Pilot Study on Next-Generation Sequencing Analysis of Vaginal Microbiota in Clinically Infertile Patients Treated with Probiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Ethics Statement
2.3. Sample Collection and DNA Extraction
2.4. Illumina Sequencing
2.5. Metagenomics Analysis
2.6. Gene Function Prediction
2.7. Statistical Analysis of Species Differences
3. Results
3.1. Patients’ Characteristics and Pregnancy Outcomes
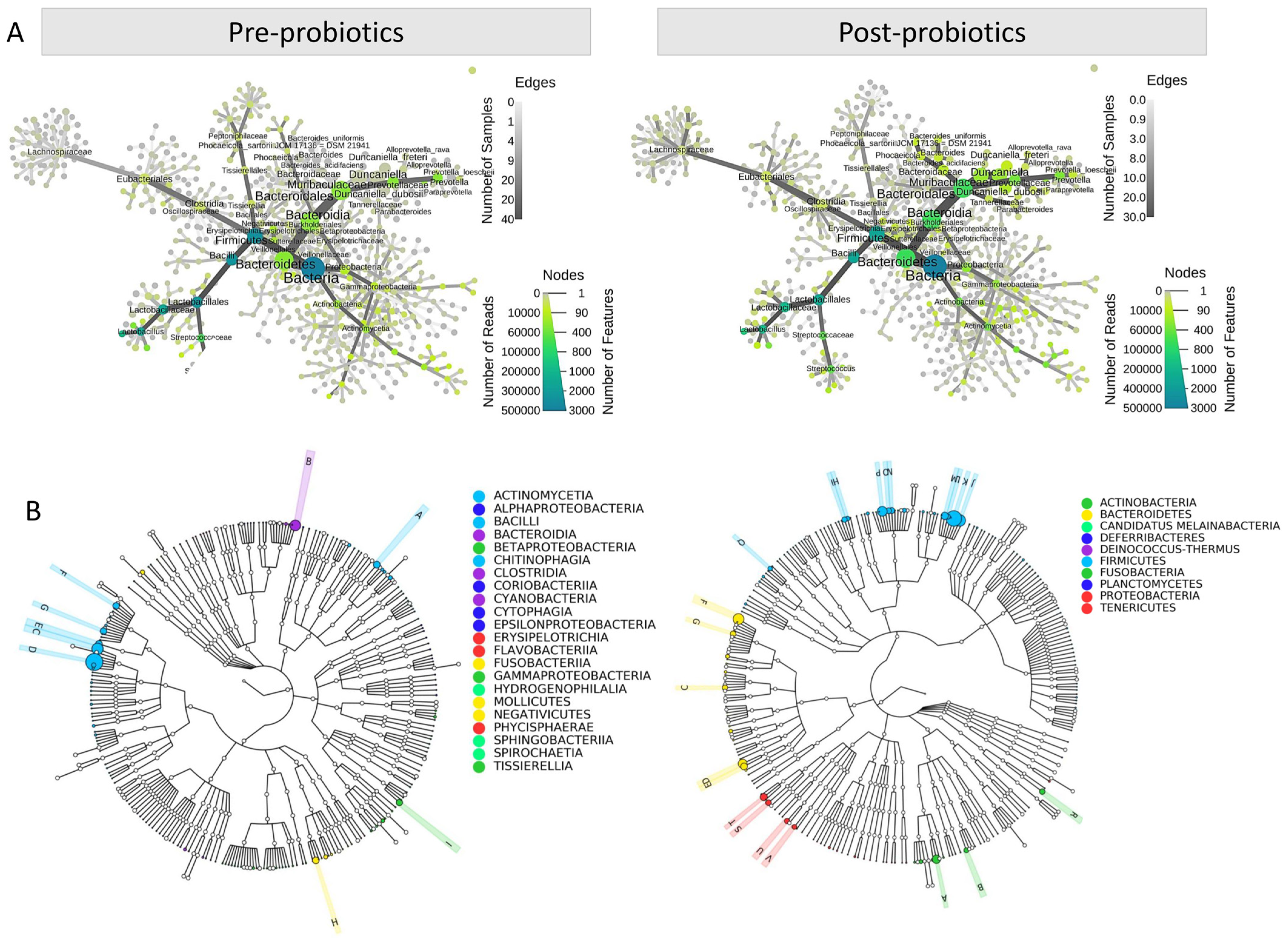
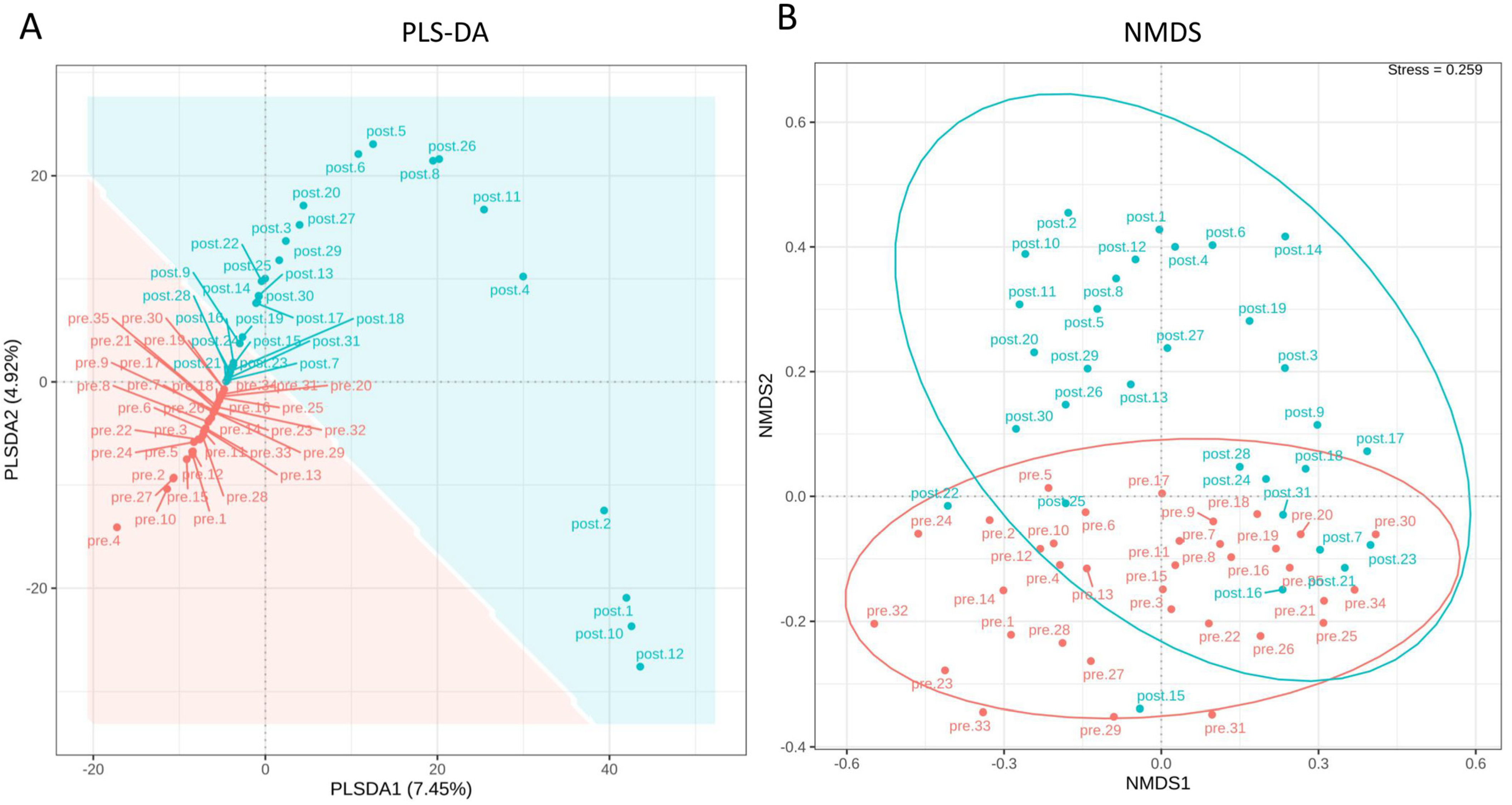
3.2. The Impact of Probiotics on the Diversity of the Female Reproductive Tract Microbiota
3.3. Richness, Diversity, and Differential Abundance of Reproductive Tract Microbiota
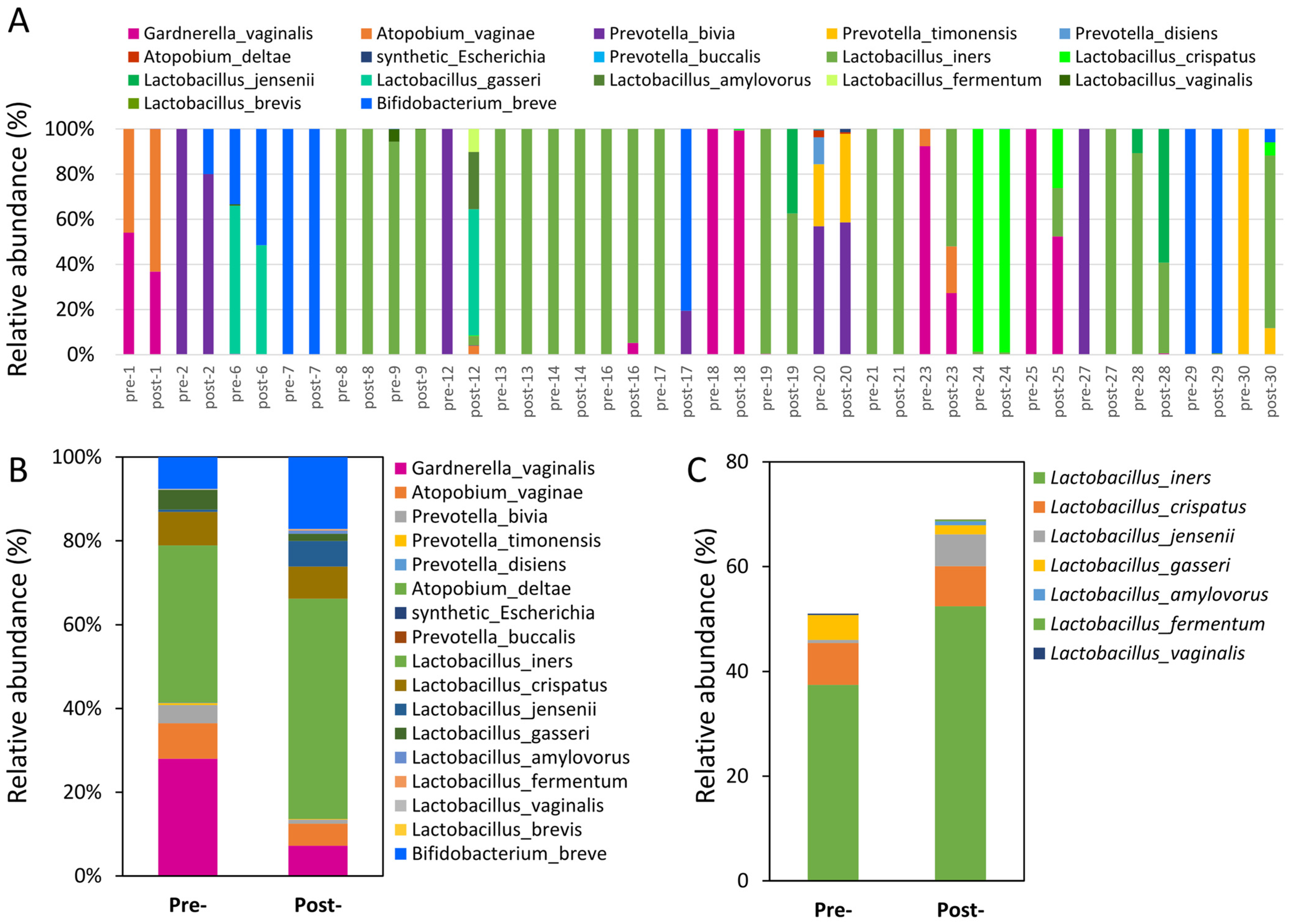
3.4. Assessment of Probiotics’ Influence on Vaginal Microbiota Composition
3.5. Functional Pathways Predicted by PiCrust2 to Intervene in Probiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larsson, P.G.; Brandsborg, E.; Forsum, U.; Pendharkar, S.; Andersen, K.K.; Nasic, S.; Hammarstrom, L.; Marcotte, H. Extended antimicrobial treatment of bacterial vaginosis combined with human lactobacilli to find the best treatment and minimize the risk of relapses. BMC Infect. Dis. 2011, 11, 223. [Google Scholar] [CrossRef]
- Huang, H.; Song, L.; Zhao, W. Effects of probiotics for the treatment of bacterial vaginosis in adult women: A meta-analysis of randomized clinical trials. Arch. Gynecol. Obstet. 2014, 289, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.; Betsi, G.I.; Athanasiou, S. Probiotics for the treatment of women with bacterial vaginosis. Clin. Microbiol. Infect. 2007, 13, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.; Singaravelu, B.G.; Srikumar, R.; Reddy, S.V.; Kokan, A. Comparative Study on the Vaginal Flora and Incidence of Asymptomatic Vaginosis among Healthy Women and in Women with Infertility Problems of Reproductive Age. J. Clin. Diagn. Res. 2017, 11, DC18–DC22. [Google Scholar] [CrossRef] [PubMed]
- Odogwu, N.M.; Olayemi, O.O.; Omigbodun, A.O. The vaginal microbiome of sub-Saharan African women: Revealing important gaps in the era of next-generation sequencing. PeerJ 2020, 8, e9684. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Redelinghuys, M.J.; Geldenhuys, J.; Jung, H.; Kock, M.M. Bacterial Vaginosis: Current Diagnostic Avenues and Future Opportunities. Front. Cell. Infect. Microbiol. 2020, 10, 354. [Google Scholar] [CrossRef]
- Kyono, K.; Hashimoto, T.; Nagai, Y.; Sakuraba, Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: A single-center pilot study. Reprod. Med. Biol. 2018, 17, 297–306. [Google Scholar] [CrossRef]
- Campisciano, G.; Florian, F.; D’Eustacchio, A.; Stankovic, D.; Ricci, G.; De Seta, F.; Comar, M. Subclinical alteration of the cervical-vaginal microbiome in women with idiopathic infertility. J. Cell. Physiol. 2017, 232, 1681–1688. [Google Scholar] [CrossRef]
- Feng, T.; Liu, Y. Microorganisms in the reproductive system and probiotic’s regulatory effects on reproductive health. Comput. Struct. Biotechnol. J. 2022, 20, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, G.; Wu, L.; Huang, X.; Li, Y.; Luo, B.; Zhu, H.; Huang, W. The Microbial Composition of Lower Genital Tract May Affect the Outcome of in vitro Fertilization-Embryo Transfer. Front. Microbiol. 2021, 12, 729744. [Google Scholar] [CrossRef] [PubMed]
- Karaer, A.; Dogan, B.; Gunal, S.; Tuncay, G.; Arda Duz, S.; Unver, T.; Tecellioglu, N. The vaginal microbiota composition of women undergoing assisted reproduction: A prospective cohort study. BJOG 2021, 128, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Haahr, T.; Jensen, J.S.; Thomsen, L.; Duus, L.; Rygaard, K.; Humaidan, P. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: A prospective study in IVF patients. Hum. Reprod. 2016, 31, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoner, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazan, J.; Alonso, R.; Alama, P.; Remohi, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Ferrari, F.; Ciebiera, M.; Zgliczynska, M.; Rapisarda, A.M.C.; Vecchio, G.M.; Pino, A.; Angelico, G.; Knafel, A.; Riemma, G.; et al. The Role of Genital Tract Microbiome in Fertility: A Systematic Review. Int. J. Mol. Sci. 2021, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Dube, R.; Kar, S.S. Genital Microbiota and Outcome of Assisted Reproductive Treatment-A Systematic Review. Life 2022, 12, 1867. [Google Scholar] [CrossRef] [PubMed]
- Koedooder, R.; Singer, M.; Schoenmakers, S.; Savelkoul, P.H.M.; Morre, S.A.; de Jonge, J.D.; Poort, L.; Cuypers, W.; Beckers, N.G.M.; Broekmans, F.J.M.; et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: A prospective study. Hum. Reprod. 2019, 34, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Dong, S.; Wang, Z.; Jiao, J.; Wang, X. Impact of a Lactobacillus dominant cervical microbiome, based on 16S-FAST profiling, on the reproductive outcomes of IVF patients. Front. Cell. Infect. Microbiol. 2023, 13, 1059339. [Google Scholar] [CrossRef]
- Villani, A.; Fontana, A.; Barone, S.; de Stefani, S.; Primiterra, M.; Copetti, M.; Panebianco, C.; Parri, C.; Scianname, N.; Quitadamo, P.A.; et al. Identifying Predictive Bacterial Markers from Cervical Swab Microbiota on Pregnancy Outcome in Woman Undergoing Assisted Reproductive Technologies. J. Clin. Med. 2022, 11, 680. [Google Scholar] [CrossRef]
- Haahr, T.; Humaidan, P.; Elbaek, H.O.; Alsbjerg, B.; Laursen, R.J.; Rygaard, K.; Johannesen, T.B.; Andersen, P.S.; Ng, K.L.; Jensen, J.S. Vaginal Microbiota and In Vitro Fertilization Outcomes: Development of a Simple Diagnostic Tool to Predict Patients at Risk of a Poor Reproductive Outcome. J. Infect. Dis. 2019, 219, 1809–1817. [Google Scholar] [CrossRef]
- Wee, B.A.; Thomas, M.; Sweeney, E.L.; Frentiu, F.D.; Samios, M.; Ravel, J.; Gajer, P.; Myers, G.; Timms, P.; Allan, J.A.; et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 341–348. [Google Scholar] [CrossRef]
- Lozano, F.M.; Lledo, B.; Morales, R.; Cascales, A.; Hortal, M.; Bernabeu, A.; Bernabeu, R. Characterization of the Endometrial Microbiome in Patients with Recurrent Implantation Failure. Microorganisms 2023, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Mandar, R.; Soerunurk, G.; Stsepetova, J.; Smidt, I.; Roop, T.; Koljalg, S.; Saare, M.; Ausmees, K.; Le, D.D.; Jaagura, M.; et al. Impact of Lactobacillus crispatus-containing oral and vaginal probiotics on vaginal health: A randomised double-blind placebo controlled clinical trial. Benef. Microbes 2023, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Son, D.; Hur, Y.M.; Park, S.; You, Y.A.; Kim, S.M.; Lee, G.; Kang, S.; Chung, Y.; Lim, S.; et al. Lactobacillus Probiotics Improve Vaginal Dysbiosis in Asymptomatic Women. Nutrients 2023, 15, 1862. [Google Scholar] [CrossRef]
- De Vrese, M.; Laue, C.; Papazova, E.; Petricevic, L.; Schrezenmeir, J. Impact of oral administration of four Lactobacillus strains on Nugent score—Systematic review and meta-analysis. Benef. Microbes 2019, 10, 483–496. [Google Scholar] [CrossRef]
- Thanaboonyawat, I.; Pothisan, S.; Petyim, S.; Laokirkkiat, P. Pregnancy outcomes after vaginal probiotic supplementation before frozen embryo transfer: A randomized controlled study. Sci. Rep. 2023, 13, 11892. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Castro, I.; Arroyo, R.; Alba, C.; Beltran, D.; Rodriguez, J.M. Immunomodulation of the Vaginal Ecosystem by Ligilactobacillus salivarius CECT 30632 Improves Pregnancy Rates among Women with Infertility of Unknown Origin or Habitual Abortions. Nutrients 2023, 15, 362. [Google Scholar] [CrossRef]
- Iniesta, S.; Esteban, S.; Armijo, O.; Lobo, S.; Manzano, S.; Espinosa, I.; Cardenas, N.; Bartha, J.L.; Jimenez, E. Ligilactobacillus salivarius PS11610 exerts an effect on the microbial and immunological profile of couples suffering unknown infertility. Am. J. Reprod. Immunol. 2022, 88, e13552. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, X.; Liang, Y.; Lin, S.; Qian, W.; Fan, S. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. mBio 2020, 11, e03242-19. [Google Scholar] [CrossRef]
- Freitas, A.C.; Chaban, B.; Bocking, A.; Rocco, M.; Yang, S.; Hill, J.E.; Money, D.M.; Group, V.R. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Sci. Rep. 2017, 7, 9212. [Google Scholar] [CrossRef] [PubMed]
- Hyman, R.W.; Herndon, C.N.; Jiang, H.; Palm, C.; Fukushima, M.; Bernstein, D.; Vo, K.C.; Zelenko, Z.; Davis, R.W.; Giudice, L.C. The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J. Assist. Reprod. Genet. 2012, 29, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, A.; Lledo, B.; Díaz, M.C.; Lozano, F.M.; Ruiz, V.; Fuentes, A.; Lopez-Pineda, A.; Moliner, B.; Castillo, J.C.; Ortiz, J.A.; et al. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J. Assist. Reprod. Genet. 2019, 36, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhu, S.; Wang, B.; Duan, L. Alterations of Gut Microbiota in Patients with Irritable Bowel Syndrome Based on 16S rRNA-Targeted Sequencing: A Systematic Review. Clin. Transl. Gastroenterol. 2019, 10, e00012. [Google Scholar] [CrossRef]
- Oliver, A.; LaMere, B.; Weihe, C.; Wandro, S.; Lindsay, K.L.; Wadhwa, P.D.; Mills, D.A.; Pride, D.T.; Fiehn, O.; Northen, T.; et al. Cervicovaginal Microbiome Composition Is Associated with Metabolic Profiles in Healthy Pregnancy. mBio 2020, 11, e01851-20. [Google Scholar] [CrossRef]


| Items | Data |
|---|---|
| Age (years) | 37.0 ± 3.8 |
| Body weight (kg) | 61.9 ± 12.5 |
| BMI (kg/m2) | 23.3 ± 3.6 |
| Infertility duration (years) | 4.1 ± 3.0 |
| Menstruation cycle | |
| Regular | 83.3% |
| Irregular | 16.7% |
| Menstruation period (days) | 29.0 ± 2.3 |
| Menstruation length (days) | 6.0 ± 1.6 |
| Type of infertility | |
| Primary | 63.3% |
| Secondary | 36.7% |
| Duration of probiotic supplementation (weeks) | 8.6 ± 1.5 |
| Pregnancy rate (%) | 56.7% |
| Time to pregnancy following probiotic intervention (weeks) | 10.5 ± 12.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.-T.; Li, C.-J.; Wu, C.-C.; Pan, L.-F.; Tsui, K.-H. Pilot Study on Next-Generation Sequencing Analysis of Vaginal Microbiota in Clinically Infertile Patients Treated with Probiotics. J. Clin. Med. 2024, 13, 3420. https://doi.org/10.3390/jcm13123420
Lin L-T, Li C-J, Wu C-C, Pan L-F, Tsui K-H. Pilot Study on Next-Generation Sequencing Analysis of Vaginal Microbiota in Clinically Infertile Patients Treated with Probiotics. Journal of Clinical Medicine. 2024; 13(12):3420. https://doi.org/10.3390/jcm13123420
Chicago/Turabian StyleLin, Li-Te, Chia-Jung Li, Chia-Chun Wu, Li-Fei Pan, and Kuan-Hao Tsui. 2024. "Pilot Study on Next-Generation Sequencing Analysis of Vaginal Microbiota in Clinically Infertile Patients Treated with Probiotics" Journal of Clinical Medicine 13, no. 12: 3420. https://doi.org/10.3390/jcm13123420
APA StyleLin, L.-T., Li, C.-J., Wu, C.-C., Pan, L.-F., & Tsui, K.-H. (2024). Pilot Study on Next-Generation Sequencing Analysis of Vaginal Microbiota in Clinically Infertile Patients Treated with Probiotics. Journal of Clinical Medicine, 13(12), 3420. https://doi.org/10.3390/jcm13123420









