ZH-ECochG Bode Plot: A Novel Approach to Visualize Electrocochleographic Data in Cochlear Implant Users
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Review
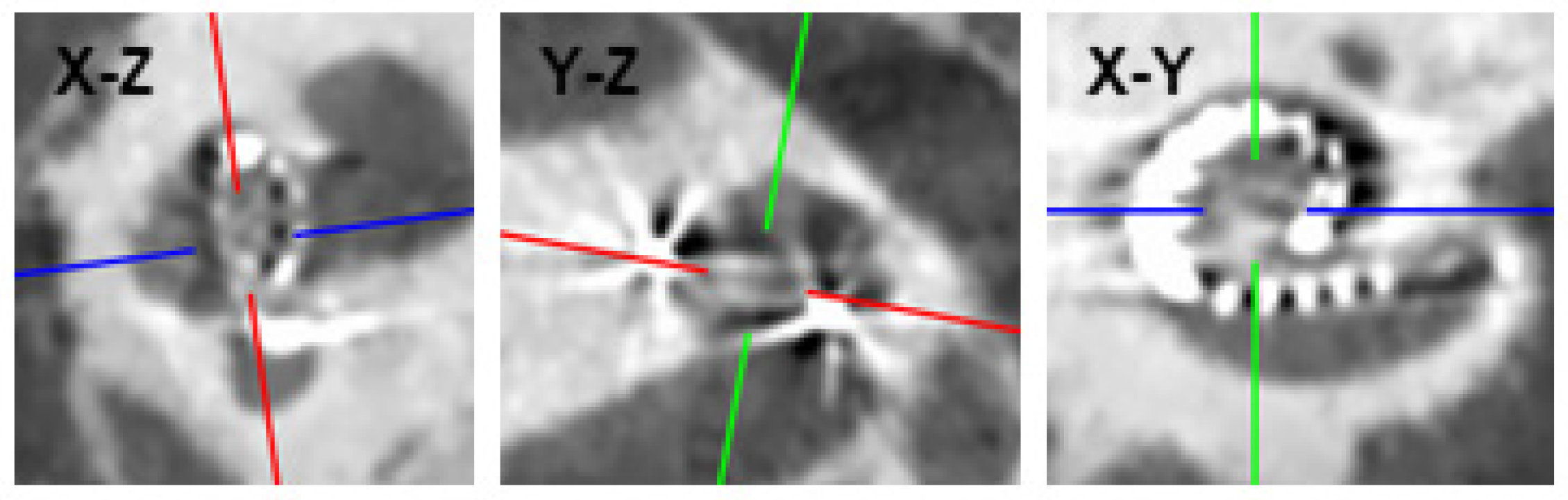
2.2. Construction of the ZH-ECochG Bode Plot
3. Results
3.1. Systematic Review
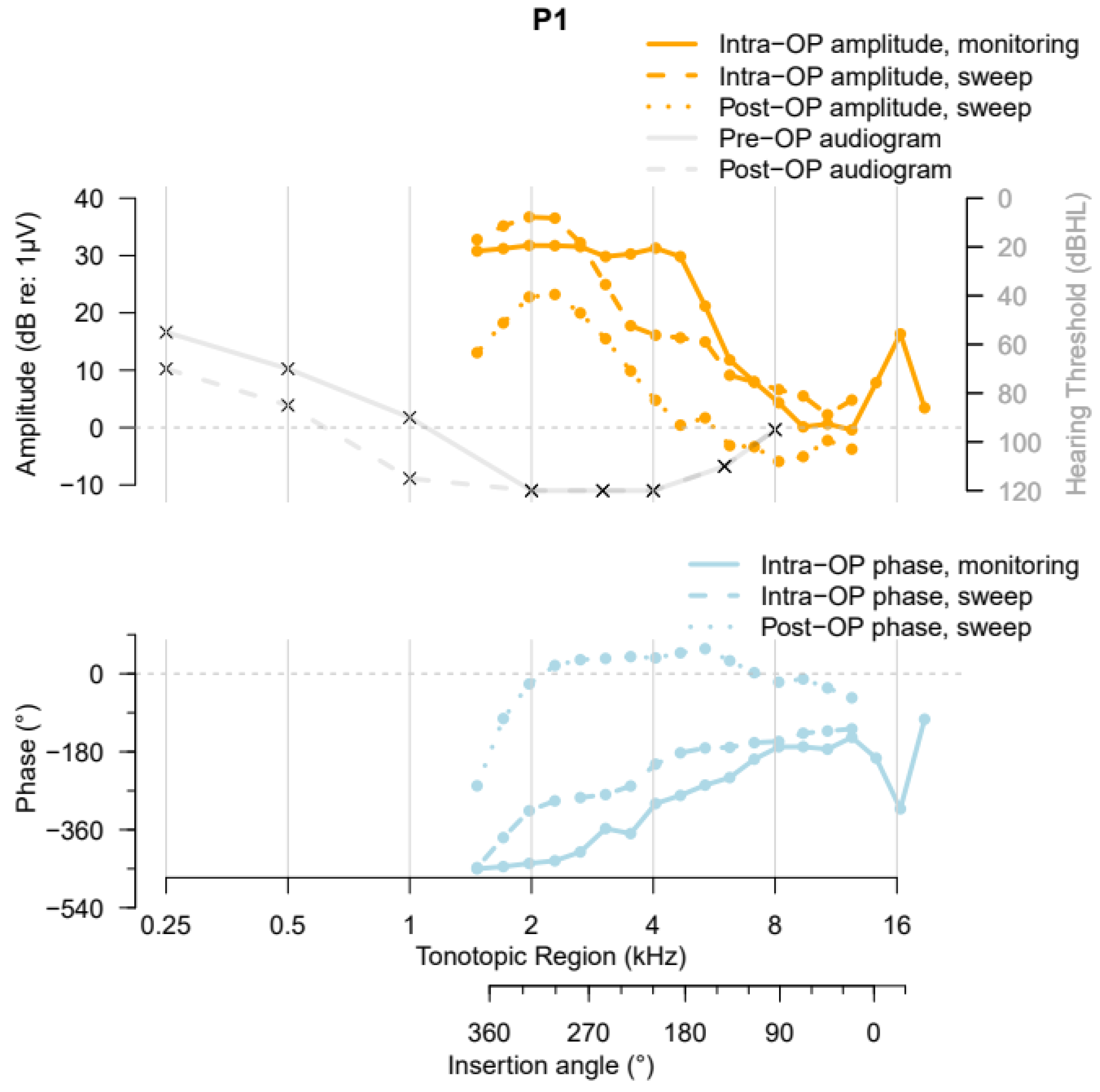
3.2. The ZH-ECochG Bode Plot in Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhury, B.; Fitzpatrick, D.C.; Buchman, C.A.; Wei, B.P.; Dillon, M.T.; He, S.; Adunka, O.F. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol. Neurotol. 2012, 33, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Dallos, P. Cochlear potentials and cochlear mechanics. In Basic Mechanisms in Hearing; MØLler, A.R., Ed.; Academic Press: New York, NY, USA, 1973; pp. 335–376. [Google Scholar]
- Eggermont, J.J. Ups and Downs in 75 Years of Electrocochleography. Front. Syst. Neurosci. 2017, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Forgues, M.; Koehn, H.A.; Dunnon, A.K.; Pulver, S.H.; Buchman, C.A.; Adunka, O.F.; Fitzpatrick, D.C. Distinguishing hair cell from neural potentials recorded at the round window. J. Neurophysiol. 2014, 111, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Pappa, A.K.; Hutson, K.A.; Scott, W.C.; Wilson, J.D.; Fox, K.E.; Masood, M.M.; Giardina, C.K.; Pulver, S.H.; Grana, G.D.; Askew, C.; et al. Hair cell and neural contributions to the cochlear summating potential. J. Neurophysiol. 2019, 121, 2163–2180. [Google Scholar] [CrossRef]
- Snyder, R.L.; Schreiner, C.E. The auditory neurophonic: Basic properties. Hear. Res. 1984, 15, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Abbas, P.J.; Tejani, V.D.; Scheperle, R.A.; Brown, C.J. Using Neural Response Telemetry to Monitor Physiological Responses to Acoustic Stimulation in Hybrid Cochlear Implant Users. Ear Hear. 2017, 38, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Adunka, O.; Roush, P.; Grose, J.; Macpherson, C.; Buchman, C.A. Monitoring of cochlear function during cochlear implantation. Laryngoscope 2006, 116, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Bester, C.W.; Campbell, L.; Dragovic, A.; Collins, A.; O’Leary, S.J. Characterizing electrocochleography in cochlear implant recipients with residual low-frequency hearing. Front. Neurosci. 2017, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Dalbert, A.; Pfiffner, F.; Hoesli, M.; Koka, K.; Veraguth, D.; Roosli, C.; Huber, A. Assessment of Cochlear Function during Cochlear Implantation by Extra- and Intracochlear Electrocochleography. Front. Neurosci. 2018, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Dalbert, A.; Pfiffner, F.; Hoesli, M.; Meerwein, C.; Veraguth, D.; Roosli, C.; Huber, A. Changes of Electrocochleographic Responses During Cochlear Implantation Presented at the Annual Meeting of ADANO 2016 in Berlin. Otol. Neurotol. 2019, 40, e424–e429. [Google Scholar] [CrossRef] [PubMed]
- Giardina, C.K.; Brown, K.D.; Adunka, O.F.; Buchman, C.A.; Hutson, K.A.; Pillsbury, H.C.; Fitzpatrick, D.C. Intracochlear Electrocochleography: Response Patterns During Cochlear Implantation and Hearing Preservation. Ear Hear. 2019, 40, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.S.; Riggs, W.J.; Giardina, C.K.; O’Connell, B.P.; Holder, J.T.; Dwyer, R.T.; Koka, K.; Labadie, R.F.; Fitzpatrick, D.C.; Adunka, O.F. Patterns Seen During Electrode Insertion Using Intracochlear Electrocochleography Obtained Directly Through a Cochlear Implant. Otol. Neurotol. 2017, 38, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.S.; Riggs, W.J.; Koka, K.; Litvak, L.M.; Malhotra, P.; Moberly, A.C.; O‘Connell, B.P.; Holder, J.; Di Lella, F.A.; Boccio, C.M.; et al. Real-Time Intracochlear Electrocochleography Obtained Directly Through a Cochlear Implant. Otol. Neurotol. 2017, 38, e107–e113. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Mylanus, E.; Venail, F.; Lenarz, T.; Birman, C.; Di Lella, F.; Roland, J.T.; Gantz, B.; Beynon, A.; Sicard, M.; et al. Monitoring Cochlear Health With Intracochlear Electrocochleography During Cochlear Implantation: Findings From an International Clinical Investigation. Ear Hear. 2023, 44, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Radeloff, A.; Shehata-Dieler, W.; Scherzed, A.; Rak, K.; Harnisch, W.; Hagen, R.; Mlynski, R. Intraoperative monitoring using cochlear microphonics in cochlear implant patients with residual hearing. Otol. Neurotol. 2012, 33, 348–354. [Google Scholar] [CrossRef]
- Saoji, A.A.; Graham, M.K.; Adkins, W.J.; Koka, K.; Carlson, M.L.; Neff, B.A.; Driscoll, C.L.W.; Fitzpatrick, D.C. Multi-Frequency Electrocochleography and Electrode Scan to Identify Electrode Insertion Trauma during Cochlear Implantation. Brain Sci. 2023, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Sijgers, L.; Pfiffner, F.; Grosse, J.; Dillier, N.; Koka, K.; Roosli, C.; Huber, A.; Dalbert, A. Simultaneous Intra- and Extracochlear Electrocochleography During Cochlear Implantation to Enhance Response Interpretation. Trends Hear. 2021, 25, 2331216521990594. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Shew, M.A.; Lefler, S.M.; Kallogjeri, D.; Wick, C.C.; Holden, T.A.; Durakovic, N.; Ortmann, A.J.; Herzog, J.A.; Buchman, C.A. Is Characteristic Frequency Limiting Real-Time Electrocochleography During Cochlear Implantation? Front. Neurosci. 2022, 16, 915302. [Google Scholar] [CrossRef] [PubMed]
- Adunka, O.F.; Mlot, S.; Suberman, T.A.; Campbell, A.P.; Surowitz, J.; Buchman, C.A.; Fitzpatrick, D.C. Intracochlear recordings of electrophysiological parameters indicating cochlear damage. Otol. Neurotol. 2010, 31, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.; Adunka, O.F.; Demason, C.E.; Ahmad, F.I.; Buchman, C.A.; Fitzpatrick, D.C. Detection of intracochlear damage with cochlear implantation in a gerbil model of hearing loss. Otol. Neurotol. 2011, 32, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Dalbert, A.; Huber, A.; Veraguth, D.; Roosli, C.; Pfiffner, F. Assessment of Cochlear Trauma During Cochlear Implantation Using Electrocochleography and Cone Beam Computed Tomography. Otol. Neurotol. 2016, 37, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Riggs, W.J.; Dwyer, R.T.; Holder, J.T.; Mattingly, J.K.; Ortmann, A.; Noble, J.H.; Dawant, B.M.; Valenzuela, C.V.; O‘Connell, B.P.; Harris, M.S.; et al. Intracochlear Electrocochleography: Influence of Scalar Position of the Cochlear Implant Electrode on Postinsertion Results. Otol. Neurotol. 2019, 40, e503–e510. [Google Scholar] [CrossRef] [PubMed]
- Koka, K.; Riggs, W.J.; Dwyer, R.; Holder, J.T.; Noble, J.H.; Dawant, B.M.; Ortmann, A.; Valenzuela, C.V.; Mattingly, J.K.; Harris, M.M.; et al. Intra-Cochlear Electrocochleography During Cochear Implant Electrode Insertion Is Predictive of Final Scalar Location. Otol. Neurotol. 2018, 39, e654–e659. [Google Scholar] [CrossRef] [PubMed]
- Saoji, A.A.; Patel, N.S.; Carlson, M.L.; Neff, B.A.; Koka, K.; Tarigoppula, V.S.A.; Driscoll, C.L.W. Multi-frequency Electrocochleography Measurements can be Used to Monitor and Optimize Electrode Placement During Cochlear Implant Surgery. Otol. Neurotol. 2019, 40, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Attias, J.; Ulanovski, D.; Hilly, O.; Greenstein, T.; Sokolov, M.; HabibAllah, S.; Mormer, H.; Raveh, E. Postoperative Intracochlear Electrocochleography in Pediatric Cochlear Implant Recipients: Association to Audiometric Thresholds and Auditory Performance. Ear Hear. 2020, 41, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Coulthurst, S.; Nachman, A.J.; Murray, M.T.; Koka, K.; Saoji, A.A. Comparison of Pure-Tone Thresholds and Cochlear Microphonics Thresholds in Pediatric Cochlear Implant Patients. Ear Hear. 2020, 41, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Koka, K.; Saoji, A.A.; Attias, J.; Litvak, L.M. An objective estimation of air-bone-gap in cochlear implant recipients with residual hearing using electrocochleography. Front. Neurosci. 2017, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Koka, K.; Saoji, A.A.; Litvak, L.M. Electrocochleography in Cochlear Implant Recipients With Residual Hearing: Comparison With Audiometric Thresholds. Ear Hear. 2017, 38, e161–e167. [Google Scholar] [CrossRef] [PubMed]
- Riggs, W.J.; Roche, J.P.; Giardina, C.K.; Harris, M.S.; Bastian, Z.J.; Fontenot, T.E.; Buchman, C.A.; Brown, K.D.; Adunka, O.F.; Fitzpatrick, D.C. Intraoperative Electrocochleographic Characteristics of Auditory Neuropathy Spectrum Disorder in Cochlear Implant Subjects. Front. Neurosci. 2017, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, R.; del Castillo, I.; Cama, E.; Scimemi, P.; Starr, A. Audibility, speech perception and processing of temporal cues in ribbon synaptic disorders due to OTOF mutations. Hear. Res. 2015, 330, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, R.; Rossi, R.; Scimemi, P.; Cama, E.; Valentino, M.L.; La Morgia, C.; Caporali, L.; Liguori, R.; Magnavita, V.; Monteleone, A.; et al. OPA1-related auditory neuropathy: Site of lesion and outcome of cochlear implantation. Brain 2015, 138, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Trecca, E.M.C.; Adunka, O.F.; Hiss, M.M.; Mattingly, J.K.; Moberly, A.C.; Dodson, E.E.; Cassano, M.; Prevedello, D.M.; Riggs, W.J. Intraoperative Electrocochleography in Subjects Affected by Vestibular Schwannoma and Meniere’s Disease: Comparison of Results. Ear Hear. 2022, 43, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Bester, C.; Dalbert, A.; Collins, A.; Razmovski, T.; Gerard, J.M.; O’Leary, S. Electrocochleographic Patterns Predicting Increased Impedances and Hearing Loss after Cochlear Implantation. Ear Hear. 2023, 44, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Kaicer, A.; Sly, D.; Iseli, C.; Wei, B.; Briggs, R.; O’Leary, S. Intraoperative Real-time Cochlear Response Telemetry Predicts Hearing Preservation in Cochlear Implantation. Otol. Neurotol. 2016, 37, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Dalbert, A.; Sim, J.H.; Gerig, R.; Pfiffner, F.; Roosli, C.; Huber, A. Correlation of Electrophysiological Properties and Hearing Preservation in Cochlear Implant Patients. Otol. Neurotol. 2015, 36, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Dalbert, A.; Rohner, P.; Roosli, C.; Veraguth, D.; Huber, A.; Pfiffner, F. Correlation Between Electrocochleographic Changes During Surgery and Hearing Outcome in Cochlear Implant Recipients: A Case Report and Systematic Review of the Literature. Otol. Neurotol. 2020, 41, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.S.; Koka, K.; Riggs, W.J.; Saleh, S.; Holder, J.T.; Dwyer, R.T.; Prentiss, S.; Lefler, S.; Kozlowski, K.; Hiss, M.M.; et al. Can Electrocochleography Help Preserve Hearing After Cochlear Implantation With Full Electrode Insertion? Otol. Neurotol. 2022, 43, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lenarz, T.; Buechner, A.; Gantz, B.; Hansen, M.; Tejani, V.D.; Labadie, R.; O‘Connell, B.; Buchman, C.A.; Valenzuela, C.V.; Adunka, O.F.; et al. Relationship Between Intraoperative Electrocochleography and Hearing Preservation. Otol. Neurotol. 2022, 43, e72–e78. [Google Scholar] [CrossRef]
- O‘Connell, B.P.; Holder, J.T.; Dwyer, R.T.; Gifford, R.H.; Noble, J.H.; Bennett, M.L.; Rivas, A.; Wanna, G.B.; Haynes, D.S.; Labadie, R.F. Intra- and postoperative electrocochleography may be predictive of final electrode position and postoperative hearing preservation. Front. Neurosci. 2017, 11, 291. [Google Scholar] [CrossRef]
- O’Leary, S.; Briggs, R.; Gerard, J.M.; Iseli, C.; Wei, B.P.C.; Tari, S.; Rousset, A.; Bester, C. Intraoperative Observational Real-time Electrocochleography as a Predictor of Hearing Loss After Cochlear Implantation: 3 and 12 Month Outcomes. Otol. Neurotol. 2020, 41, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Sijgers, L.; Sorensen, T.; Soulby, A.; Boyle, P.; Dalbert, A.; Röösli, C.; Jablonski, G.E.; Hamacher, V.; Greisiger, R.; Jiang, D.; et al. Classification of Acoustic Hearing Preservation After Cochlear Implantation Using Electrocochleography. Trends Hear. 2023, 27, 23312165231220997. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, M.W.; O‘Connell, B.P.; Giardina, C.K.; Buss, E.; Brown, K.D.; Dillon, M.T.; Rooth, M.A.; Pillsbury, H.C.; Buchman, C.A.; Adunka, O.F.; et al. Relationship Between Electrocochleography, Angular Insertion Depth, and Cochlear Implant Speech Perception Outcomes. Ear Hear. 2021, 42, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, D.C.; Campbell, A.T.; Choudhury, B.; Dillon, M.P.; Forgues, M.; Buchman, C.A.; Adunka, O.F. Round window electrocochleography just before cochlear implantation: Relationship to word recognition outcomes in adults. Otol. Neurotol. 2014, 35, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, T.E.; Giardina, C.K.; Dillon, M.; Rooth, M.A.; Teagle, H.F.; Park, L.R.; Brown, K.D.; Adunka, O.F.; Buchman, C.A.; Pillsbury, H.C.; et al. Residual Cochlear Function in Adults and Children Receiving Cochlear Implants: Correlations With Speech Perception Outcomes. Ear Hear. 2019, 40, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Formeister, E.J.; McClellan, J.H.; Merwin, W.H.; Iseli, C.E.; Calloway, N.H.; Teagle, H.F.; Buchman, C.A.; Adunka, O.F.; Fitzpatrick, D.C. Intraoperative round window electrocochleography and speech perception outcomes in pediatric cochlear implant recipients. Ear Hear. 2015, 36, 249–260. [Google Scholar] [CrossRef] [PubMed]
- McClellan, J.H.; Formeister, E.J.; Merwin, W.H.; Dillon, M.T.; Calloway, N.; Iseli, C.; Buchman, C.A.; Fitzpatrick, D.C.; Adunka, O.F. Round window electrocochleography and speech perception outcomes in adult cochlear implant subjects: Comparison with audiometric and biographical information. Otol. Neurotol. 2014, 35, e245–e252. [Google Scholar] [CrossRef]
- Walia, A.; Shew, M.A.; Kallogjeri, D.; Wick, C.C.; Durakovic, N.; Lefler, S.M.; Ortmann, A.J.; Herzog, J.A.; Buchman, C.A. Electrocochleography and cognition are important predictors of speech perception outcomes in noise for cochlear implant recipients. Sci. Rep. 2022, 12, 3083. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Shew, M.A.; Lee, D.S.; Lefler, S.M.; Kallogjeri, D.; Wick, C.C.; Durakovic, N.; Fitzpatrick, D.C.; Ortmann, A.J.; Herzog, J.A.; et al. Promontory Electrocochleography Recordings to Predict Speech-Perception Performance in Cochlear Implant Recipients. Otol. Neurotol. 2022, 43, 915–923. [Google Scholar] [CrossRef]
- Rüegg, U.; Dalbert, A.; Veraguth, D.; Röösli, C.; Huber, A.; Pfiffner, F. Correlation between Speech Perception Outcomes after Cochlear Implantation and Postoperative Acoustic and Electric Hearing Thresholds. J. Clin. Med. 2021, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Koch, R.W.; Ladak, H.M.; Elfarnawany, M.; Agrawal, S.K. Measuring Cochlear Duct Length–a historical analysis of methods and results. J. Otolaryngol. Head. Neck Surg. 2017, 46, 19. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- ISO 8253-1 2010; Acoustics—Audiometric Test Methods—Part 1: Pure-Tone Air and Bone Conduction Audiometry. ISO: New York, NY, USA, 2010.
- Skarzynski, H.; van de Heyning, P.; Agrawal, S.; Arauz, S.L.; Atlas, M.; Baumgartner, W.; Caversaccio, M.; de Bodt, M.; Gavilan, J.; Godey, B.; et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol. Suppl. 2013, 133, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.L.; Polterauer, D.; Hempel, J.M.; Canis, M.; Spiro, J.E.; Müller, J. Variation of the cochlear anatomy and cochlea duct length: Analysis with a new tablet-based software. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Dhanasingh, A.; Jolly, C. An overview of cochlear implant electrode array designs. Hear. Res. 2017, 356, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.D. A cochlear frequency-position function for several species--29 years later. J. Acoust. Soc. Am. 1990, 87, 2592–2605. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.N.; Tavora-Vieira, D.; Rajan, G.P. Using the Implant Electrode Array to Conduct Real-time Intraoperative Hearing Monitoring During Pediatric Cochlear Implantation: Preliminary Experiences. Otol. Neurotol. 2016, 37, e148–e153. [Google Scholar] [CrossRef]
- Adel, Y.; Tillein, J.; Petzold, H.; Weissgerber, T.; Baumann, U. Band-Limited Chirp-Evoked Compound Action Potential in Guinea Pig: Comprehensive Neural Measure for Cochlear Implantation Monitoring. Ear Hear. 2021, 42, 142–162. [Google Scholar] [CrossRef] [PubMed]
- Andonie, R.R.; Wimmer, W.; Wildhaber, R.A.; Caversaccio, M.; Weder, S. Real-Time Feature Extraction from Electrocochleography with Impedance Measurements during Cochlear Implantation using Linear State-Space Models. IEEE Trans. Biomed. Eng. 2023, 70, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Arweiler-Harbeck, D.; D‘Heygere, V.; Meyer, M.; Hans, S.; Waschkies, L.; Lang, S.; Anton, K.; Hessel, H.; Schneider, A.; Heiler, T.; et al. Digital Live Imaging of Intraoperative Electrocochleography—First Description of Feasibility and Hearing Preservation During Cochlear Implantation. Otol. Neurotol. 2021, 42, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.H.; Yin, L.X.; Saoji, A.A.; Carlson, M.L. Electrocochleography in cochlear implantation: Development, applications, and future directions. World J. Otorhinolaryngol. Head. Neck Surg. 2021, 7, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Baumhoff, P.; Rahbar Nikoukar, L.; de Andrade, J.S.C.; Lenarz, T.; Kral, A. Summating Potential as Marker of Intracochlear Position in Bipolar Electrocochleography. Ear Hear. 2023, 44, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Bayri Ulukan, M.; Ciprut, A. Intracochlear electrocochleography findings in cochlear implant recipients with auditory neuropathy spectrum disorder. Int. J. Pediatr. Otorhinolaryngol. 2023, 170, 111596. [Google Scholar] [CrossRef] [PubMed]
- Bester, C.; Weder, S.; Collins, A.; Dragovic, A.; Brody, K.; Hampson, A.; O’Leary, S. Cochlear microphonic latency predicts outer hair cell function in animal models and clinical populations. Hear. Res. 2020, 398, 108094. [Google Scholar] [CrossRef] [PubMed]
- Bester, C.; Collins, A.; Razmovski, T.; Weder, S.; Briggs, R.J.; Wei, B.; Zakaria, A.F.; Gerard, J.M.; Mitchell-Innes, A.; Tykocinski, M.; et al. Electrocochleography triggered intervention successfully preserves residual hearing during cochlear implantation: Results of a randomised clinical trial. Hear. Res. 2022, 426, 108353. [Google Scholar] [CrossRef] [PubMed]
- Buechner, A.; Bardt, M.; Haumann, S.; Geissler, G.; Salcher, R.; Lenarz, T. Clinical experiences with intraoperative electrocochleography in cochlear implant recipients and its potential to reduce insertion trauma and improve postoperative hearing preservation. PLoS ONE 2022, 17, e0266077. [Google Scholar] [CrossRef]
- Buhle, A.C.; McCrary, H.C.; Gordon, S.A.; Johnson, K.M.; Babajanian, E.E.; Patel, N.S. Expanding Understanding of Electrocochleography in Cochlear Implantation: Auditory Neuropathy Spectrum Disorder With Normal Pure Tone Average. Otol. Neurotol. Open 2023, 3, e035. [Google Scholar] [CrossRef]
- Calloway, N.H.; Fitzpatrick, D.C.; Campbell, A.P.; Iseli, C.; Pulver, S.; Buchman, C.A.; Adunka, O.F. Intracochlear Electrocochleography During Cochlear Implantation. Otol. Neurotol. 2014, 35, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.P.; Suberman, T.A.; Buchman, C.A.; Fitzpatrick, D.C.; Adunka, O.F. Correlation of early auditory potentials and intracochlear electrode insertion properties: An animal model featuring near real-time monitoring. Otol. Neurotol. 2010, 31, 1391–1398. [Google Scholar] [CrossRef]
- Campbell, L.; Bester, C.; Iseli, C.; Sly, D.; Dragovic, A.; Gummer, A.W.; O’Leary, S. Electrophysiological Evidence of the Basilar-Membrane Travelling Wave and Frequency Place Coding of Sound in Cochlear Implant Recipients. Audiol. Neurootol. 2017, 22, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Kaicer, A.; Briggs, R.; O’Leary, S. Cochlear response telemetry: Intracochlear electrocochleography via cochlear implant neural response telemetry pilot study results. Otol. Neurotol. 2015, 36, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Dalbert, A.; Pfiffner, F.; Roosli, C.; Thoele, K.; Sim, J.H.; Gerig, R.; Huber, A.M. Extra- and Intracochlear Electrocochleography in Cochlear Implant Recipients. Audiol. Neurootol. 2015, 20, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Dalbert, A.; Sijgers, L.; Grosse, J.; Veraguth, D.; Roosli, C.; Huber, A.; Pfiffner, F. Simultaneous Intra- and Extracochlear Electrocochleography During Electrode Insertion. Ear Hear. 2021, 42, 414–424. [Google Scholar] [CrossRef] [PubMed]
- DeMason, C.; Choudhury, B.; Ahmad, F.; Fitzpatrick, D.C.; Wang, J.; Buchman, C.A.; Adunka, O.F. Electrophysiological properties of cochlear implantation in the gerbil using a flexible array. Ear Hear. 2012, 33, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Eichler, T.; Lakomek, A.; Waschkies, L.; Meyer, M.; Sadok, N.; Lang, S.; Arweiler-Harbeck, D. Two different methods to digitally visualize continuous electrocochleography potentials during cochlear implantation: A first description of feasibility. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Gawęcki, W.; Balcerowiak, A.; Podlawska, P.; Borowska, P.; Gibasiewicz, R.; Szyfter, W.; Wierzbicka, M. Robot-Assisted Electrode Insertion in Cochlear Implantation Controlled by Intraoperative Electrocochleography—A Pilot Study. J. Clin. Med. 2022, 11, 7045. [Google Scholar] [CrossRef] [PubMed]
- Giardina, C.K.; Khan, T.E.; Pulver, S.H.; Adunka, O.F.; Buchman, C.A.; Brown, K.D.; Pillsbury, H.C.; Fitzpatrick, D.C. Response Changes During Insertion of a Cochlear Implant Using Extracochlear Electrocochleography. Ear Hear. 2018, 39, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Greisiger, R.; Bester, C.; Sørensen, T.; Korslund, H.; Bunne, M.; O’Leary, S.; Jablonski, G.E. Intraoperative Measured Electrocochleography and Fluoroscopy Video to Detect Cochlea Trauma. Otol. Neurotol. 2024, 45, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Cruise, A.; Gibson, W.; Bate, K.; Sanli, H. Preliminary results and technique for electrophysiological intra-operative monitoring of residual hearing during cochlear implantation. Cochlear Implant. Int. 2011, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Helmstaedter, V.; Lenarz, T.; Erfurt, P.; Kral, A.; Baumhoff, P. The Summating Potential Is a Reliable Marker of Electrode Position in Electrocochleography: Cochlear Implant as a Theragnostic Probe. Ear Hear. 2018, 39, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Henslee, A.M.; Kaufmann, C.R.; Andrick, M.D.; Reineke, P.T.; Tejani, V.D.; Hansen, M.R. Development and Characterization of an Electrocochleography-Guided Robotics-Assisted Cochlear Implant Array Insertion System. Otolaryngol. Head Neck Surg. 2021, 167, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Imsiecke, M.; Büchner, A.; Lenarz, T.; Nogueira, W. Psychoacoustic and electrophysiological electric-acoustic interaction effects in cochlear implant users with ipsilateral residual hearing. Hear. Res. 2020, 386, 107873. [Google Scholar] [CrossRef] [PubMed]
- Jwair, S.; Ramekers, D.; Thomeer, H.G.X.M.; Versnel, H. Acute effects of cochleostomy and electrode-array insertion on compound action potentials in normal-hearing guinea pigs. Front. Neurosci. 2023, 17, 978230. [Google Scholar] [CrossRef] [PubMed]
- Kashani, R.G.; Kocharyan, A.; Bennion, D.M.; Scheperle, R.A.; Etler, C.; Oleson, J.; Dunn, C.C.; Claussen, A.D.; Gantz, B.J.; Hansen, M.R. Combining Intraoperative Electrocochleography with Robotics-Assisted Electrode Array Insertion. Otol. Neurotol. 2024, 45, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lenarz, T.; Buechner, A.; Lesinski-Schiedat, A.; Timm, M.; Salcher, R. Hearing Preservation With a New Atraumatic Lateral Wall Electrode. Otol. Neurotol. 2020, 41, e993–e1003. [Google Scholar] [CrossRef] [PubMed]
- Linder, P.; Iso-Mustajarvi, M.; Dietz, A. A Comparison of ECochG With the Subjective Sound Perception During Cochlear Implantation Under Local Anesthesia-A Case Series Study. Otol. Neurotol. 2022, 43, e540–e547. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Bester, C.; Collins, A.; Newbold, C.; Hampson, A.; Chambers, S.; Eastwood, H.; O’Leary, S. Intraoperative force and electrocochleography measurements in an animal model of cochlear implantation. Hear. Res. 2018, 358, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Lorens, A.; Walkowiak, A.; Polak, M.; Kowalczuk, A.; Furmanek, M.; Skarzynski, H.; Obrycka, A. Cochlear Microphonics in Hearing Preservation Cochlear Implantees. J. Int. Adv. Otol. 2019, 15, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Mandala, M.; Colletti, L.; Tonoli, G.; Colletti, V. Electrocochleography during cochlear implantation for hearing preservation. Otolaryngol. Head. Neck Surg. 2012, 146, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Lu, T.; Chen, M.; Mao, J.; Hu, X.; Li, S. Forward Electric Stimulation-Induced Interference in Intracochlear Electrocochleography of Acoustic Stimulation in the Cochlea of Guinea Pigs. Front. Neurosci. 2022, 16, 853275. [Google Scholar] [CrossRef] [PubMed]
- Panario, J.; Bester, C.; O’Leary, S.J. Characteristics of the Summating Potential Measured Across a Cochlear Implant Array as an Indicator of Cochlear Function. Ear Hear. 2023, 44, 1088–1106. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Lorens, A.; Walkowiak, A.; Furmanek, M.; Skarzynski, P.H.; Skarzynski, H. In Vivo Basilar Membrane Time Delays in Humans. Brain Sci. 2022, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Macias, A.; O’Leary, S.; Ramos-deMiguel, A.; Bester, C.; Falcon-González, J.C. Intraoperative Intracochlear Electrocochleography and Residual Hearing Preservation Outcomes When Using Two Types of Slim Electrode Arrays in Cochlear Implantation. Otol. Neurotol. 2019, 40, S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Saoji, A.A.; Graham, M.K.; Adkins, W.J.; Nassiri, A.M.; Neff, B.A.; Carlson, M.L.; Driscoll, C.L.W. Relationship Between Intraoperative Electrocochleography Responses and Immediate Postoperative Bone Conduction Thresholds in Cochlear Implantation. Otol. Neurotol. 2022, 43, e880–e887. [Google Scholar] [CrossRef] [PubMed]
- Scheperle, R.; Etler, C.; Oleson, J.; Dunn, C.; Kashani, R.; Claussen, A.; Gantz, B.J.; Hansen, M.R. Evaluation of Real-Time Intracochlear Electrocochleography for Guiding Cochlear Implant Electrode Array Position. J. Clin. Med. 2023, 12, 7409. [Google Scholar] [CrossRef] [PubMed]
- Schuerch, K.; Waser, M.; Mantokoudis, G.; Anschuetz, L.; Caversaccio, M.; Wimmer, W.; Weder, S. Increasing the reliability of real-time electrocochleography during cochlear implantation: A standardized guideline. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4655–4665. [Google Scholar] [CrossRef] [PubMed]
- Schuerch, K.; Waser, M.; Mantokoudis, G.; Anschuetz, L.; Wimmer, W.; Caversaccio, M.; Weder, S. Performing Intracochlear Electrocochleography during Cochlear Implantation. J. Vis. Exp. JoVE 2022, 8, e63153. [Google Scholar] [CrossRef] [PubMed]
- Schuerch, K.; Wimmer, W.; Rummel, C.; Caversaccio, M.D.; Weder, S. Objective evaluation of intracochlear electrocochleography: Repeatability, thresholds, and tonotopic patterns. Front. Neurol. 2023, 14, 1181539. [Google Scholar] [CrossRef] [PubMed]
- Skarżyński, P.H.; Lorens, A.; Walkowiak, A.; Polak, M.; Skarżyński, H. Multi-Frequency Intraoperative Monitoring of Hearing Preservation during Cochlear Implantation. Life 2022, 12, 636. [Google Scholar] [CrossRef]
- Soulby, A.; Connor, S.; Jiang, D.; Nunn, T.; Boyle, P.; Pai, I. Establishing Reproducibility and Correlation of Cochlear Microphonic Amplitude to Implant Electrode Position Using Intraoperative Electrocochleography and Postoperative Cone Beam Computed Tomography. Ear Hear. 2021, 42, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Tejani, V.D.; Carroll, R.L.; Abbas, P.J.; Brown, C.J. Impact of stimulus frequency and recording electrode on electrocochleography in Hybrid cochlear implant users. Hear. Res. 2019, 384, 107815. [Google Scholar] [CrossRef] [PubMed]
- van Gendt, M.J.; Koka, K.; Kalkman, R.K.; Stronks, H.C.; Briaire, J.J.; Litvak, L.; Frijns, J.H.M. Simulating intracochlear electrocochleography with a combined model of acoustic hearing and electric current spread in the cochlea. J. Acoust. Soc. Am. 2020, 147, 2049. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.J.; Walia, A.; Lefler, S.M.; Ortmann, A.J.; Shew, M.A.; Durakovic, N.; Wick, C.C.; Herzog, J.A.; Buchman, C.A. Identifying Slim Modiolar Electrode Tip Fold-Over With Intracochlear Electrocochleography. Otolaryngol. Head Neck Surg. 2024, 170, 1124–1132. [Google Scholar] [CrossRef]
- Walia, A.; Shew, M.A.; Ettyreddy, A.; Lefler, S.M.; Jiramongkolchai, P.; Wick, C.C.; Durakovic, N.; Buchman, C.A.; Herzog, J.A. Early Hearing Preservation Outcomes following Cochlear Implantation with New Slim Lateral Wall Electrode Using Electrocochleography. Otol. Neurotol. 2022, 43, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Shew, M.A.; Ortmann, A.J.; Buchman, C.A.; Herzog, J.A. Hearing Preservation After Cochlear Reimplantation Using Electrocochleography: A Case Report. Laryngoscope 2021, 131, 2348–2351. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Shew, M.A.; Varghese, J.; Ioerger, P.; Lefler, S.M.; Ortmann, A.J.; Herzog, J.A.; Buchman, C.A. Improved Cochlear Implant Performance Estimation Using Tonotopic-Based Electrocochleography. JAMA Otolaryngol. Head. Neck Surg. 2023, 149, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Weder, S.; Bester, C.; Collins, A.; Shaul, C.; Briggs, R.J.; O’Leary, S. Toward a Better Understanding of Electrocochleography: Analysis of Real-Time Recordings. Ear Hear. 2020, 41, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Wijewickrema, S.; Bester, C.; Gerard, J.M.; Collins, A.; O’Leary, S. Automatic analysis of cochlear response using electrocochleography signals during cochlear implant surgery. PLoS ONE 2022, 17, e0269187. [Google Scholar] [CrossRef] [PubMed]
- Trecca, E.M.C.; Riggs, W.J.; Mattingly, J.K.; Hiss, M.M.; Cassano, M.; Adunka, O.F. Electrocochleography and Cochlear Implantation: A Systematic Review. Otol. Neurotol. 2020, 41, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Kontorinis, G.; Lenarz, T.; Stöver, T.; Paasche, G. Impact of the insertion speed of cochlear implant electrodes on the insertion forces. Otol. Neurotol. 2011, 32, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Franke-Trieger, A.; Jolly, C.; Darbinjan, A.; Zahnert, T.; Muerbe, D. Insertion Depth Angles of Cochlear Implant Arrays With Varying Length: A Temporal Bone Study. Otol. Neurotol. 2013, 35, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, M.A.; Naik, K.; Dallos, P. Using the cochlear microphonic as a tool to evaluate cochlear function in mouse models of hearing. J. Assoc. Res. Otolaryngol. 2011, 12, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.; Alzhrani, F. Comparison of cochlear duct length between the Saudi and non-Saudi populations. Ann. Saudi Med. 2018, 38, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Gee, A.H.; Zhao, Y.; Treece, G.M.; Bance, M.L. Practicable assessment of cochlear size and shape from clinical CT images. Sci. Rep. 2021, 11, 3448. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Kashyap, R.L.; Chu, C.N. Building Skeleton Models via 3-D Medial Surface Axis Thinning Algorithms. CVGIP Graph. Models Image Process. 1994, 56, 462–478. [Google Scholar] [CrossRef]
- Kollmannsberger, P.; Kerschnitzki, M.; Repp, F.; Wagermaier, W.; Weinkamer, R.; Fratzl, P. The small world of osteocytes: Connectomics of the lacuno-canalicular network in bone. New J. Phys. 2017, 19, 073019. [Google Scholar] [CrossRef]
- Kjer, H.M.; Fagertun, J.; Vera, S.; Gil, D.; González Ballester, M.Á.; Paulsen, R.R. Free-form image registration of human cochlear μ CT data using skeleton similarity as anatomical prior. Pattern Recognit. Lett. 2016, 76, 76–82. [Google Scholar] [CrossRef]
- Breitsprecher, T.; Dhanasingh, A.; Schulze, M.; Kipp, M.; Dakah, R.A.; Oberhoffner, T.; Dau, M.; Frerich, B.; Weber, M.A.; Langner, S.; et al. CT imaging-based approaches to cochlear duct length estimation-a human temporal bone study. Eur. Radiol. 2022, 32, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Ortmann, A.J.; Lefler, S.; Holden, T.A.; Puram, S.V.; Herzog, J.A.; Buchman, C.A. Place Coding in the Human Cochlea. Medrxiv 2023. [Google Scholar] [CrossRef] [PubMed]

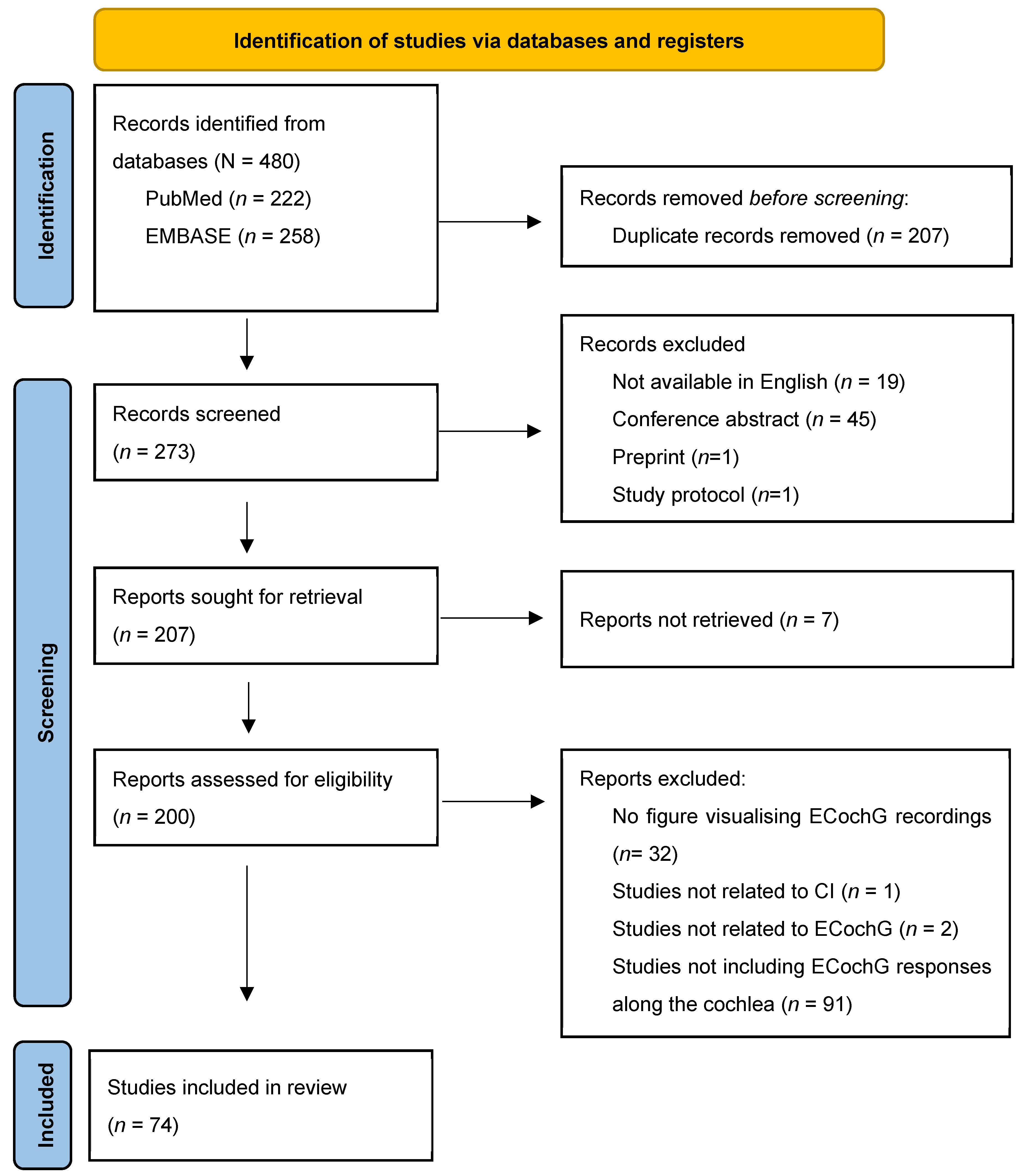


| Parameter | N | Parameter | N | ||
|---|---|---|---|---|---|
| Time of recording | Intraoperative | 98 * | Recording location | Intracochlear | 92 † |
| Intra- and postoperative | 2 * | Intra- and extracochlear | 11 † | ||
| Postoperative | 15 | Extracochlear | 12 | ||
| * Intraoperative | † Intracochlear | ||||
| During insertion | 70 | CI apical electrode | 48 | ||
| During and after insertion | 6 | Various CI electrodes | 39 | ||
| After insertion | 24 | Custom electrode | 14 | ||
| CI apical and various electrodes | 2 | ||||
| x-axis used | Insertion time | 34 | y-axis used | Waveform amplitude | 23 |
| Time waveform | 19 | Response magnitude | 62 | ||
| Recording electrode | 23 | Phase | 7 | ||
| Different steps | 6 | Stimulus frequency | 1 | ||
| Insertion depth | 13 | Sound pressure level | 1 | ||
| Tonotopic position | 3 | Participants | 1 | ||
| Stimulus frequency | 2 | Two or more y-axes | 20 | ||
| Sound pressure level | 1 | ||||
| Number of intracochlear electrodes | 1 | ||||
| Angle of rotation | 1 | ||||
| Two x-axes | 12 | ||||
| ECochG component | CM/DIF/FFT F0 | 71 | Extra | Audiogram included | 2 |
| Rarefaction/condensation | 3 | Notes during insertion | 16 | ||
| ANN/SUM | 2 | Noise floor included | 7 | ||
| CAP | 4 | Exemplary waveforms | 8 | ||
| SP | 2 | Color bar (amplitude) | 4 | ||
| Two or more components | 17 | Subplots | 66 | ||
| Sum of spectral peaks of the harmonics | 14 | Multiple curves | 75 | ||
| Not further specified | 2 | 3D visualization | 2 | ||
| Participant | Age at Implantation (Years) | Sex | Side | Etiology | Preoperative PTA (dB HL) | Hearing Preservation (%) | Length of Cochlear Duct (mm) | Electrode Array | Angular Insertion Depth (°) |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 65 | Male | Left | Progressive SNHL, unknown cause | 83.75 | 57.1 | 36.2 | HiFocus Mid-Scala (Advanced Bionics) | 374° |
| P2 | 59 | Male | Left | Ménière’s disease | 63.75 | 37.1 | 36.9 | CI 622 (Cochlear) | 410° |
| P3 | 83 | Male | Left | Progressive SNHL S/P chronic inflammation of the middle ear | 97.5 | 11.3 | 37.0 | CI 612 (Cochlear) | 362° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geys, M.; Sijgers, L.; Dobrev, I.; Dalbert, A.; Röösli, C.; Pfiffner, F.; Huber, A. ZH-ECochG Bode Plot: A Novel Approach to Visualize Electrocochleographic Data in Cochlear Implant Users. J. Clin. Med. 2024, 13, 3470. https://doi.org/10.3390/jcm13123470
Geys M, Sijgers L, Dobrev I, Dalbert A, Röösli C, Pfiffner F, Huber A. ZH-ECochG Bode Plot: A Novel Approach to Visualize Electrocochleographic Data in Cochlear Implant Users. Journal of Clinical Medicine. 2024; 13(12):3470. https://doi.org/10.3390/jcm13123470
Chicago/Turabian StyleGeys, Marlies, Leanne Sijgers, Ivo Dobrev, Adrian Dalbert, Christof Röösli, Flurin Pfiffner, and Alexander Huber. 2024. "ZH-ECochG Bode Plot: A Novel Approach to Visualize Electrocochleographic Data in Cochlear Implant Users" Journal of Clinical Medicine 13, no. 12: 3470. https://doi.org/10.3390/jcm13123470
APA StyleGeys, M., Sijgers, L., Dobrev, I., Dalbert, A., Röösli, C., Pfiffner, F., & Huber, A. (2024). ZH-ECochG Bode Plot: A Novel Approach to Visualize Electrocochleographic Data in Cochlear Implant Users. Journal of Clinical Medicine, 13(12), 3470. https://doi.org/10.3390/jcm13123470






