Artificial Intelligence in Coronary Artery Calcium Scoring
Abstract
1. Introduction
2. Deep Learning and Artificial Neural Networks
3. Studies of CACS Automation
3.1. ECG-Gated and Non-Gated NCCT
3.2. PET/CT Attenuation Correction
3.3. Low-Dose Chest CT and Transthoracic Echocardiogram
3.4. Multiple CT Protocols
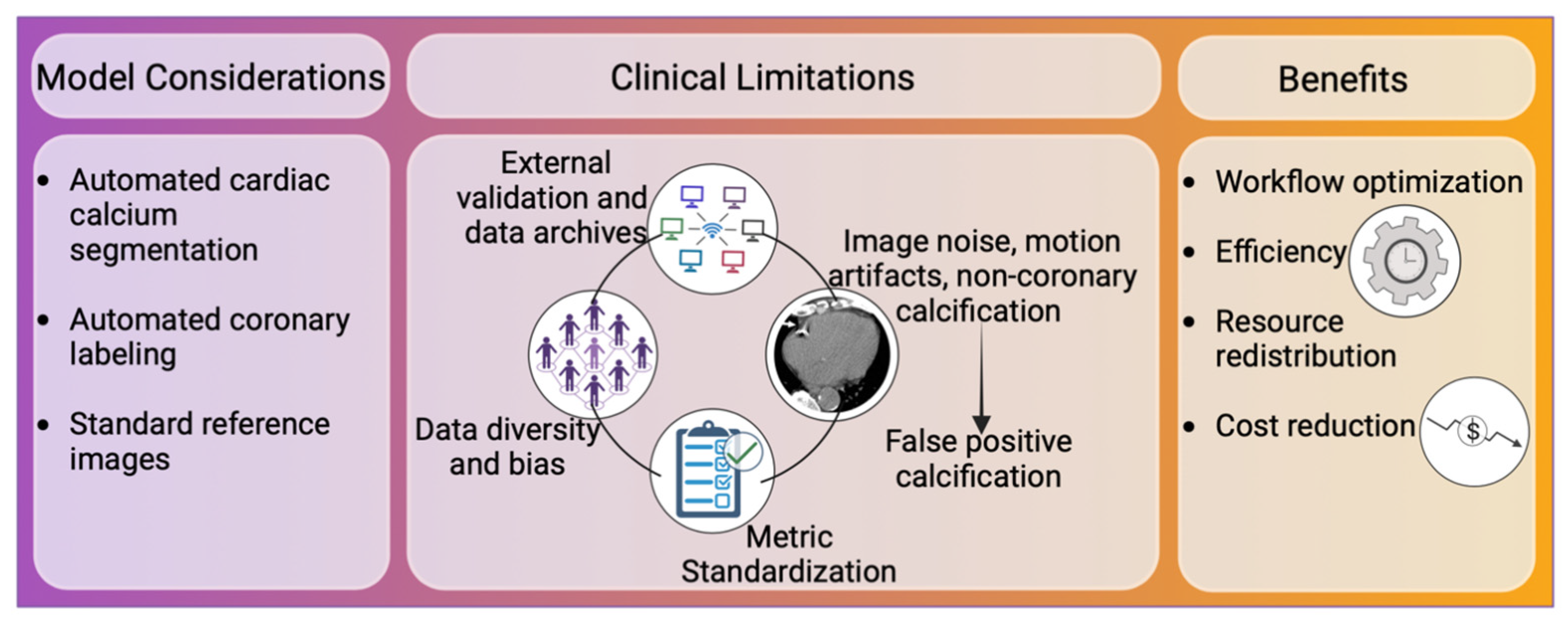
4. Towards Clinical Implementation
4.1. Workflow Optimization
4.2. Image Considerations
4.3. External Validation and Data Diversity
4.4. Metrics Standardization
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Greenland, P.; Alpert, J.S.; Beller, G.A.; Benjamin, E.J.; Budoff, M.J.; Fayad, Z.A.; Foster, E.; Hlatky, M.A.; Hodgson, J.M.; Kushner, F.G.; et al. 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults. J. Am. Coll. Cardiol. 2010, 56, e50–e103. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and Its Progression. JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Razavi, A.C.; Agatston, A.S.; Shaw, L.J.; De Cecco, C.N.; Van Assen, M.; Sperling, L.S.; Bittencourt, M.S.; Daubert, M.A.; Nasir, K.; Blumenthal, R.S.; et al. Evolving Role of Calcium Density in Coronary Artery Calcium Scoring and Atherosclerotic Cardiovascular Disease Risk. JACC Cardiovasc. Imaging 2022, 15, 1648–1662. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of Coronary Artery Calcium Using Ultrafast Computed Tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Kumar, P.; Bhatia, M. Coronary Artery Calcium Data and Reporting System (CAC-DRS): A Primer. J. Cardiovasc. Imaging 2023, 31, 1–17. [Google Scholar] [CrossRef]
- Obisesan, O.H.; Osei, A.D.; Uddin, S.M.I.; Dzaye, O.; Blaha, M.J. An Update on Coronary Artery Calcium Interpretation at Chest and Cardiac CT. Radiol. Cardiothorac. Imaging 2021, 3, e200484. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Einstein, A.J.; Johnson, L.L.; Bokhari, S.; Son, J.; Thompson, R.C.; Bateman, T.M.; Hayes, S.W.; Berman, D.S. Agreement of Visual Estimation of Coronary Artery Calcium from Low-Dose CT Attenuation Correction Scans in Hybrid PET/CT and SPECT/CT with Standard Agatston Score. J. Am. Coll. Cardiol. 2010, 56, 1914–1921. [Google Scholar] [CrossRef]
- Chiles, C.; Duan, F.; Gladish, G.W.; Ravenel, J.G.; Baginski, S.G.; Snyder, B.S.; DeMello, S.; Desjardins, S.S.; Munden, R.F.; NLST Study Team. Association of Coronary Artery Calcification and Mortality in the National Lung Screening Trial: A Comparison of Three Scoring Methods. Radiology 2015, 276, 82–90. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Wu, F.-Z.; Wang, Y.-C.; Ju, Y.-J.; Mar, G.-Y.; Chuo, C.-C.; Lin, H.-S.; Wu, M.-T. Reliable Categorisation of Visual Scoring of Coronary Artery Calcification on Low-Dose CT for Lung Cancer Screening: Validation with the Standard Agatston Score. Eur. Radiol. 2013, 23, 1226–1233. [Google Scholar] [CrossRef]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef]
- Makimoto, H.; Kohro, T. Adopting Artificial Intelligence in Cardiovascular Medicine: A Scoping Review. Hypertens. Res. 2024, 47, 685–699. [Google Scholar] [CrossRef]
- Van den Eynde, J.; Lachmann, M.; Laugwitz, K.-L.; Manlhiot, C.; Kutty, S. Successfully Implemented Artificial Intelligence and Machine Learning Applications in Cardiology: State-of-the-Art Review. Trends Cardiovasc. Med. 2023, 33, 265–271. [Google Scholar] [CrossRef]
- Siemen Healthineers. Computed Tomography. Available online: https://www.siemens-healthineers.com/en-us/computed-tomography (accessed on 25 February 2023).
- Litjens, G.; Ciompi, F.; Wolterink, J.M.; de Vos, B.D.; Leiner, T.; Teuwen, J.; Išgum, I. State-of-the-Art Deep Learning in Cardiovascular Image Analysis. JACC Cardiovasc. Imaging 2019, 12, 1549–1565. [Google Scholar] [CrossRef]
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of Deep Learning: Concepts, CNN Architectures, Challenges, Applications, Future Directions. J. Big Data 2021, 8, 53. [Google Scholar] [CrossRef]
- Recht, M.P.; Dewey, M.; Dreyer, K.; Langlotz, C.; Niessen, W.; Prainsack, B.; Smith, J.J. Integrating Artificial Intelligence into the Clinical Practice of Radiology: Challenges and Recommendations. Eur. Radiol. 2020, 30, 3576–3584. [Google Scholar] [CrossRef]
- Lee, J.H.; Joo, I.; Kang, T.W.; Paik, Y.H.; Sinn, D.H.; Ha, S.Y.; Kim, K.; Choi, C.; Lee, G.; Yi, J.; et al. Deep Learning with Ultrasonography: Automated Classification of Liver Fibrosis Using a Deep Convolutional Neural Network. Eur. Radiol. 2020, 30, 1264–1273. [Google Scholar] [CrossRef]
- Lessmann, N.; van Ginneken, B.; Zreik, M.; de Jong, P.A.; de Vos, B.D.; Viergever, M.A.; Isgum, I. Automatic Calcium Scoring in Low-Dose Chest CT Using Deep Neural Networks with Dilated Convolutions. IEEE Trans. Med. Imaging 2018, 37, 615–625. [Google Scholar] [CrossRef]
- Wolterink, J.M.; Leiner, T.; Takx, R.A.P.; Viergever, M.A.; Isgum, I. Automatic Coronary Calcium Scoring in Non-Contrast-Enhanced ECG-Triggered Cardiac CT with Ambiguity Detection. IEEE Trans. Med. Imaging 2015, 34, 1867–1878. [Google Scholar] [CrossRef]
- Ihdayhid, A.R.; Lan, N.S.R.; Williams, M.; Newby, D.; Flack, J.; Kwok, S.; Joyner, J.; Gera, S.; Dembo, L.; Adler, B.; et al. Evaluation of an Artificial Intelligence Coronary Artery Calcium Scoring Model from Computed Tomography. Eur. Radiol. 2023, 33, 321–329. [Google Scholar] [CrossRef]
- Xu, C.; Guo, H.; Xu, M.; Duan, M.; Wang, M.; Liu, P.; Luo, X.; Jin, Z.; Liu, H.; Wang, Y. Automatic Coronary Artery Calcium Scoring on Routine Chest Computed Tomography (CT): Comparison of a Deep Learning Algorithm and a Dedicated Calcium Scoring CT. Quant. Imaging Med. Surg. 2022, 12, 2684–2695. [Google Scholar] [CrossRef]
- Wolterink, J.M.; Leiner, T.; De Vos, B.D.; Van Hamersvelt, R.W.; Viergever, M.A.; Išgum, I. Automatic Coronary Artery Calcium Scoring in Cardiac CT Angiography Using Paired Convolutional Neural Networks. Med. Image Anal. 2016, 34, 123–136. [Google Scholar] [CrossRef]
- Eng, D.; Chute, C.; Khandwala, N.; Rajpurkar, P.; Long, J.; Shleifer, S.; Khalaf, M.H.; Sandhu, A.T.; Rodriguez, F.; Maron, D.J.; et al. Automated Coronary Calcium Scoring Using Deep Learning with Multicenter External Validation. npj Digit. Med. 2021, 4, 88. [Google Scholar] [CrossRef]
- Pieszko, K.; Shanbhag, A.; Killekar, A.; Miller, R.J.H.; Lemley, M.; Otaki, Y.; Singh, A.; Kwiecinski, J.; Gransar, H.; Van, K.S.D.; et al. Deep Learning of Coronary Calcium Scores from PET/CT Attenuation Maps Accurately Predicts Adverse Cardiovascular Events. JACC Cardiovasc. Imaging 2023, 16, 675–687. [Google Scholar] [CrossRef]
- Morf, C.; Sartoretti, T.; Gennari, A.G.; Maurer, A.; Skawran, S.; Giannopoulos, A.A.; Sartoretti, E.; Schwyzer, M.; Curioni-Fontecedro, A.; Gebhard, C.; et al. Diagnostic Value of Fully Automated Artificial Intelligence Powered Coronary Artery Calcium Scoring from 18F-FDG PET/CT. Diagnostics 2022, 12, 1876. [Google Scholar] [CrossRef]
- Suh, Y.J.; Kim, C.; Lee, J.-G.; Oh, H.; Kang, H.; Kim, Y.-H.; Yang, D.H. Fully Automatic Coronary Calcium Scoring in Non-ECG-Gated Low-Dose Chest CT: Comparison with ECG-Gated Cardiac CT. Eur. Radiol. 2023, 33, 1254–1265. [Google Scholar] [CrossRef]
- Sabia, F.; Balbi, M.; Ledda, R.E.; Milanese, G.; Ruggirello, M.; Valsecchi, C.; Marchianò, A.; Sverzellati, N.; Pastorino, U. Fully Automated Calcium Scoring Predicts All-Cause Mortality at 12 Years in the MILD Lung Cancer Screening Trial. PLoS ONE 2023, 18, e0285593. [Google Scholar] [CrossRef]
- Yuan, N.; Kwan, A.C.; Duffy, G.; Theurer, J.; Chen, J.H.; Nieman, K.; Botting, P.; Dey, D.; Berman, D.S.; Cheng, S.; et al. Prediction of Coronary Artery Calcium Using Deep Learning of Echocardiograms. J. Am. Soc. Echocardiogr. 2023, 36, 474–481.e3. [Google Scholar] [CrossRef]
- Zeleznik, R.; Foldyna, B.; Eslami, P.; Weiss, J.; Alexander, I.; Taron, J.; Parmar, C.; Alvi, R.M.; Banerji, D.; Uno, M.; et al. Deep Convolutional Neural Networks to Predict Cardiovascular Risk from Computed Tomography. Nat. Commun. 2021, 12, 715. [Google Scholar] [CrossRef]
- Sandhu, A.T.; Rodriguez, F.; Ngo, S.; Patel, B.N.; Mastrodicasa, D.; Eng, D.; Khandwala, N.; Balla, S.; Sousa, D.; Maron, D.J. Incidental Coronary Artery Calcium: Opportunistic Screening of Previous Nongated Chest Computed Tomography Scans to Improve Statin Rates (NOTIFY-1 Project). Circulation 2023, 147, 703–714. [Google Scholar] [CrossRef]
- Peng, A.W.; Dudum, R.; Jain, S.S.; Maron, D.J.; Patel, B.N.; Khandwala, N.; Eng, D.; Chaudhari, A.S.; Sandhu, A.T.; Rodriguez, F. Association of Coronary Artery Calcium Detected by Routine Ungated CT Imaging with Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2023, 82, 1192–1202. [Google Scholar] [CrossRef]
- van Velzen, S.G.M.; Lessmann, N.; Velthuis, B.K.; Bank, I.E.M.; van den Bongard, D.H.J.G.; Leiner, T.; de Jong, P.A.; Veldhuis, W.B.; Correa, A.; Terry, J.G.; et al. Deep Learning for Automatic Calcium Scoring in CT: Validation Using Multiple Cardiac CT and Chest CT Protocols. Radiology 2020, 295, 66–79. [Google Scholar] [CrossRef]
- Yu, J.; Qian, L.; Sun, W.; Nie, Z.; Zheng, D.; Han, P.; Shi, H.; Zheng, C.; Yang, F. Automated Total and Vessel-Specific Coronary Artery Calcium (CAC) Quantification on Chest CT: Direct Comparison with CAC Scoring on Non-Contrast Cardiac CT. BMC Med. Imaging 2022, 22, 177. [Google Scholar] [CrossRef]
- Hong, J.-S.; Tzeng, Y.-H.; Yin, W.-H.; Wu, K.-T.; Hsu, H.-Y.; Lu, C.-F.; Liu, H.-R.; Wu, Y.-T. Automated Coronary Artery Calcium Scoring Using Nested U-Net and Focal Loss. Comput. Struct. Biotechnol. J. 2022, 20, 1681–1690. [Google Scholar] [CrossRef]
- Gogin, N.; Viti, M.; Nicodème, L.; Ohana, M.; Talbot, H.; Gencer, U.; Mekukosokeng, M.; Caramella, T.; Diascorn, Y.; Airaud, J.-Y.; et al. Automatic Coronary Artery Calcium Scoring from Unenhanced-ECG-Gated CT Using Deep Learning. Diagn. Interv. Imaging 2021, 102, 683–690. [Google Scholar] [CrossRef]
- Sandstedt, M.; Henriksson, L.; Janzon, M.; Nyberg, G.; Engvall, J.; De Geer, J.; Alfredsson, J.; Persson, A. Evaluation of an AI-Based, Automatic Coronary Artery Calcium Scoring Software. Eur. Radiol. 2020, 30, 1671–1678. [Google Scholar] [CrossRef]
- Willemink, M.J.; Leiner, T.; de Jong, P.A.; de Heer, L.M.; Nievelstein, R.A.J.; Schilham, A.M.R.; Budde, R.P.J. Iterative Reconstruction Techniques for Computed Tomography Part 2: Initial Results in Dose Reduction and Image Quality. Eur. Radiol. 2013, 23, 1632–1642. [Google Scholar] [CrossRef]
- Den Harder, A.M.; Willemink, M.J.; De Ruiter, Q.M.B.; De Jong, P.A.; Schilham, A.M.R.; Krestin, G.P.; Leiner, T.; Budde, R.P.J. Dose Reduction with Iterative Reconstruction for Coronary CT Angiography: A Systematic Review and Meta-Analysis. Br. J. Radiol. 2016, 89, 20150068. [Google Scholar] [CrossRef]
- Garmer, M.; Lehrenfeld, C.; Metz, F.; Klein-Wiele, O.; Brandts, B.; Grönemeyer, D. False-Positive Calcifications and Radiation Dose in Coronary Artery Calcium Scoring Using Iterative Reconstruction on the Basis of a Noise Threshold. Radiol. Med. Diagn. Imaging 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Rossi, A.; Gennari, A.G.; Etter, D.; Benz, D.C.; Sartoretti, T.; Giannopoulos, A.A.; Mikail, N.; Bengs, S.; Maurer, A.; Gebhard, C.; et al. Impact of Deep Learning Image Reconstructions (DLIR) on Coronary Artery Calcium Quantification. Eur. Radiol. 2023, 33, 3832–3838. [Google Scholar] [CrossRef]
- Gong, Z.; Zhong, P.; Hu, W. Diversity in Machine Learning. IEEE Access 2019, 7, 64323–64350. [Google Scholar] [CrossRef]
- Bunkerhill Health | Advancing AI Healthcare from Concept to Clinic. Available online: https://www.bunkerhillhealth.com/ (accessed on 2 June 2024).
- Kalderstam, J.; Edén, P.; Bendahl, P.-O.; Strand, C.; Fernö, M.; Ohlsson, M. Training Artificial Neural Networks Directly on the Concordance Index for Censored Data Using Genetic Algorithms. Artif. Intell. Med. 2013, 58, 125–132. [Google Scholar] [CrossRef]
- Li, M.; Gao, Q.; Yu, T. Kappa Statistic Considerations in Evaluating Inter-Rater Reliability between Two Raters: Which, When and Context Matters. BMC Cancer 2023, 23, 799. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Shrestha, S.; Berthon, B.; Messas, E.; Donal, E.; Tison, G.H.; Min, J.K.; D’hooge, J.; Voigt, J.-U.; Dudley, J.; et al. Proposed Requirements for Cardiovascular Imaging-Related Machine Learning Evaluation (PRIME): A Checklist. JACC Cardiovasc. Imaging 2020, 13, 2017–2035. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]

| Company | Software | Applications |
|---|---|---|
| General Electric Healthcare, IL, United States | CardIQ Suite | Coronary calcium segmentation and coronary artery labeling with total and per-region scoring. |
| Arytra Ltd., West Perth, Australia | DeepC architecture | Coronary artery calcium detection and plaque volume measurement. |
| Coreline Soft, Seoul, Korea | AVIEW CAC | Coronarartery segmentation, calcium analysis, and automatic report generation. |
| Nanox AI, Petah Tikva, Israel | HealthCCSng | Coronary calcium quantification and categorization. |
| Siemens Healthineers, PA, United State | AI-Rad Companion (Cardiovascular) | Coronary calcium and heart volume quantification in gated and non-gated NCCT. Does not perform Agatston scoring [15]. |
| Author | N | Test Image Type/Protocol | Reference Image | Results | Model |
|---|---|---|---|---|---|
| Eng et al., 2021 [25] | Gated CT model (retrospective 866 scans, prospective test 55 scans) Non-gated routine chest CT (341 training, Stanford total 215 scans [42 test], MESA total 232 scans [46 test], external validation 303 scans) | - Gated NCCT - Non-gated routine chest CT | - Gated NCCT manual - Non-gated manual NCCT | For CACS ≥ 100 in non-gated CT model: - Sensitivity = 71–94% - PPV = 88–100% For gated NCCT: - Cohen’s kappa = 0.89, p <0.0001 - AI processing time vs. manual scoring = 3.5 ± 2.1 s vs. 261 s | CNN |
| Ihdayhid et al., 2023 [22] | Training 2439 cardiac CT scans Validation 771 scans Test set 1849 cardiac CT scans | - ECG-gated NCCT scans | - ECG-gated NCCT | AI vs. Manual CACS: - Spearman’s r = 0.90 [95% CI, 0.89–0.91], p < 0.001 - ICC = 0.98 [95% CI, 0.98–0.99], p < 0.001 - Bland–Altman = 1.69 - κ = 0.90 [95 CI, 0.88–0.9], p < 0.001 - Analysis time = 13.1 ± 3.2 s/scan | DeepC Architecture (3D-CNN) |
| Pieszko et al., 2023 [26] | Training and internal testing 9543 (1827 gated CT and 7716 CTAC) External validation 4331 (2737 had ECG-gated NCCT) | - CT attenuation correction scans for AI - ECG-gated NCCT | - ECG-gated NCCT | - Automated scoring time = <6 s/scan - Net reclassification improvement = −0.02 [95% CI, −0.11–0.07] - NPV of DL CTAC = 83% | DL |
| Morf et al., 2022 [27] | Test set 100 patients | - non-gated NCCT for AI - ECG-gated CT | - ECG-gated NCCT | Per-patient AI CACS: - sensitivity = 85% - specificity = 90% Inter-score agreement = 0.88 [95% CI: 0.827, 0.918] κ = 0.9 Interscore agreement per-vessel = 0.716 | AVIEW CAC (U-Net) |
| Wolterink et al., 2016 [24] | 250 CCTA and 250 gated NCCT scans | - CCTA - ECG-gated NCCT | - Manual CCTA and ECG-gated NCCT | Pearson’s = 0.950 ICC = 0.944 CVD risk accuracy = 83% κ = 0.83 Sensitivity/FP = 0.72/0.48 Bland–Altmann = −0.2 (−38.7–38.3) | ConvPairs (CNN) |
| Suh et al., 2023 [28] | 452 subjects (across 3 institutions) | - LDCT - ECG-gated NCCT | - Manual ECG-gated NCCT - Manual LDCT | Comparison of automatic and manual LDCT: κ = 0.972–0.918 Comparison of automatic and manual gated NCCT κ = 0.748–0.924 | AVIEW CAC, Coreline Soft |
| Sabia et al., 2022 [29] | 1129 subjects (Multicenter Italian Lung Disease Trial) | - LDCT | - Manual LDCT scoring | All-cause mortality CAC > 400: Hazard ratio = 5.75 [95% CI, 2.08–15.92] | AViEW, Coreline Soft (U-Net structure) |
| Yuan et al., 2023 [30] | 2831 TTE videos paired with gated NCCT | - 32-frame TTEs in parasternal long-axis view | - Manual CACS by gated NCCT | AI TTE zero CACS vs. high CACS - AUC = 0.81 [95% CI, 0.74–0.88] vs. 0.74 [0.68–0.8] - F1 score = 0.95 vs. 0.74 | CNN |
| Zeleznik at al., 2021 [31] | Test set 1857 ECG-gated CTs and LDCT NLST 14,959 patients FHS-CT2 663 PROMISE 4021 ROMICAT-II 441 | - LD chest CT (NLST) - ECG-gated (FHS-CT2, PROMISE and ROMICAT-II) | - Manual CACS by LDCT and gated NCCT | Automatic = 1.938 s per scan κ = 0.70 | U-Net |
| Xu at al., 2022 [22] | Training set 150 (group A—1 mm slice thickness) and 170 (group B—3 mm) chest CTs Test set 144 (1 mm) and 144 (3 mm) chest CTs External validation 344 paired scans | - ECG-gated NCCT - non-gated chest CT | - manual CACS by gated NCCT | Agreement between AI and gold standard manual CACS: - ICC Group A = 0.9 [95% CI, 0.85–0.93] - ICC Group B = 0.94 [95% CI, 0.92–0.96] Risk category classification: κ Group A = 0.72 κ Group B = 0.82 PPV, NPV, and accuracy: Group A = 90%, 83%, and 88% Group B = 93%, 98%, and 94% | U-Net |
| Sandhu et al., 2023 [32] | Test set 173 patients | Non-gated NCCT | Manual non-gated NCCT | Statin prescription in notification group vs. usual care group: 51.2% vs. 6.9% | DL |
| Peng et al., 2023 [33] | Test set 5678 adults | Non-gated NCCT | N/A | DL-CAC ≥ 100: - Mean 10 yrs ASCVD = 24% - 26% pts on statins DL-CAC ≥ 100 vs. DL-CAC = 0: - HR death = 1.51 [95% CI, 1.28–1.79] - HR death/MI/stroke = 1.57 [95% CI, 1.33–1.84] - HR death/MI/stroke/revascularization = 1.69 [95% CI, 1.45–1.98] | CNN |
| Van Velzen et al., 2020 [34] | Total 7240 ECG-gated standard CAC CT 902, CTAC 399, 1409 CTs radiation treatment planning (RadTherapy) 1409, 470 diagnostic Chest CTs, 2879 ECG-gated from JHS, 1181 NLST | LDCT ECG-gated CTAC | - Semi-automatic | Data for CAC CT, CTAC, diagnostic CT, and RadTherapy: ICC for CAC volume = 0.98, 0.97, 0.98, and 0.92, respectively Overall κ = 0.92 (95% CI, 0.91–0.93) | CNN |
| Yu et al., 2022 [35] | 405 LDCT and 405 gated CT | LDCT Gated NCCT | LDCT and gated NCCT | Comparison with LDCT: | CACScoreDoc |
| Hong et al., 2022 [36] | 1811 cases Training 754 Test 1 = 215 Test 2 = 744 Validation = 98 | Gated NCCT | Semi-automated clinical software (syngo.CT CaScoring, Siemens) | Data for training dataset 1 ICC 1.00, p < 0.001 κ = 0.931 U-Net vs. U-Net++ - Dice = 0.54 vs. 0.86 - IoU = 0.54 vs. 0.84 - Precision = 0.54 vs. 0.88 Analysis time 50 ms per scan | U-Net++ (U-Net with immediate upsampling after downsampling) |
| Gogin et al., 2021 [37] | Test set 783 CT (SFR data challenge set) External validation 98 CTs (orCaScore challenge set) | Gated NCCT | - Manual CACS by gated NCCT | A five-ensemble model trained on all datasets: ICC = 0.970 κ = 0.894 Accuracy = 85.7% | CNN with 3D U-Net structure |
| Sandstedt et al., 2020 [38] | 315 scans from SWEDEHEART registry | Gated NCCT | Semiautomatic and manual CACS | Correlation for CACS: Pearson’s = 0.935 ICC = 0.996 Bland–Altman = −8.2 (−115.1 to 98.2) κ = 0.919 Median analysis time: - semi-automatic = 59 s - automatic = 36 s | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aromiwura, A.A.; Kalra, D.K. Artificial Intelligence in Coronary Artery Calcium Scoring. J. Clin. Med. 2024, 13, 3453. https://doi.org/10.3390/jcm13123453
Aromiwura AA, Kalra DK. Artificial Intelligence in Coronary Artery Calcium Scoring. Journal of Clinical Medicine. 2024; 13(12):3453. https://doi.org/10.3390/jcm13123453
Chicago/Turabian StyleAromiwura, Afolasayo A., and Dinesh K. Kalra. 2024. "Artificial Intelligence in Coronary Artery Calcium Scoring" Journal of Clinical Medicine 13, no. 12: 3453. https://doi.org/10.3390/jcm13123453
APA StyleAromiwura, A. A., & Kalra, D. K. (2024). Artificial Intelligence in Coronary Artery Calcium Scoring. Journal of Clinical Medicine, 13(12), 3453. https://doi.org/10.3390/jcm13123453










