microsurgical Breast Reconstruction. J. Clin. Med. 2023, 12, 5246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janhofer, D.E.; Lakhiani, C.; Kim, P.J.; Akbari, C.; Naz, I.; Tefera, E.A.; Attinger, C.E.; Evans, K.K. The Utility of Preoperative Arteriography for Free Flap Planning in Patients with Chronic Lower Extremity Wounds. Plast. Reconstr. Surg. 2019, 143, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Zolper, E.G.B.; Sharif-Askary, B.; Abdou, S.A.; Charipova, K.B.; Bekeny, J.C.B.; Fan, K.L.; Steinberg, J.S.D.; Attinger, C.E.; Evans, K.K. Expanding Criteria for Limb Salvage in Comorbid Patients with Nonhealing Wounds: The MedStar Georgetown Protocol and Lessons Learned after 200 Lower Extremity Free Flaps. Plast. Reconstr. Surg. 2022, 150, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Giacalone, G.; Yamamoto, T.; Belva, F.; Visconti, G.; Hayashi, N.; Handa, M.; Yoshimatsu, H.; Salgarello, M. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast. Reconstr. Surg.-Glob. Open 2019, 7, e2086. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Visconti, G.; Giacalone, G.; Hayashi, N.; Yoshimatsu, H. Recent Advances in Ultrasound Technology: Ultra-High Frequency Ultrasound for Reconstructive Supermicrosurgery. J. Reconstr. Microsurg. 2022, 38, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Visconti, G.; Hayashi, A.; Santoro, A.; Longo, V.; Salgarello, M. Ultra-High frequency ultrasound imaging of lymphatic channels correlates with their histological features: A step forward in lymphatic surgery. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Neligan, P.C.; Mathes, D.W. Current techniques in preoperative imaging for abdomen-based perforator flap microsurgical breast reconstruction. J. Reconstr. Microsurg. 2010, 26, 003–010. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, A.; Sachanadani, N.S.; da Silva, N.P.B.; Lonic, D.; Heidekrueger, P.; Taeger, C.D.; Klein, S.; Jung, E.M.; Prantl, L.; Hong, J.-P. Step-by-step guide to ultrasound-based design of alt flaps by the microsurgeon—Basic and advanced applications and device settings. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Koshima, I.; Imai, H.; Uchiki, T.; Sasaki, A.; Nagamatsu, S.; Yokota, K. Investigation of flow velocity in recipient perforator artery for a reliable indicator for the flap transfer with perforator to perforator anastomosis. Microsurgery 2021, 41, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Figus, A.M.; Ramakrishnan, V.M.; Rubino, C.M. Hemodynamic changes in the microcirculation of DIEP flaps. Ann. Plast. Surg. 2008, 60, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Dusseldorp, J.R.; Pennington, D.G. Quantifying Blood Flow in the DIEP Flap. Plast. Reconstr. Surg.-Glob. Open 2014, 2, e228. [Google Scholar] [CrossRef]
- Visconti, G.; Bianchi, A.; Hayashi, A.; Cina, A.; Maccauro, G.; Almadori, G.; Salgarello, M. Thin and superthin perforator flap elevation based on preoperative planning with ultrahigh-frequency ultrasound. Arch. Plast. Surg. 2020, 47, 365–370. [Google Scholar] [CrossRef]
- Schiltz, D.; Lenhard, J.; Klein, S.; Anker, A.; Lonic, D.; Heidekrueger, P.I.; Prantl, L.; Jung, E.-M.; Da Silva, N.P.B.; Kehrer, A. Do-It-Yourself Preoperative High-Resolution Ultrasound-Guided Flap Design of the Superficial Circumflex Iliac Artery Perforator Flap (SCIP). J. Clin. Med. 2021, 10, 2427. [Google Scholar] [CrossRef] [PubMed]
- Visconti, G.; Bianchi, A.; Hayashi, A.; Salgarello, M. Designing an Anterolateral Thigh Flap Using Ultrasound. J. Reconstr. Microsurg. 2021, 38, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.H.; Maselli, A.M.; Manstein, S.; Shiah, E.; Slatnick, B.L.; Dowlatshahi, A.S.; Cauley, R.; Lee, B.T. Comparison of Various Modalities Utilized for Preoperative Planning in Microsurgical Reconstructive Surgery. J. Reconstr. Microsurg. 2021, 38, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Gravvanis, A.; Petrocheilou, G.; Tsoutsos, D.; Delikonstantinou, I.; Karakitsos, D. Integrating imaging techniques in lower limb microsurgical reconstruction: Focusing on ultrasonography versus computed tomography angiography. In Vivo 2013, 27, 371–375. [Google Scholar] [PubMed]
- Mijuskovic, B.; Tremp, M.; Heimer, M.; Boll, D.; Aschwanden, M.; Zeindler, J.; Kurzeder, C.; Schaefer, D.; Haug, M.D.; Kappos, E. Color Doppler ultrasound and computed tomographic angiography for perforator mapping in DIEP flap breast reconstruction revisited: A cohort study. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Bajus, A.; Streit, L.; Kubek, T.; Novák, A.; Vaníček, J.; Šedivý, O.; Berkeš, A.; Bayezid, K.C.; Kunovský, L.; Dražan, L. Color Doppler ultrasound versus CT angiography for DIEP flap planning: A randomized controlled trial. J. Plast. Reconstr. Aesthetic Surg. 2023, 86, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Jeong, H.H.; Lee, Y.; Powers, H.; Suh, Y.C.; Christoffi, P.; Suh, H.P.; Hong, J.P.J. Direction of Flap Rotation in Propeller Flaps: Does It Really Matter? J. Reconstr. Microsurg. 2019, 35, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-H.; Kim, K.-J.; Jung, K.-Y.; Baek, S.-K.; Park, S.-H.; Yoon, E.-S. Recipient vessel selection for head and neck reconstruction: A 30-year experience in a single institution. Arch. Craniofacial Surg. 2020, 21, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.P.; Park, C.J.; Suh, H.P. Importance of Vascularity and Selecting the Recipient Vessels of Lower Extremity Reconstruction. J. Reconstr. Microsurg. 2021, 37, 083–088. [Google Scholar] [CrossRef]
- Hong, J.P.; Kim, H.B.; Park, C.J.; Suh, H.P. Using Duplex Ultrasound for Recipient Vessel Selection. J. Reconstr. Microsurg. 2022, 38, 200–205. [Google Scholar] [CrossRef]
- Ochoa, O.; Pisano, S.; Chrysopoulo, M.; Ledoux, P.; Arishita, G.; Nastala, C. Salvage of intraoperative deep inferior epigastric perforator flap venous congestion with augmentation of venous outflow. Plast. Reconstr. Surg.-Glob. Open 2013, 1, e52. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.S.; Oh, T.S.; Hong, J.P. Innovations in diabetic foot reconstruction using supermicrosurgery. Diabetes Metab. Res. Rev. 2016, 32, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.S.; Oh, T.S.; Lee, H.S.; Lee, S.H.; Cho, Y.P.; Park, J.R.; Hong, J.P. A New Approach for Reconstruction of Diabetic Foot Wounds Using the Angiosome and Supermicrosurgery Concept. Plast. Reconstr. Surg. 2016, 138, 702e–709e. [Google Scholar] [CrossRef] [PubMed]
- Shinomiya, R.; Sunagawa, T.; Nakashima, Y.; Kodama, A.; Hayashi, Y.; Tokumoto, M.; Adachi, N. Monitoring Vascular Compromise Using Ultrasound After Free Tissue Transfer. J. Ultrasound Med. 2020, 39, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.J.; Shin, J.Y.; Roh, S.-G.; Lee, N.-H.; Chung, Y.K. Clinical analysis of factors affecting the failure of free flaps used in head and neck reconstruction. Arch. Craniofac. Surg. 2023, 24, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Maselli, A.; Barron, S.; Morgenstern, M.; Comer, C.D.; Chow, K.; Cauley, R.; Lee, B.T. Applications of Ultrasound in the Postoperative Period: A Review. J. Reconstr. Microsurg. 2022, 38, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Gardikiotis, I.; Ruiz-Moya, A.; Fontdevila, J.; Weshahy, O.; Palacin, J.; Vinals, J.; Hong, J.P. Duplex echography as an adjuvant tool to clinical examination to detect early postoperative free flap vascular compromise. Microsurgery 2021, 41, 109–118. [Google Scholar] [CrossRef]
- Knoedler, S.; Hoch, C.C.; Huelsboemer, L.; Knoedler, L.; Stögner, V.A.; Pomahac, B.; Kauke-Navarro, M.; Colen, D. Postoperative free flap monitoring in reconstructive surgery—Man or machine? Front. Surg. 2023, 10, 1130566. [Google Scholar] [CrossRef] [PubMed]
- Kohlert, S.; Quimby, A.E.; Saman, M.; Ducic, Y. Postoperative Free-Flap Monitoring Techniques. Semin. Plast. Surg. 2019, 33, 013–016. [Google Scholar] [CrossRef]
- Pratt, G.F.; Rozen, W.M.; Chubb, D.; Whitaker, I.S.; Grinsell, D.; Ashton, M.W.; Acosta, R. Modern adjuncts and technologies in microsurgery: An historical and evidence-based review. Microsurgery 2010, 30, 657–666. [Google Scholar] [CrossRef]
- Henderson, P.W.; Fernandez, J.G.; Cemal, Y.; Mehrara, B.J.; Pusic, A.L.; McCarthy, C.M.; Matros, E.; Cordeiro, P.G.; Disa, J.J. Successful Salvage of Late Anastomotic Thrombosis after Free Tissue Transfer. J. Reconstr. Microsurg. 2016, 32, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Coriddi, M.; Mehrara, B.; Skoracki, R.; Singhal, D.; Dayan, J.H. Immediate Lymphatic Reconstruction: Technical Points and Literature Review. Plast. Reconstr. Surg.-Glob. Open 2021, 9, e3431. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, M.; Arrault, M.; Vignes, S. Positive impact of delayed breast reconstruction on breast-cancer treatment-related arm lymphoedema. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Campisi, C.; Boccardo, F. Lymphedema and microsurgery. Microsurgery 2002, 22, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Cagnati, P.; Gulli, R.; Spanò, F.; Puppo, F.; Campisi, C.; Boccardo, F. Current Views on Diagnostic Approach and Treatment of Lymphedema. Am. J. Med. 2012, 125, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Singhal, D. Immediate lymphatic reconstruction. J. Surg. Oncol. 2018, 118, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; Singhal, D. Advances in lymphedema: An under-recognized disease with a hopeful future for patients. Vasc. Med. 2024, 29, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Coriddi, M.; Dayan, J.; Bloomfield, E.N.; McGrath, L.N.; Diwan, R.; Monge, J.B.; Gutierrez, J.B.; Brown, S.; Boe, L.; Mehrara, B. Efficacy of Immediate Lymphatic Reconstruction to Decrease Incidence of Breast Cancer-related Lymphedema: Preliminary Results of Randomized Controlled Trial. Ann. Surg. 2023, 278, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.I.; Mohos, B.; Tzou, C.-H.J. Imaging Modalities for Evaluating Lymphedema. Medicina 2023, 59, 2016. [Google Scholar] [CrossRef]
- Cuccurullo, V.; Rapa, M.; Catalfamo, B.; Gatta, G.; Di Grezia, G.; Cascini, G.L. The Role of Imaging of Lymphatic System to Prevent Cancer Related Lymphedema. Bioengineering 2023, 10, 1407. [Google Scholar] [CrossRef]
- Czedik-Eysenberg, M.; Steinbacher, J.; Obermayer, B.; Yoshimatsu, H.; Hara, H.; Mihara, M.; Tzou, C.J.; Meng, S. Exclusive use of ultrasound for locating optimal LVA sites—A descriptive data analysis. J. Surg. Oncol. 2019, 121, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Kajikawa, A.; Yamamoto, T.; Takeuchi, T.; Terashima, T.; Kurogi, N. The dynamic-lymphaticovenular anastomosis method for breast cancer treatment-related lymphedema: Creation of functional lymphaticovenular anastomoses with use of preoperative dynamic ultrasonography. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kondra, K.; Stanton, E.; Salibian, A.A.; Patel, K.M. Imaging in lymphedema management: A narrative review of preoperative assessment & surgical planning. Ann. Breast Surg. 2024, 8, 5. [Google Scholar] [CrossRef]
- Ogata, F.; Azuma, R.; Kikuchi, M.; Koshima, I.; Morimoto, Y. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann. Plast. Surg. 2007, 58, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Unno, N.; Inuzuka, K.; Suzuki, M.; Yamamoto, N.; Sagara, D.; Nishiyama, M.; Konno, H. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J. Vasc. Surg. 2007, 45, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yamamoto, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. Indocyanine green–enhanced lymphography for upper extremity lymphedema. Plast. Reconstr. Surg. 2011, 128, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yamamoto, N.; Yoshimatsu, H.; Narushima, M.; Koshima, I. Factors Associated with Lymphosclerosis: An Analysis on 962 Lymphatic Vessels. Plast. Reconstr. Surg. 2017, 140, 734–741. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Hermann, A.P.; Madsen, A.R.; Christensen, S.; Sørensen, J.A. Indocyanine green lymphangiography is superior to clinical staging in breast cancer-related lymphedema. Sci. Rep. 2021, 11, 21103. [Google Scholar] [CrossRef]
- Cha, H.G.; Oh, T.M.; Cho, M.-J.; Pak, C.S.J.M.; Suh, H.P.M.; Jeon, J.Y.M.; Hong, J.P.M. Changing the Paradigm: Lymphovenous Anastomosis in Advanced Stage Lower Extremity Lymphedema. Plast. Reconstr. Surg. 2020, 147, 199–207. [Google Scholar] [CrossRef]
- Chang, E.I.M.; Schaverien, M.V.M.; Hanson, S.E.M.; Chu, C.K.M.; Hanasono, M.M.M. Evolution in Surgical Management of Breast Cancer-related Lymphedema: The MD Anderson Cancer Center Experience. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e2674. [Google Scholar] [CrossRef] [PubMed]
- Visconti, G.; Hayashi, A.; Bianchi, A.; Tartaglione, G.; Bartoletti, R.; Salgarello, M. Lymphaticovenular Anastomosis for Advanced-Stage Peripheral Lymphedema: Expanding Indication and Introducing the Hand/Foot Sign. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Salgarello, M.; Hayashi, A.; Yang, J.C.-S.; Visconti, G. Recipient Venule Selection and Anastomosis Configuration for Lymphaticovenular Anastomosis in Extremity Lymphedema: Algorithm Based on 1,000 Lymphaticovenular Anastomosis. J. Reconstr. Microsurg. 2021, 38, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.-A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef] [PubMed]
- Heneweer, C.; Zirk, M.M.; Safi, A.M.; Smeets, R.M.; Malter, W.; Kröger, N.; Zöller, J.E.M.; Maintz, D.; Zinser, M.M. An Innovative Approach for Preoperative Perforator Flap Planning Using Contrast-enhanced B-flow Imaging. Plast. Reconstr. Surg.-Glob. Open 2021, 9, e3547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zinser, M.J.; Kröger, N.; Malter, W.; Schulz, T.; Puesken, M.; Mallmann, P.; Zirk, M.; Schröder, K.; Andree, C.; Seidenstuecker, K.; et al. Preoperative Perforator Mapping in DIEP Flaps for Breast Reconstruction. The Impact of New Contrast-Enhanced Ultrasound Techniques. J. Pers. Med. 2022, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Seth, I.; Bulloch, G.; Joseph, K.; Hunter-Smith, D.J.; Rozen, W.M. Use of Artificial Intelligence in the Advancement of Breast Surgery and Implications for Breast Reconstruction: A Narrative Review. J. Clin. Med. 2023, 12, 5143. [Google Scholar] [CrossRef] [PubMed]
- Mullaney, P. Qualitative ultrasound training: Defining the learning curve. Clin. Radiol. 2019, 74, 327.e7–327.e19. [Google Scholar] [CrossRef]
- Malagón, P.; Hayashi, A.; Del Río, M.; Vilà, J.; García, O.; Carrasco, C.; Higueras, C. Improving the learning process of ultrasound in plastic surgery: How easy is to read ultrasound images? J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2831–2870. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cowan, R.; Mann, G.; Salibian, A.A. Ultrasound in Microsurgery: Current Applications and New Frontiers. J. Clin. Med. 2024, 13, 3412. https://doi.org/10.3390/jcm13123412
Cowan R, Mann G, Salibian AA. Ultrasound in Microsurgery: Current Applications and New Frontiers. Journal of Clinical Medicine. 2024; 13(12):3412. https://doi.org/10.3390/jcm13123412
Chicago/Turabian StyleCowan, Rachel, Gursimran Mann, and Ara A. Salibian. 2024. "Ultrasound in Microsurgery: Current Applications and New Frontiers" Journal of Clinical Medicine 13, no. 12: 3412. https://doi.org/10.3390/jcm13123412



