Nanoengineering Solutions for Cancer Therapy: Bridging the Gap between Clinical Practice and Translational Research
Abstract
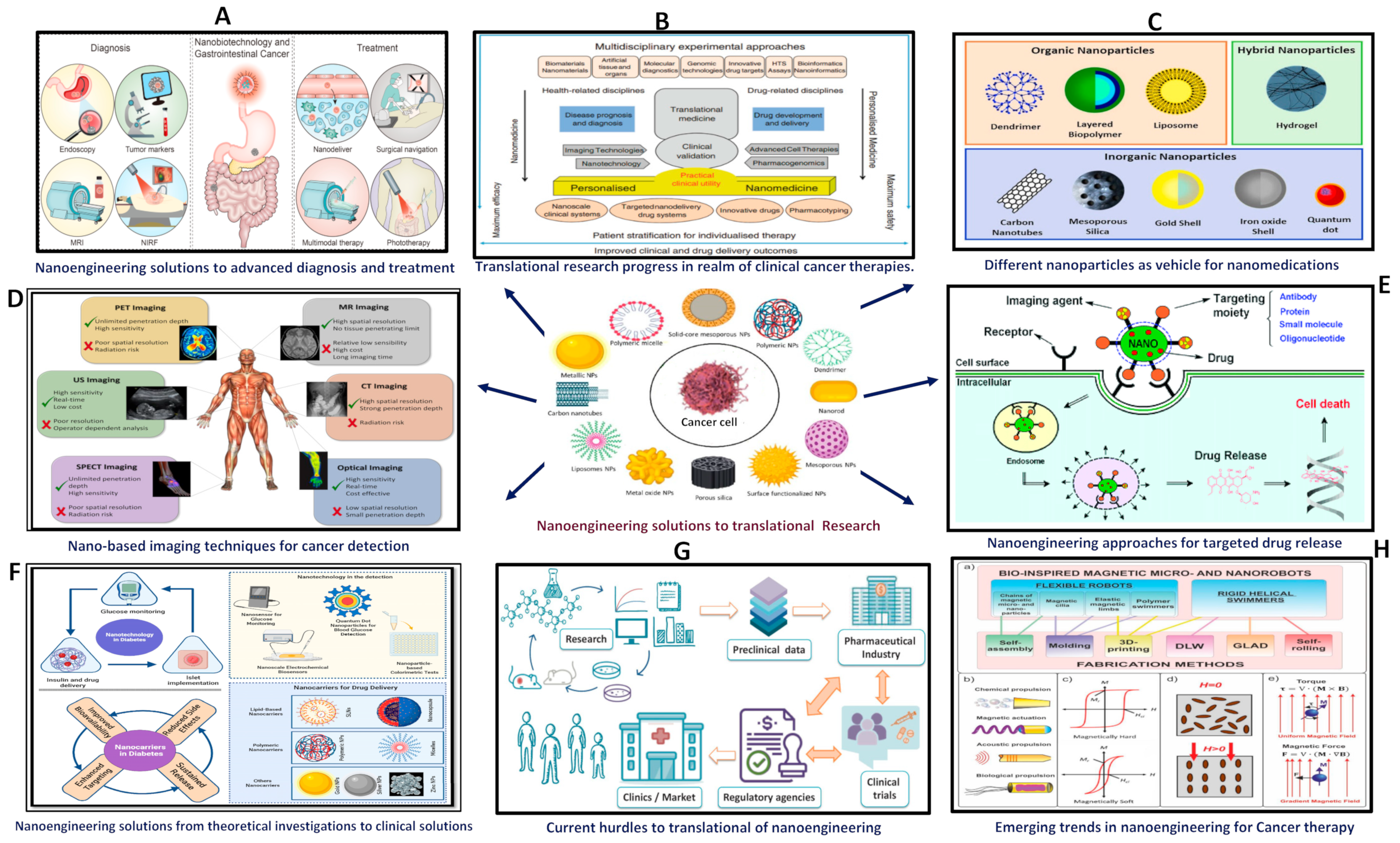
1. Introduction
2. Nanoengineering Approaches for Targeted Drug Delivery
2.1. Nanoparticles as Vehicles for Medications
2.2. Liposomes for Enclosing Hydrophobic and Hydrophilic Medications
2.3. Polymeric Nanoparticles for Regulated Release
2.4. Dendrimers for Accurate Medication Delivery
2.5. Nanoparticle Surface Functionalization for Targeting
2.6. Stimuli-Responsive Nanoparticles for Induced Drug Release
3. Nano-Based Imaging Techniques for Cancer Detection and Monitoring
3.1. Fluorescence Imaging with Quantum Dots
3.2. Magnetic Resonance Imaging (MRI) with Iron Oxide Nanoparticles
3.3. Gold Nanoparticles for Surface-Enhanced Raman Scattering Imaging
3.4. Photoacoustic Imaging with Nanoparticles
4. Emerging Trends in Nanoengineering for Cancer Therapy
4.1. Personalized Nanomedicine
4.2. Multifunctional Nanoparticles
4.3. Stimuli-Responsive Nanomaterials
4.4. Targeting the Tumor Microenvironment
4.5. Nanotechnology in Immunotherapy
4.6. Bioinspired Nanomaterials
4.7. Nanotechnology in Combination Therapies
5. The importance of Translational Research Progress in the Realm of Clinical Cancer Therapies
5.1. Identification and Validation of Biomarkers
5.2. Utilization of Preclinical Models for Drug Development
5.3. Development of Biomaterials and Drug Delivery Systems
5.4. Progress in Immunotherapy and Precision Medicine
5.5. Execution of Clinical Trials and Negotiation of Regulatory Pathways
6. Challenges Associated with Progressing Cancer Therapies via Translational Research
6.1. Complexity of Tumor Biology
6.2. Development of Drug Resistance
6.3. Design and Implementation of Clinical Trials
6.4. Regulatory and Ethical Considerations
6.5. Equity and Accessibility
7. Conclusions and Future Prospective
8. Clinical Significance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemp, J.A.; Kwon, Y.J. Cancer nanotechnology: Current status and perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Patel, H.; Li, J.; Bo, L.; Mehta, R.; Ashby, C.R., Jr.; Wang, S.; Cai, W.; Chen, Z.S. Nanotechnology-based delivery systems to overcome drug resistance in cancer. Med. Rev. 2024, 4, 5–30. [Google Scholar] [CrossRef]
- Kashyap, B.K.; Singh, V.V.; Solanki, M.K.; Kumar, A.; Ruokolainen, J.; Kesari, K.K. Smart Nanomaterials in Cancer Theranostics: Challenges and Opportunities. ACS Omega 2023, 8, 14290–14320. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Gao, Y.; Meng, F.; Luo, L. Recent progress of nanotechnology-based theranostic systems in cancer treatments. Cancer Biol. Med. 2021, 18, 336–351. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Salvati, E.; Stellacci, F.; Krol, S. Nanosensors for early cancer detection and for therapeutic drug monitoring. Nanomedicine 2015, 10, 3495–3512. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef]
- Neetika; Sharma, M.; Thakur, P.; Gaur, P.; Rani, G.M.; Rustagi, S.; Talreja, R.K.; Chaudhary, V. Cancer treatment and toxicity outlook of nanoparticles. Environ. Res. 2023, 237 Pt 1, 116870. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Viegas, L.; Seck, F.; Fonte, P. An insight on lipid nanoparticles for therapeutic proteins delivery. J. Drug Deliv. Sci. Technol. 2022, 77, 103839. [Google Scholar] [CrossRef]
- Basu, B.; Prajapati, B.; Dutta, A.; Paliwal, H. Medical application of liposomes. J. Explor. Res. Pharmacol. 2024, 9, 13–22. [Google Scholar] [CrossRef]
- Çagdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as Potential Drug Carrier Systems for Drug Delivery. In Application of Nanotechnology in Drug Delivery; InTech: London, UK, 2014. [Google Scholar] [CrossRef]
- Ansary, R.H.; Awang, M.B.; Rahman, M.K. Biodegradable Poly (D,L-lactic-co-glycolic acid)-Based Micro/Nanoparticles for Sustained Release of Protein Drugs—A Review. Trop. J. Pharm. Res. 2014, 13, 1179–1190. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Lin, Z.; Wei, Q.; Qian, J.; Ruan, R.; Jiang, X.; Hou, L.; Song, J.; Ding, J.; et al. Stimuli-Responsive Nanoparticles for Controlled Drug Delivery in Synergistic Cancer Immunotherapy. Adv. Sci. 2022, 9, 2103444. [Google Scholar] [CrossRef]
- Mosleh-Shirazi, S.; Abbasi, M.; Moaddeli, M.R.; Vaez, A.; Shafiee, M.; Kasaee, S.R.; Amani, A.M.; Hatam, S. Nanotechnology Advances in the Detection and Treatment of Cancer: An Overview. Nanotheranostics 2022, 6, 400–423. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The Promise of Nanotechnology in Personalized Medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar] [CrossRef]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 2013, 15, 253–282. [Google Scholar] [CrossRef]
- Srinivasan, M.; Rajabi, M.; Mousa, S.A. Multifunctional Nanomaterials and Their Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2015, 5, 1690–1703. [Google Scholar] [CrossRef]
- Murugan, B.; Suresh, S.; Fatimah, I.; Johan, M.R. Smart stimuli-responsive nanocarriers for the cancer therapy—Nanomedicine. Nanotechnol. Rev. 2021, 10, 933–953. [Google Scholar] [CrossRef]
- Raju, G.S.R.; Pavitra, E.; Varaprasad, G.L.; Bandaru, S.S.; Nagaraju, G.P.; Farran, B.; Huh, Y.S.; Han, Y.-K. Nanoparticles mediated tumor microenvironment modulation: Current advances and applications. J. Nanobiotechnol. 2022, 20, 274. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med. Drug Discov. 2020, 6, 100024. [Google Scholar] [CrossRef]
- Zhou, L.; Zou, M.; Xu, Y.; Lin, P.; Lei, C.; Xia, X. Nano Drug Delivery System for Tumor Immunotherapy: Next-Generation Therapeutics. Front. Oncol. 2022, 12, 864301. [Google Scholar] [CrossRef] [PubMed]
- Combes, G.F.; Vučković, A.M.; Perić Bakulić, M.; Antoine, R.; Bonačić-Koutecky, V.; Trajković, K. Nanotechnology in Tumor Biomarker Detection: The Potential of Liganded Nanoclusters as Nonlinear Optical Contrast Agents for Molecular Diagnostics of Cancer. Cancers 2021, 13, 4206. [Google Scholar] [CrossRef]
- Arnal-Estapé, A.; Foggetti, G.; Starrett, J.H.; Nguyen, D.X.; Politi, K. Preclinical Models for the Study of Lung Cancer Pathogenesis and Therapy Development. Cold Spring Harb. Perspect. Med. 2021, 11, a037820. [Google Scholar] [CrossRef]
- Sridharan, B.; Lim, H.G. Advances in photoacoustic imaging aided by nano contrast agents: Special focus on role of lymphatic system imaging for cancer theranostics. J. Nanobiotechnol. 2023, 21, 437. [Google Scholar] [CrossRef]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface enhanced Raman scattering with gold nanoparticles: Effect of particle shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef]
- Pompili, L.; Porru, M.; Caruso, C.; Biroccio, A.; Leonetti, C. Patient-derived xenografts: A relevant preclinical model for drug development. J. Exp. Clin. Cancer Res. 2016, 35, 189. [Google Scholar] [CrossRef] [PubMed]
- Rana, I.; Oh, J.; Baig, J.; Moon, J.H.; Son, S.; Nam, J. Nanocarriers for cancer nano-immunotherapy. Drug Deliv. Transl. Res. 2023, 13, 1936–1954. [Google Scholar] [CrossRef] [PubMed]
- Tandale, P.; Choudhary, N.; Singh, J.; Sharma, A.; Shukla, A.; Sriram, P.; Soni, U.; Singla, N.; Barnwal, R.P.; Singh, G.; et al. Fluorescent quantum dots: An insight on synthesis and potential biological application as drug carrier in cancer. Biochem. Biophys. Rep. 2021, 26, 100962. [Google Scholar] [CrossRef]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef]
- Rahman, M. Magnetic Resonance Imaging and Iron-oxide Nanoparticles in the era of Personalized Medicine. Nanotheranostics 2023, 7, 424–449. [Google Scholar] [CrossRef]
- Aldosari, F.M.M. Characterization of Labeled Gold Nanoparticles for Surface-Enhanced Raman Scattering. Molecules 2022, 27, 892. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.Y.; Choi, H.K.; Lee, J.Y.; Choi, J.W. Application of Gold Nanoparticle to Plasmonic Biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef]
- Steinbrueck, A.; Karges, J. Metal Complexes and Nanoparticles for Photoacoustic Imaging. ChemBioChem 2023, 24, e202300079. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Al-Najar, B.T.; Bououdina, M. Bioinspired Nanoparticles for Efficient Drug Delivery System. In Robotic Systems: Concepts, Methodologies, Tools, and Applications; IGI Global: Hershey, PA, USA, 2020; pp. 540–574. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- De Maria Marchiano, R.; Di Sante, G.; Piro, G.; Carbone, C.; Tortora, G.; Boldrini, L.; Pietragalla, A.; Daniele, G.; Tredicine, M.; Cesario, A.; et al. Translational Research in the Era of Precision Medicine: Where We Are and Where We Will Go. J. Pers. Med. 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381–400. [Google Scholar] [CrossRef]
- Trucillo, P. Biomaterials for Drug Delivery and Human Applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef]
- Rawat, N.; Ahmad, N.; Raturi, P.; Singhvi, N.; Sahai, N.; Kothiyal, P. Nanobiomaterials: Exploring mechanistic roles in combating microbial infections and cancer. Discov. Nano 2023, 18, 158. [Google Scholar] [CrossRef]
- Al-Haideri, M.; Tondok, S.B.; Safa, S.H.; Maleki, A.H.; Rostami, S.; Jalil, A.T.; Al-Gazally, M.E.; Alsaikhan, F.; Rizaev, J.A.; Mohammad, T.A.M.; et al. CAR-T cell combination therapy: The next revolution in cancer treatment. Cancer Cell Int. 2022, 22, 365. [Google Scholar] [CrossRef]
- Kiyotani, K.; Toyoshima, Y.; Nakamura, Y. Personalized immunotherapy in cancer precision medicine. Cancer Biol. Med. 2021, 18, 955–965. [Google Scholar] [CrossRef]
- Moore, D.C.; Guinigundo, A.S. Biomarker-Driven Oncology Clinical Trials: Novel Designs in the Era of Precision Medicine. J. Adv. Pract. Oncol. 2023, 14 (Suppl. S1), 9–13. [Google Scholar] [CrossRef]
- Spreafico, A.; Hansen, A.R.; Abdul Razak, A.R.; Bedard, P.L.; Siu, L.L. The Future of Clinical Trial Design in Oncology. Cancer Discov. 2021, 11, 822–837. [Google Scholar] [CrossRef]
- Austin, C.P. Opportunities and challenges in translational science. Clin. Transl. Sci. 2021, 14, 1629–1647. [Google Scholar] [CrossRef]
- Amirmahani, F.; Ebrahimi, N.; Molaei, F.; Faghihkhorasani, F.; Jamshidi, K.; Seyede, G.; Mirtaghi, M.; Borjian-Boroujeni, M. Approaches for the integration of big data in translational medicine: Single-cell and computational methods. Ann. N. Y. Acad. Sci. 2021, 493, 3–28. [Google Scholar] [CrossRef]
- Jacquemin, V.; Antoine, M.; Dom, G.; Detours, V.; Maenhaut, C.; Dumont, J.E. Dynamic Cancer Cell Heterogeneity: Diagnostic and Therapeutic Implications. Cancers 2022, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Gao, W.; Su, M.; Nice, E.C.; Zhang, W.; Lin, J.; Xie, N. Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 641469. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, E.; Tsimberidou, A.M.; Vo, H.H.; Kurzrock, R. Clinical trial design in the era of precision medicine. Genome Med. 2022, 14, 101. [Google Scholar] [CrossRef]
- Li, A.; Bergan, R.C. Clinical trial design: Past, present, and future in the context of big data and precision medicine. Cancer 2020, 126, 4838–4846. [Google Scholar] [CrossRef] [PubMed]
- McNair, L. Ethical and regulatory oversight of clinical research: The role of the Institutional Review Board. Exp. Biol. Med. 2022, 247, 561–566. [Google Scholar] [CrossRef]
- Castonguay, L.G.; Muran, J.C. Fostering collaboration between researchers and clinicians through building practice-oriented research: An introduction. Psychother. Res. 2015, 25, 1–5. [Google Scholar] [CrossRef]
- Dos-Santos-Silva, I.; Gupta, S.; Orem, J.; Shulman, L.N. Global disparities in access to cancer care. Commun. Med. 2022, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Pillai, G.G.; Andrade, C.J.; Ligibel, J.A.; Basu, P.; Cohen, L.; Khan, I.A.; Mustian, K.M.; Puthiyedath, R.; Dhiman, K.S.; et al. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J. Clin. 2022, 72, 144–164. [Google Scholar] [CrossRef]
- Tiwari, H.; Rai, N.; Singh, S.; Gupta, P.; Verma, A.; Singh, A.K.; Kajal; Salvi, P.; Singh, S.K.; Gautam, V. Recent Advances in Nanomaterials-Based Targeted Drug Delivery for Preclinical Cancer Diagnosis and Therapeutics. Bioengineering 2023, 10, 760. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
| Aspect of Nanoengineering | Description | Impact on Cancer Therapy | Reference Cited |
|---|---|---|---|
| Targeted Drug Delivery | Nanostructured systems such as nanoparticles, liposomes, and dendrimers facilitate the accurate administration of therapeutic substances to tumor locations, thus reducing overall systemic toxicity. | Improves the effectiveness of treatment, diminishes adverse outcomes, and circumvents resistance to medication. | [10] |
| Personalized Nanomedicine | Utilizing translational research findings, personalized treatments are customized based on individual patient profiles, biomarkers, and tumor characteristics. | Enhances treatment outcomes, increases patient response rates, and promotes personalized medicine strategies. | [21] |
| Multifunctional Nanoparticles | The integration of drug delivery, imaging capabilities, targeting ligands, and therapeutic payloads into a unified platform facilitates theranostic applications. | Enables the process of continuous monitoring, timely identification, and tailored therapeutic protocols. | [22,23] |
| Stimuli-Responsive Nanomaterials | Designed with the capability to react to specific signals in the tumor microenvironment, leading to controlled drug release and improved treatment specificity. | Minimizing off-target effects, overcoming drug resistance, and optimizing therapeutic efficacy are crucial objectives in pharmacology. | [24] |
| Targeting Tumor Microenvironment | Strategies in nanoengineering are centered on regulating elements of the tumor microenvironment to create an inhospitable condition for tumor proliferation and to stimulate immune reactions. | Improves the effectiveness of immunotherapy, hinders the advancement of tumors, and averts the spread of metastases. | [25] |
| Combination Therapies | Facilitating the creation of combination therapies, nanoengineered platforms incorporate various therapeutic approaches, synergistically targeting diverse pathways within tumors. | Enhances the synergy of treatment, surmounts mechanisms of resistance, and augments therapeutic results. | [26] |
| Immunotherapy Enhancement | Enhancements in immunotherapy are achieved through nanoengineered methods that optimize drug delivery to immune cells, stimulate immune responses, and overcome suppressive mechanisms in the tumor microenvironment. | Enhances the immune responses against tumors, extends the duration of responses to treatment, and diminishes the toxicity associated with immunotherapy. | [27] |
| Nanoengineering Solution | Translational Research Contribution | Clinical Impact | Key Clinical Findings and Patient Outcomes | Reference Cited |
|---|---|---|---|---|
| Nanoparticle Drug Delivery | Generation of nanocarriers and kinetics tuning for medication release. | Improved medication efficiency and focused administration. | longer lifetimes, less systemic toxicity, and increased tumor response rates | [14] |
| Preclinical research on pharmacokinetics and biodistribution | improved treatment tolerability and elevated therapeutic index | [15] | ||
| Nanomedicine | Finding biomarkers unique to tumors and optimizing nanomaterial design | Precision targeting of tumors and diseased cells | Reduced off-target effects and customized treatment methods. | [28] |
| Clinical studies on the tolerance, safety, and effectiveness of nanomedicines | Enhanced disease management and extended progression-free survival | [29] | ||
| Nanoparticle-based Imaging | Creation of contrast agents and surface changes for nanoparticles | Improved diagnostic accuracy and imaging quality | Early tumor diagnosis, correct staging, well-planned therapy, | [30] |
| Clinical verification of medicines based on nanoparticles for imaging modalities | Better treatment monitoring, and enhanced tumor margin visibility | [31] | ||
| Nanotechnology in Radiotherapy | Radiosensitization via nanoparticles, focusing on hypoxic areas in cancers | Enhanced radiation delivery and efficacy | Increased local tumor control, reduced radiation-related toxicity | [11] |
| Preclinical research on tumor radiosensitivity and radiation dose enhancement | Improved tumor response, potential for dose escalation | [32] | ||
| Theranostic Nanoparticles | Imaging and therapeutic agents incorporated into a single nanoparticle | Simultaneous diagnosis and therapy | Personalized therapy modifications and real-time treatment response monitoring | [5] |
| Theranostic agent clinical trials and patient-specific therapy planning | Better therapeutic results, less side effects, and shorter treatment times | [4] | ||
| Nanoparticle-based Immunotherapy | Nanoformulations of immunomodulatory agents, targeted immune cell delivery | Enhanced immune activation and response | Enhanced reaction times, long-lasting immunological recall, and decreased systemic adverse effects | [19] |
| Clinical research on immune cell interactions and the effectiveness of immunotherapy | Increased tumor regression, extended survival, and possibility for long-term disease management | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, P.; Pareek, S.; Kulkarni, P.; Salgia, R.; Singhal, S.S. Nanoengineering Solutions for Cancer Therapy: Bridging the Gap between Clinical Practice and Translational Research. J. Clin. Med. 2024, 13, 3466. https://doi.org/10.3390/jcm13123466
Garg P, Pareek S, Kulkarni P, Salgia R, Singhal SS. Nanoengineering Solutions for Cancer Therapy: Bridging the Gap between Clinical Practice and Translational Research. Journal of Clinical Medicine. 2024; 13(12):3466. https://doi.org/10.3390/jcm13123466
Chicago/Turabian StyleGarg, Pankaj, Siddhika Pareek, Prakash Kulkarni, Ravi Salgia, and Sharad S. Singhal. 2024. "Nanoengineering Solutions for Cancer Therapy: Bridging the Gap between Clinical Practice and Translational Research" Journal of Clinical Medicine 13, no. 12: 3466. https://doi.org/10.3390/jcm13123466
APA StyleGarg, P., Pareek, S., Kulkarni, P., Salgia, R., & Singhal, S. S. (2024). Nanoengineering Solutions for Cancer Therapy: Bridging the Gap between Clinical Practice and Translational Research. Journal of Clinical Medicine, 13(12), 3466. https://doi.org/10.3390/jcm13123466








