Gelatin-Based Liver Phantoms for Training Purposes: A Cookbook Approach
Abstract
:1. Introduction
2. Materials and Methods
- Criteria 1: hardness—how hard/soft is the model when handling;
- Criteria 2: friability—how easy or not the model fracture when it is used for training;
- Criteria 3: handling—how easy or not the model can be handled without being damaged;
- Criteria 4: optimal characteristics for ultrasound—how optimal/not optimal is the model for ultrasound examination;
- Criteria 5: optimal characteristics for elastography—how optimal/not optimal is the model for ultrasound elastography;
- Criteria 6: optimal characteristics for Fibroscan—how optimal/not optimal is the model for Fibroscan examination;
- Criteria 7: optimal for multiple punctures—how well/not well does the model behave for multiple puncture;
- Criteria 8: puncture resistance—how easy/difficult is to puncture the model;
- Criteria 9: optimal for training in ultrasound-guided procedures—how optimal/not optimal is the model for ultrasound-guided procedures.
- Criteria 1: contrast to the surrounding tissues—how good/poor is the contrast to the surrounding tissues.
- Criteria 2: visible limit compared to the surrounding tissues—how easy/difficult is to identify the limit between the contrast and non-contrast tissue.
- Criteria 3: easy to identify from the surrounding tissues (even at small sizes)—how easy/difficult is to identify a contrast tissue inside the model.
- Criteria 4: homogeneity—how homogeneous is the gelatin-based contrast solution.
- G8: 8 g gelatin/100 mL liquid (water) + 12.5 g sugar
- G12: 12 g gelatin/100 mL liquid (water) + 15 g sugar
- G14: 14 g gelatin/100 mL liquid (water) + 17.5 g sugar
- G16: 16 g gelatin/100 mL liquid (water) + 20 g sugar
- G14i5: 14 g gelatin/100 mL liquid (95% water, 5% intravenous lipid solution) + 17.5 g sugar
- G14i10: 14 g gelatin/100 mL liquid (90% water, 10% intravenous lipid solution) + 17.5 g sugar
- G14i15: 14 g gelatin/100 mL liquid (85% water, 15% intravenous lipid solution) + 17.5 g sugar
- G14alc10: 14 g gelatin/100 mL liquid (90% water, 10% technic alcohol) + 17.5 g sugar
- G14alc20: 14 g gelatin/100 mL liquid (80% water, 20% technic alcohol) + 17.5 g sugar
- G14s32:17.5: 14 g gelatin/100 mL liquid (75% water, 25% cream solution) + 17.5 g sugar
- G14s32:15: 14 g gelatin/100 mL liquid (75% water, 25% cream solution) + 15 g sugar
- G14s32:12.5: 14 g gelatin/100 mL liquid (75% water, 25% cream solution) + 12.5 g sugar
- G14s32:10: 14 g gelatin/100 mL liquid (75% water, 25% cream solution) + 10 g sugar
- G14s32:7.5: 14 g gelatin/100 mL liquid (75% water, 25% cream solution) + 7.5 g sugar
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| G8 | G12 | G14 | |
| Ultrasound | 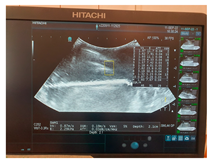 | 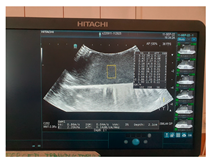 | 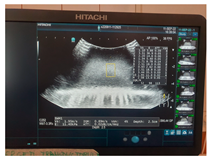 |
| CT-scan |  |  |  |
| MRI–T1 |  |  |  |
| MRI–T2 |  |  |  |
| Fracture force graphic | 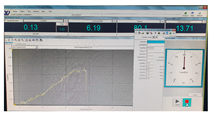 |  |  |
| G16 | G14Alc10 | G14Alc20 | |
| Ultrasound |  | 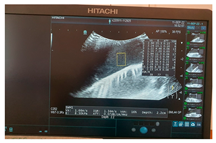 | 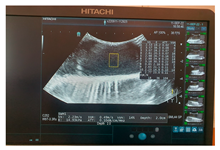 |
| CT-scan | 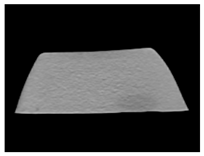 |  | 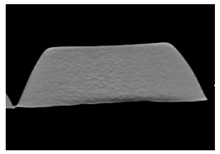 |
| MRI–T1 | 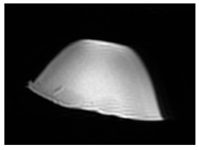 | 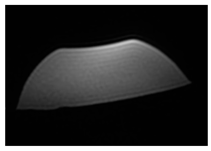 | 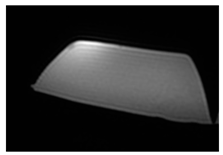 |
| MRI–T2 | 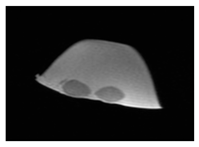 | 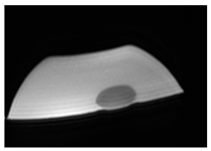 |  |
| Fracture force graphic |  |  |  |
| G14i5 | G14i10 | G14i15 | |
| Ultrasound | 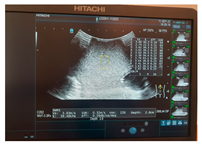 | 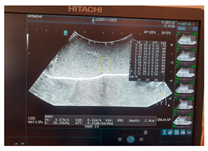 | 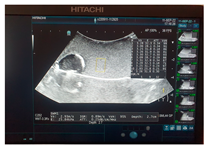 |
| CT-scan |  | 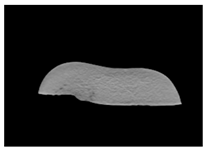 |  |
| MRI–T1 |  |  |  |
| MRI–T2 |  | 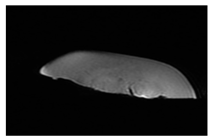 |  |
| Fracture force graphic |  |  |  |
| G14S32:25-17.5 | G14S32:25-15 | G14S32:25-12.5 | |
| Ultrasound | 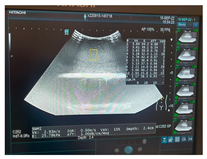 | 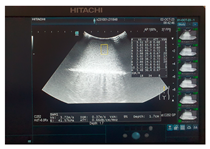 |  |
| CT-scan | 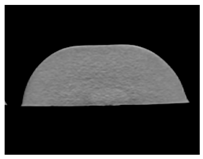 |  | 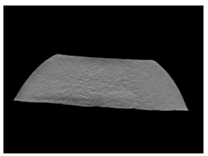 |
| MRI–T1 |  |  |  |
| MRI–T2 | 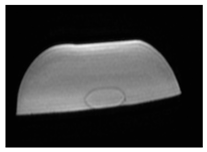 | 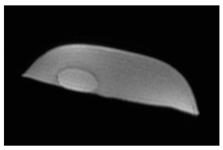 |  |
| Fracture force graphic |  |  | 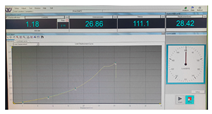 |
| G14S32:25-10 | G14S32:25-7.5 | Pig liver (ex vivo) | |
| Ultrasound | 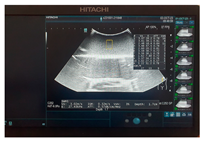 | 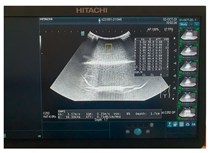 | 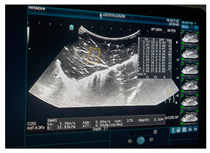 |
| CT-scan |  |  |  |
| MRI–T1 | 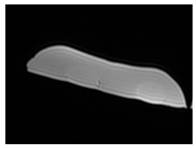 |  | 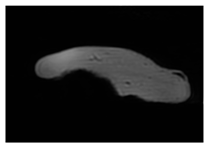 |
| MRI–T2 | 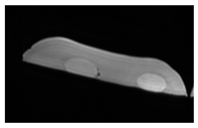 |  | 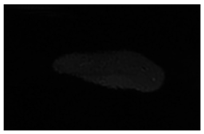 |
| Fracture force graphic |  |  |  |
| Human normal liver | Human fatty liver | Human cirrhotic liver | |
| Ultrasound | 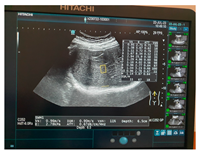 | 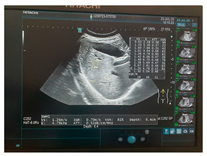 | 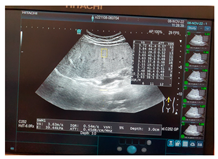 |
| CT-scan | 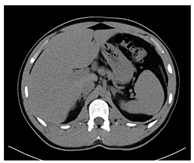 |  | 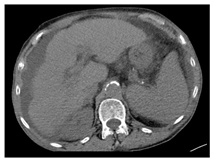 |
| MRI–T1 | 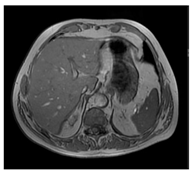 | 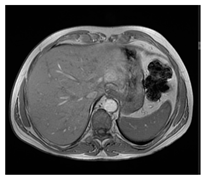 | 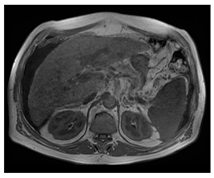 |
| MRI–T2 | 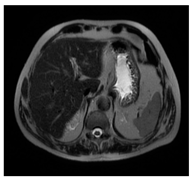 |  | 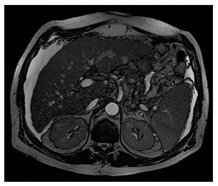 |
| Fracture force graphic | N.A. | N.A. | N.A. |
References
- Kyoung, M.I.; Eun, Y.K. Impact of 8-Week Bedside Ultrasound Training for Surgical Residents in the Intensive Care Unit of a Tertiary Care Hospital—A Pilot Study. J. Surg. Ultrasound 2021, 8, 6–18. [Google Scholar] [CrossRef]
- Torzilli, G.; McCormack, L.; Pawlik, T. Parenchyma-sparing liver resections. Int. J. Surg. 2020, 82, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D. American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009, 49, 1017–1044. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ms.ro/media/documents/Publica%C8%9Bia_de_examen_pentru_ob%C8%9Binerea_atestatelor_de_studii_.pdf (accessed on 15 March 2023).
- Park, R.; Lee, S.M.; Kim, S.; Park, S.; Choe, J.; Do, K.H.; Seo, J.B. Learning curve for CT-guided percutaneous transthoracic needle biopsy: Retrospective evaluation among 17 thoracic imaging fellows at a tertiary referral hospital. Am. J. Roentgenol. 2022, 218, 112–123. [Google Scholar] [CrossRef] [PubMed]
- CIRS Inc.—Computerized Imaging Reference Systems, Tissue Simulation & Phantom Technology (2021): Triple Modality 3D Abdominal Phantom—Model 057A. Available online: https://www.cirsinc.com/products/ultrasound/zerdine-hydrogel/triple-modality-3d-abdominal-phantom/ (accessed on 2 August 2021).
- Kyoto Kagaku Co., Ltd. Abdominal Intraoperative & Laparoscopic Ultrasound Phantom—IOUSFAN, Update 7 August 2020. Available online: https://www.kyotokagaku.com/en/products_data/us-3/ (accessed on 2 August 2021).
- MDD Inc.—Medical Device Depot: Triple Modality 3D abdominal Phantom—Model 057A. Available online: https://www.medicaldevicedepot.com/Triple-Modality-3D-Abdominal-Phantom-p/057a.htm (accessed on 2 August 2021).
- Limbs&Things: IOUSFAN—Abdominal Intraoperative & Laparoscopic Ultrasound Phantom. Available online: https://limbsandthings.com/au/products/KKUS-3/kkus-3-iousfan-abdominal-intraoperative-laparoscopic-ultrasound-phantom (accessed on 2 August 2021).
- Culjat, M.O.; Goldenberg, D.; Tewari, P.; Singh, R.S. A review of tissue substitutes for ultrasound imaging. Ultrasound Med. Biol. 2010, 36, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Chmarra, M.K.; Hansen, R.; Mårvik, R.; Langø, T. Multimodal phantom of liver tissue. PLoS ONE 2013, 8, e64180. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, N.; Schwaiger, J.; Markert, M.; Flatz, W.; Lueth, T.C. Evaluation of a resectable ultrasound liver phantom for testing of surgical navigation systems. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 916–919. [Google Scholar] [CrossRef]
- Bao, P.; Warmath, J.; Galloway, R.; Herline, A. Ultrasound to computer-tomography registration for image-guided laparoscopic liver surgery. Surg. Endosc. 2005, 19, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Luddington, O.S.; Culleton, S.R.; Francis, R.J.; Boucek, J.A. A gelatin liver phantom of suspended 90Y resin microspheres to simulate the physiologic microsphere biodistribution of a postradioembolization liver. J. Nucl. Med. Technol. 2014, 42, 265–268. [Google Scholar] [CrossRef]
- Schwaiger, J.; Markert, M.; Shevchenko, N.; Lueth, T.C. The effects of real-time image navigation in operative liver surgery. Int. J. Comput. Assist. Radiol. Surg. 2011, 6, 785–796. [Google Scholar] [CrossRef]
- Sugimoto, K.; Moriyasu, F.; Shiraishi, J.; Yamada, M.; Imai, Y. A phantom study comparing ultrasound-guided liver tumor puncture using newreal-time 3Dultrasound and conventional 2Dultrasound. Am. J. Roentgenol. 2011, 196, W753–W757. [Google Scholar] [CrossRef]
- Pacioni, A.; Carbone, M.; Freschi, C.; Viglialoro, R.; Ferrari, V.; Ferrari, M. Patient-specific ultrasound liver phantom: Materials and fabricationmethod. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Banovac, F.; Tang, J.; Xu, S.; Lindisch, D.; Chung, H.Y.; Levy, E.B.; Chang, T.; McCullough, M.F.; Yaniv, Z.; Wood, B.J.; et al. Precision targeting of liver lesions using a novel electromagnetic navigation evice in physiologic phantom and swine. Med. Phys. 2005, 32, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Joe, E.; Kim, S.H.; Lee, K.B.; Jang, J.-J.; Lee, J.Y.; Lee, J.M.; Han, J.K.; Choi, B.I. Feasibility and accuracy of dual-source dual-energy CT for noninvasive determination of hepatic iron accumulation. Radiology 2012, 262, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Murotani, K.; Kazuhiro, M.; Kawai, N.; Sato, M.; Minamiguchi, H.; Nakai, M.; Sonomura, T.; Hosokawa, S.; Nishioku, T. Optimum CT reconstruction parameters for vascular and hepatocellular carcinoma models in a liver phantom with multi-level dynamic computed tomography with 64 detector rows: A basic study. Radiol. Phys. Technol. 2013, 6, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Widmann, G.; Wallach, D.; Toporek, G.; Schullian, P.; Weber, S.; Bale, R. Angiographic C-arm CT- versus MDCT-guided stereotactic punctures of liver lesions: Nonrigid phantom study. Am. J. Roentgenol. 2013, 201, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- In, E.; Naguib, H.; Haider, M. Mechanical stability analysis of carrageenan-based polymer gel for magnetic resonance imaging liver phantom with lesion particles. J. Med. Imaging 2014, 1, 035502. [Google Scholar] [CrossRef] [PubMed]
- Rube, M.A.; Holbrook, A.B.; Cox, B.F.; Buciuc, R.; Melzer, A. Wireless mobile technology to improve workflow and feasibility of MR-guided percutaneous interventions. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.; Lalwani, R.; Asghar, A.; Sahai, A.; Sharma, P.; Singh, R. Assessment of liver volume with spiral computerized tomography scanning in north indian adults. Internet J. Radiol. 2009, 13, 1. [Google Scholar]
- Andersen, V.; Sonne, J.; Sletting, S.; Prip, A. The volume of the liver in patients correlates to body weight and alcohol consumption. Alcohol Alcohol. 2000, 35, 531–532. [Google Scholar] [CrossRef]
- Available online: https://www.wikihow.com/Make-Ballistics-Gel (accessed on 2 August 2021).
- Barr, R.G. Shear wave liver elastography. Abdom. Radiol. 2018, 43, 800–807. [Google Scholar] [CrossRef]
- Petzold, G.; Hofer, J.; Ellenrieder, V.; Neesse, A.; Kunsch, S. Liver Stiffness Measured by 2-Dimensional Shear Wave Elastography: Prospective Evaluation of Healthy Volunteers and Patients With Liver Cirrhosis. J. Ultrasound Med. 2019, 38, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Maklad, N.F.; Ophir, J.; Balsara, V. Attenuation of ultrasound in normal liver and diffuse liver disease in vivo. Ultrason. Imaging 1984, 6, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Kumar, V.; Ozturk, A.; Nam, K.; de Korte, C.L.; Barr, R.G. US Attenuation for Liver Fat Quantification: An AIUM-RSNA QIBA Pulse-Echo Quantitative Ultrasound Initiative. Radiology 2022, 302, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Starekova, J.; Hernando, D.; Pickhardt, P.J.; Reeder, S.B. Quantification of Liver Fat Content with CT and MRI: State of the Art. Radiology 2021, 301, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Tisch, C.; Brencicova, E.; Schwendener, N.; Lombardo, P.; Jackowski, C.; Zech, W.D. Hounsfield unit values of liver pathologies in unenhanced post-mortem computed tomography. Int. J. Legal Med. 2019, 133, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Kim, S.Y.; Kim, K.W.; Lim, Y.S.; Lee, S.J.; Lee, M.G.; Lee, J.; Lee, S.G.; Yu, E. Determination of normal hepatic elasticity by using real-time shear-wave elastography. Radiology 2014, 271, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Reis, J.; Oppenheimer, D.C.; Rubens, D.J.; Ormachea, J.; Hah, Z.; Parker, K.J. Attenuation of Shear Waves in Normal and Steatotic Livers. Ultrasound Med. Biol. 2019, 45, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Guibal, A.; Renosi, G.; Rode, A.; Scoazec, J.Y.; Guillaud, O.; Chardon, L.; Munteanu, M.; Dumortier, J.; Collin, F.; Lefort, T. Shear wave elastography: An accurate technique to stage liver fibrosis in chronic liver diseases. Diagn. Interv. Imaging 2016, 97, 91–99. [Google Scholar] [CrossRef]
- Foucher, J.; Chanteloup, E.; Vergniol, J.; Castéra, L.; Le Bail, B.; Adhoute, X.; Bertet, J.; Couzigou, P.; de Lédinghen, V. Diagnosis of cirrhosis by transient elastography (FibroScan): A prospective study. Gut 2006, 55, 403–408. [Google Scholar] [CrossRef]
- Madsen, E.L.; Zagzebski, J.A.; Banjavie, R.A.; Jutila, R.E. Tissue mimicking materials for ultrasound phantoms. Med. Phys. 1978, 5, 391–394. [Google Scholar] [CrossRef]
- Burlew, M.M.; Madsen, E.L.; Zagzebski, J.A.; Banjavic, R.A.; Sum, S.W. A new ultrasound tissue-equivalent material. Radiology 1980, 134, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Ophir, J. Ultrasound Phantom. U.S. Patent 4,286,455, 1 September 1981. [Google Scholar]
- Madsen, E.L.; Frank, G.R.; Dong, F. Liquid or solid ultrasonically tissue-mimicking materials with very low scatter. Ultrasound Med. Biol. 1998, 24, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.L.; Frank, G.R.; Krouskop, T.A.; Varghese, T.; Kallel, F.; Ophir, J. Tissue-mimicking oil-in-gelatin dispersions for use in heterogeneous elastography phantoms. Ultrason Imaging 2003, 25, 17–38. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, W.D.; Madsen, E.L.; Unal, O.; Vigen, K.K.; Frank, G.R.; Thomadsen, B.R. Tissue mimicking materials for a multi-imaging modality prostate phantom. Med. Phys. 2001, 28, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Ramnarine, K.V.; Anderson, T.; Hoskins, P.R. Construction and geometric stability of physiological flow rate wall-less stenosis phantoms. Ultrasound Med. Biol. 2001, 27, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Brewin, M.P.; Pike, L.C.; Rowland, D.E.; Birch, M.J. The acoustic properties, centered on 20 MHZ, of an IEC agar-based tissue-mimicking material and its temperature, frequency and age dependence. Ultrasound Med. Biol. 2008, 34, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.; Duck, F.A. Ultrasonic tissue-equivalent materials using inorganic gel mixtures. Br. J. Radiol. 1982, 55, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kitatuji, M.; Kanda, H. New tissue mimicking materials for ultrasound phantoms. In Proceedings of the 2005 IEEE Ultrasonics Symposium, Rotterdam, The Netherlands, 18–21 September 2005; pp. 1664–1667. [Google Scholar]
- Dong, F.; Madsen, E.L.; MacDonald, M.C.; Zagzebski, J.A. Nonlinearity parameter for tissue-mimicking materials. Ultrasound Med. Biol. 1999, 25, 831–838. [Google Scholar] [CrossRef]
- Ophir, J. Ultrasound phantom material. Br. J. Radiol. 1984, 57, 1161. [Google Scholar] [CrossRef]
- Lerski, R.A.; Duggan, T.C.; Christie, J. A simple tissue-like ultrasound phantom material. Br. J. Radiol. 1982, 55, 156–157. [Google Scholar] [CrossRef]
- Zell, K.; Sperl, J.I.; Vogel, M.W.; Niessner, R.; Haisch, C. Acoustical properties of selected tissue phantom materials for ultrasound imaging. Phys. Med. Biol. 2007, 52, N475–N484. [Google Scholar] [CrossRef] [PubMed]
- Fromageau, J.; Brusseau, E.; Vray, D.; Gimenez, G.; Delachartre, P. Characterization of PVA cryogel for intravascular ultrasound elasticity imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2003, 50, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Kharine, A.; Manohar, S.; Seeton, R.; Kolkman, R.G.; Bolt, R.A.; Steenbergen, W.; de Mul, F.F. Poly(vinyl alcohol) gels for use as tissue phantoms in photoacoustic mammography. Phys. Med. Biol. 2003, 48, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Surry, K.J.; Austin, H.J.; Fenster, A.; Peters, T.M. Poly(vinyl alcohol) cryogel phantoms for use in ultrasound and MR imaging. Phys. Med. Biol. 2004, 49, 5529–5546. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Leen, E.; Goldberg, J.A.; Angerson, W.J.; Sutherland, G.R.; McArdle, C.S. Flow measurement using duplex Doppler ultrasound: Haemodynamic changes in patients with colorectal liver metastases. Clin. Phys. Physiol. Meas. 1992, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Radiation Units and Measurements. Tissue Substitutes, Phantoms, and Computational Modelling in Medical Ultrasound; ICRU: Bethesda, MD, USA, 1998. [Google Scholar]
- Wojcik, G.; Szabo, T.; Mould, J.; Carcione, L.; Clougherty, F. Nonlinear pulse calculations and data in water and a tissue mimic. In Proceedings of the 1999 IEEE Ultrasonics Symposium. Proceedings. International Symposium (Cat. No.99CH37027), Tahoe, NV, USA, 17–20 October 1999; Volume 2, pp. 1521–1526. [Google Scholar]
- Kim, Y.T.; Kim, H.C.; Inada-Kim, M.; Jung, S.S.; Yun, Y.H.; Jho, M.J.; Sandstrom, K. Evaluation of tissue mimicking quality of tofu for biomedical ultrasound. Ultrasound Med. Biol. 2009, 35, 472–481. [Google Scholar] [CrossRef]
- Davies, R.P.; Kew, J. Tissue phantom for learning US-guided vascular punctures. J. Vasc. Interv. Radiol. 2001, 12, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Abbas, S.; Chan, V.W. Ultrasound phantom for hands-on practice. Reg. Anesth. Pain. Med. 2005, 30, 593–594. [Google Scholar] [CrossRef]
- Nicotra, J.J.; Gay, S.B.; Wallace, K.K.; McNulty, B.C.; Dameron, R.D. Evaluation of a breast biopsy phantom for learning freehand ultrasound-guided biopsy of the liver. Acad. Radiol. 1994, 1, 385–387. [Google Scholar] [CrossRef]
- Bude, R.O.; Adler, R.S. An easily made, low-cost, tissue-like ultrasound phantom material. J. Clin. Ultrasound 1995, 23, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Rethy, A.; Sæternes, J.O.; Halgunset, J.; Mårvik, R.; Hofstad, E.F.; Sánchez-Margallo, J.A.; Langø, T. Anthropomorphic liver phantom with flow for multimodal image-guided liver therapy research and training. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 61–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ursu, C.-P.; Ciocan, A.; Ursu, Ș.; Ciocan, R.A.; Gherman, C.D.; Cordoș, A.-A.; Vălean, D.; Pop, R.S.; Furcea, L.E.; Procopeț, B.; et al. Prognostic Indicators of Overall Survival in Hepatocellular Carcinoma Patients Undergoing Liver Resection. Cancers 2024, 16, 1427. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Kizawa, S.; Daimon, M.; Minami, H.; Ueda, Y.; Tomioka, A.; Komeda, K.; Asakuma, M.; Tomiyama, H.; Lee, S.W. Surgical management of right hepatectomy after coronary artery bypass grafting using the right gastroepiploic artery: A case report and literature review. World J. Surg. Oncol. 2024, 22, 119. [Google Scholar] [CrossRef]
- Ciocan, A.; Mărgărit, S.; Bartoș, A.; Ciobanu, L.; Iancu, I.; Ciocan, R.A.; Al Hajjar, N. Emergency liver resection for non-traumatic lesions: A systematic review. Ann. Ital. Chir. 2023, 94, 580–586. [Google Scholar]
- Shrimal, P.; Thakur, N.; Gopinath, B.; Mishra, P.R.; Rajalekshmi, R.; Bhoi, S.; Aggarwal, P.; Jamshed, N.; Upadhyay, A.D. Comparing commercial versus low-cost gelatinous phantoms for ultrasound-guided needle tracking: A randomized crossover trial, among emergency medicine residents. Turk. J. Emerg. Med. 2024, 24, 103–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richardson, C.; Bernard, S.; Dinh, V.A. A Cost-effective, Gelatin-Based Phantom Model for Learning Ultrasound-Guided Fine-Needle Aspiration Procedures of the Head and Neck. J. Ultrasound Med. 2015, 34, 1479–1484. [Google Scholar] [CrossRef] [PubMed]

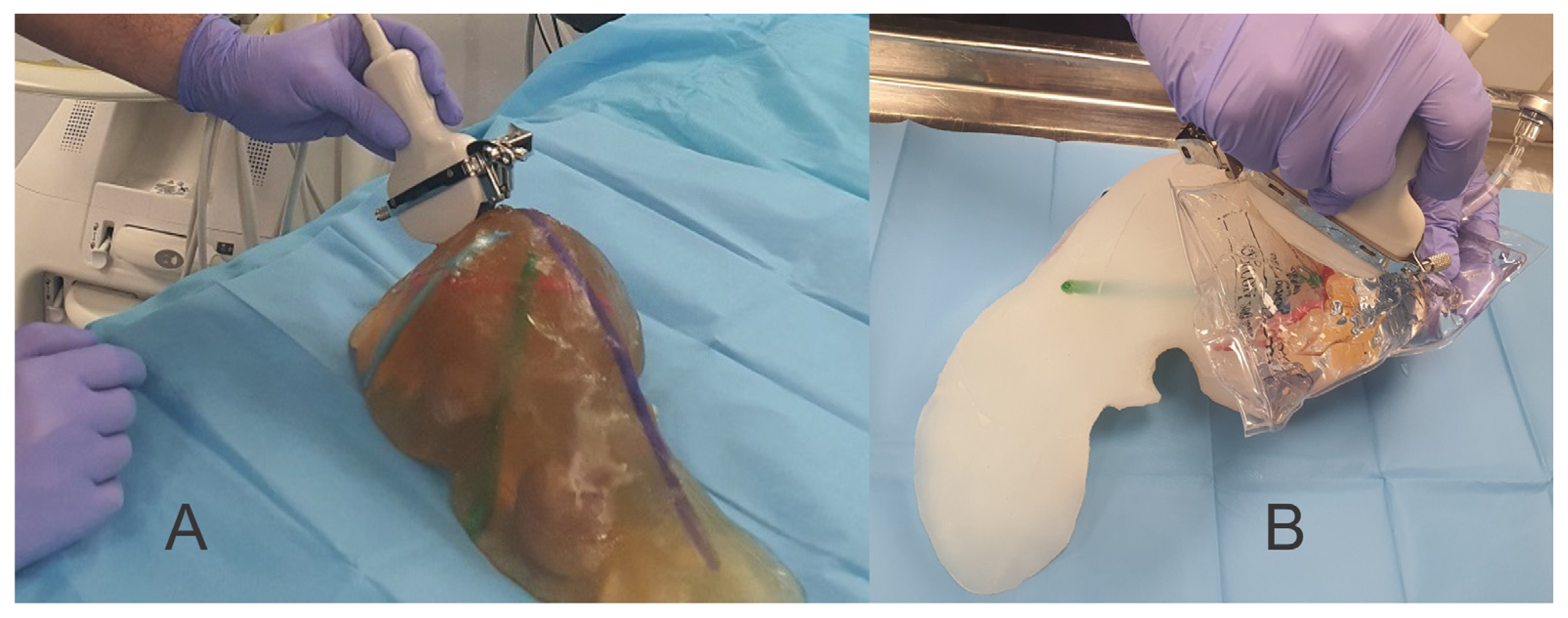
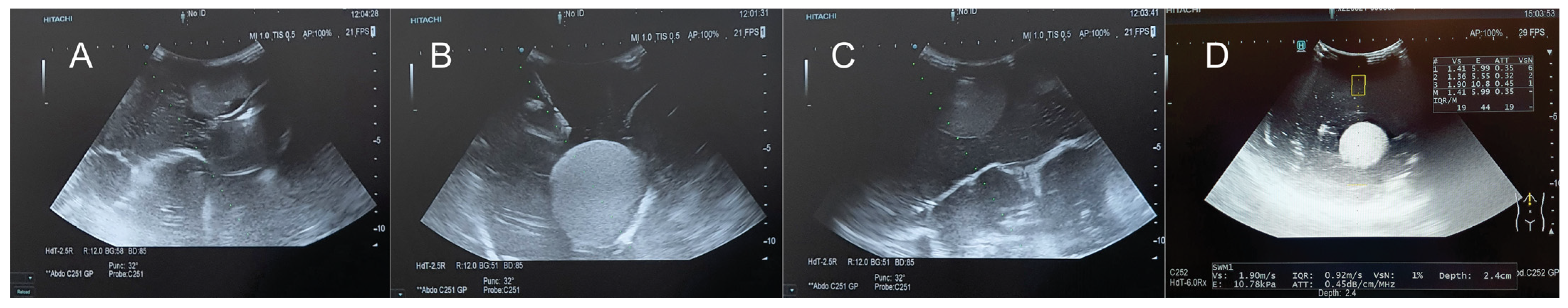
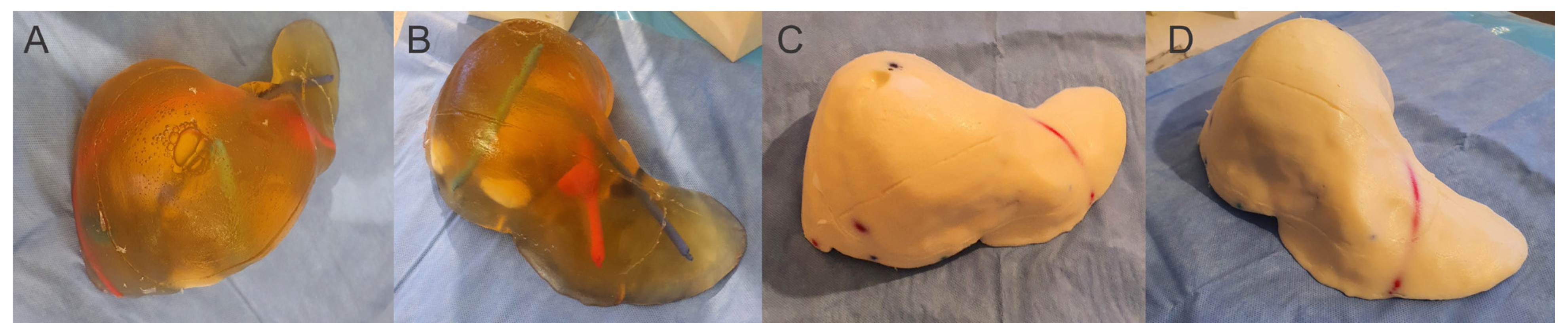
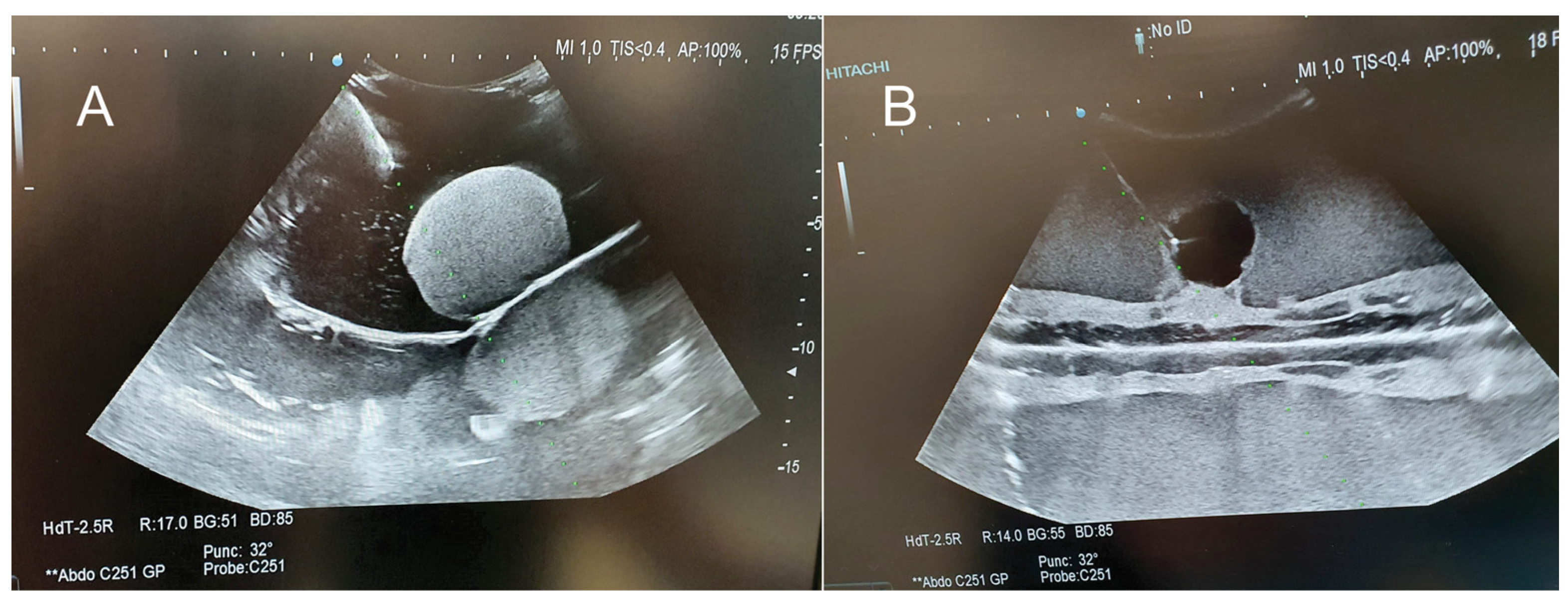
| Criteria | Ex Vivo Pig Liver | Silicone Liver Shore A 13 | Gelatin Liver—16 g | Gelatin Liver—14 g | Gelatin Liver—12 g | Gelatin Liver—8 g | |
|---|---|---|---|---|---|---|---|
| 1 | Hardness | 3 | 5 | 4 | 4 | 3 | 2 |
| 2 | Friability | 3 | 1 | 4 | 3 | 4 | 5 |
| 3 | Handling | 4 | 5 | 3 | 4 | 3 | 1 |
| 4 | Optimal characteristics for ultrasound | 5 | 2 | 3 | 4 | 3 | 2 |
| 5 | Optimal characteristics for elastography | 5 | 2 | 3 | 4 | 4 | 2 |
| 6 | Optimal characteristics for Fibriscan | 5 | 0 | 0 | 0 | 3 | 0 |
| 7 | Optimal for multiple punctures | 4 | 5 | 3 | 4 | 3 | 2 |
| 8 | Resistance to punctures | 3 | 5 | 4 | 3 | 2 | 2 |
| 9 | Optimal for training in US-guided procedure | 5 | 2 | 4 | 5 | 4 | 2 |
| Total | 37 | 27 | 28 | 31 | 29 | 18 |
| Criteria | Wheat Flour | Corn Starch | Talcum Powder | 32% Fat Bovine Milk/i.v. Lipid Solution | |
|---|---|---|---|---|---|
| 1 | Contrast to the surrounding tissues | 4 | 3 | 2 | 5 |
| 2 | Visible limit compared to the surrounding tissues | 5 | 3 | 3 | 5 |
| 3 | Easy to identify from the surrounding tissues (even at small sizes) | 5 | 3 | 2 | 5 |
| 4 | Homogeneity | 3 | 2 | 2 | 5 |
| Total | 17 | 11 | 9 | 20 |
| Elasticity (Kilopascal—kPa) | Ultrasounds Attenuation (dB/cm/MHz) | Shear Wave Speed (m/s) | CT-Scan Density (Hounsfield Unit—HU) | MRI–T1 Signal Intensity (SI-a.u.) | MRI–T2 Signal Intensity (SI-a.u.) | Fracture Force (Kilonewton—kN) | |
|---|---|---|---|---|---|---|---|
| G8 | 7.67 | 0.04 | 1.60 | 52.29 | 1012.7 | 1355.4 | 0.23 |
| G12 | 11 | 0.10 | 1.9 | 71 | 1329.1 | 808.5 | 0.30 |
| G14 | 13 | 0.09 | 2.08 | 82.07 | 1458.1 | 1103.3 | 0.55 |
| G16 | 5.84 | 0.12 | 1.39 | 92.32 | 1607.6 | 890.1 | 0.38 |
| G14i5 | 30.9 | 0.15 | 3.21 | 83.25 | 1489.4 | 1642.7 | 0.50 |
| G14i10 | 33 | 0.20 | 3.32 | 80.73 | 1547.6 | 1289.6 | 0.52 |
| G14i15 | 39.2 | 0.28 | 3.62 | 71.5 | 1223.6 | 1863.7 | 0.67 |
| G14alc10 | 14.9 | 0.08 | 2.23 | 70.75 | 917.5 | 2162.3 | 0.38 |
| G14alc20 | 23 | 0.11 | 2.77 | 60.43 | 1054.4 | 1904.5 | 0.38 |
| G14s32:17.5 | 38.3 | 0.93 | 3.57 | 74.87 | 1025.6 | 1574.6 | 1.13 |
| G14s32:15 | 32.7 | 0.71 | 3.30 | 65.69 | 993.3 | 1414.6 | 2.01 |
| G14s32:12.5 | 35.45 | 0.71 | 3.44 | 64.75 | 1333.6 | 1336.2 | 2.16 |
| G14s32:10 | 27.1 | 0.75 | 3 | 50.85 | 1144.6 | 1314.8 | 2.28 |
| G14s32:7.5 | 35.15 | 0.67 | 3.42 | 49.9 | 1398.8 | 1397.4 | 1.81 |
| Pig liver (ex vivo) | 32.1 | 0.94 | 3.27 | 76.06 | 1131.5 | 176.1 | 1.26 |
| Human normal liver | 4.5 4.8 [27] 4.93 [28] | 0.5 0.552 ± 0.03 [29] 0.5 (normal)–1.1 (severe steatosis) [30] | 1.23 1.3 [27] | 51.02 42 [31]–58.32 [32] | 402.1 | 215.4 | N.A. |
| Human fatty liver (with no fibrosis) | 4.47 No significant difference compared to normal liver [33] | 0.56 0.69 [34] | 1.22 3.42 [34] | 36.1 32.44 [32]–64 [32] | 453.83 | 282.7 | N.A. |
| Human cirrhotic liver | 35.8 14 [27] 25.8 [35] 27.5–62.7 [36] 13.29 [28] | 0.5 0.58 [34] | 3.45 2.2 [27] 2.61 [34] | 50.46 50.59 [32] | 363.38 | 188.95 | N.A. |
| G8 G12 | G14 G16 | G14i5 G14i10 G14i15 | G14alc10 G14alc20 | G14s32:7.5 G14s32:10 G14s32:12.5 | G14s32:15 G14s32:17.5 | |
|---|---|---|---|---|---|---|
| Cost (euro) | 10–11 | 11–12 | 11–14 | 12–13 | 14–17 | 17–19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elisei, R.C.; Graur, F.; Szold, A.; Melzer, A.; Moldovan, S.C.; Motrescu, M.; Moiş, E.; Popa, C.; Pîsla, D.; Vaida, C.; et al. Gelatin-Based Liver Phantoms for Training Purposes: A Cookbook Approach. J. Clin. Med. 2024, 13, 3440. https://doi.org/10.3390/jcm13123440
Elisei RC, Graur F, Szold A, Melzer A, Moldovan SC, Motrescu M, Moiş E, Popa C, Pîsla D, Vaida C, et al. Gelatin-Based Liver Phantoms for Training Purposes: A Cookbook Approach. Journal of Clinical Medicine. 2024; 13(12):3440. https://doi.org/10.3390/jcm13123440
Chicago/Turabian StyleElisei, Radu Claudiu, Florin Graur, Amir Szold, Andreas Melzer, Sever Cãlin Moldovan, Mihai Motrescu, Emil Moiş, Cãlin Popa, Doina Pîsla, Cãlin Vaida, and et al. 2024. "Gelatin-Based Liver Phantoms for Training Purposes: A Cookbook Approach" Journal of Clinical Medicine 13, no. 12: 3440. https://doi.org/10.3390/jcm13123440
APA StyleElisei, R. C., Graur, F., Szold, A., Melzer, A., Moldovan, S. C., Motrescu, M., Moiş, E., Popa, C., Pîsla, D., Vaida, C., Tudor, T., Coţe, A., & Al-Hajjar, N. (2024). Gelatin-Based Liver Phantoms for Training Purposes: A Cookbook Approach. Journal of Clinical Medicine, 13(12), 3440. https://doi.org/10.3390/jcm13123440









