Assessing Functional Capacity in Myalgic Encephalopathy/Chronic Fatigue Syndrome: A Patient-Informed Questionnaire
Abstract
:1. Introduction
2. Methods
2.1. Questionnaire Development
2.2. Initial Survey Rounds Developing FUNCAP Questionnaire Items
2.3. Strategy for Shortened Questionnaire Version
2.4. Statistical Evaluation of Questionnaire Sub-Scores
2.4.1. Descriptive Statistics
2.4.2. Reliability
Internal Consistency
Round 6: Test–Retest Reliability
2.4.3. Validation
3. Results
3.1. Respondent Demographic Information
3.2. Initial Survey Rounds
3.3. Final Survey Rounds
3.3.1. Round 4—English Version
3.3.2. Main Round 5 Respondent Characteristics
3.4. Round 5 Item Scores
3.4.1. Creating a Shortened Version of FUNCAP55 (FUNCAP27)
3.4.2. FUNCAP Item Score Statistics
3.4.3. Round 5 FUNCAP55 Respondent Evaluation
3.5. Statistical Evaluation of Questionnaire Domain Sub-Scores
3.5.1. Descriptive Statistics
Floor and Ceiling Effects
Correlations among Sub-Scores
3.5.2. Reliability
Consistency
Round 6: FUNCAP27 Test–Retest Reliability
3.5.3. Validation
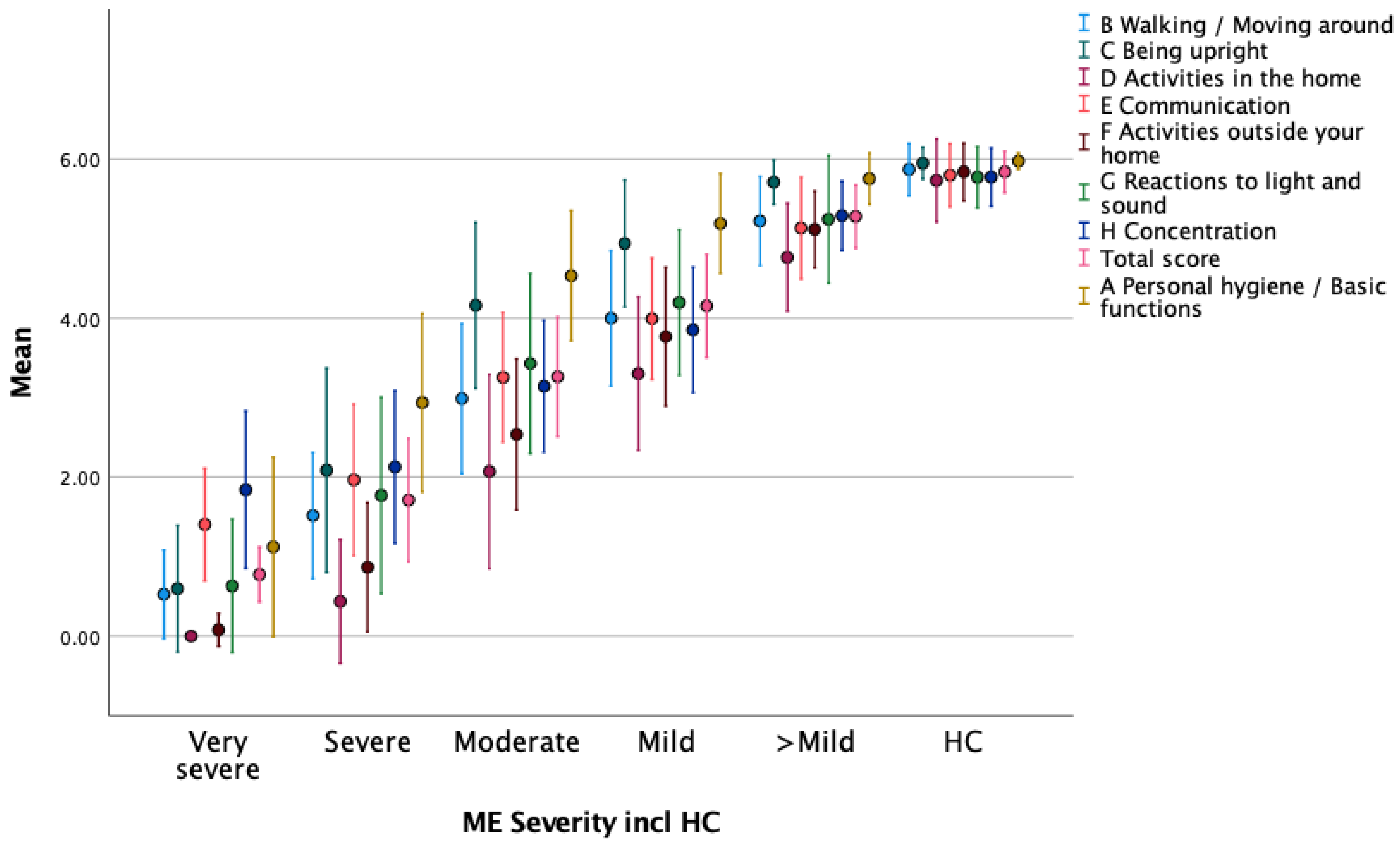
Known Group Validation—FUNCAP Sub-Scores vs. HCs
Content Validation—FUNCAP Items vs. WHO ICF Functions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A., Jr.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front. Pediatr. 2018, 6, 412. [Google Scholar] [CrossRef]
- Nacul, L.C.; Lacerda, E.M.; Campion, P.; Pheby, D.; Drachler Mde, L.; Leite, J.C.; Poland, F.; Howe, A.; Fayyaz, S.; Molokhia, M. The functional status and well being of people with myalgic encephalomyelitis/chronic fatigue syndrome and their carers. BMC Public Health 2011, 11, 402. [Google Scholar] [CrossRef]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- NICE. Myalgic Encephalomyelitis (or Encephalopathy)/Chronic Fatigue Syndrome: Diagnosis and Management. NICE Guideline [NG206]. Available online: https://www.nice.org.uk/guidance/ng206 (accessed on 1 May 2024).
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Jason, L.A.; Jordan, K.; Miike, T.; Bell, D.S.; Lapp, C.; Torres-Harding, S.; Rowe, K.; Gurwitt, A.; De Meirleir, K.; Van Hoof, E.L.S. A Pediatric Case Definition for Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2006, 13, 1–44. [Google Scholar] [CrossRef]
- Helsedirektoratet. Nasjonal Veileder. Pasienter med CFS/ME: Utredning, Diagnostikk, Behandling, Rehabilitering, Pleie og Omsorg. 2015. Available online: www.helsedirektoratet.no (accessed on 1 May 2024).
- Vernon, S.D.; Hartle, M.; Sullivan, K.; Bell, J.; Abbaszadeh, S.; Unutmaz, D.; Bateman, L. Post-exertional malaise among people with long COVID compared to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Work 2023, 74, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.; Jason, L.A.; Unutmaz, D.; Bateman, L.; Vernon, S.D. Improvement of Long COVID symptoms over one year. Front. Med. 2022, 9, 1065620. [Google Scholar] [CrossRef]
- Haywood, K.L.; Staniszewska, S.; Chapman, S. Quality and acceptability of patient-reported outcome measures used in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): A systematic review. Qual. Life Res. 2012, 21, 35–52. [Google Scholar] [CrossRef]
- Vergauwen, K.; Huijnen, I.P.; Kos, D.; Van de Velde, D.; van Eupen, I.; Meeus, M. Assessment of activity limitations and participation restrictions with persons with chronic fatigue syndrome: A systematic review. Disabil. Rehabil. 2015, 37, 1706–1716. [Google Scholar] [CrossRef]
- McHorney, C.W.; Johne, W., Jr.; Anastasiae, R. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and Clinical Tests of Validity in Measuring Physical and Mental Health Constructs. Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef]
- Nijs, J.; Vaes, P.; McGregor, N.; Van Hoof, E.; Meirleir, K.D. Psychometric Properties of the Dutch Chronic Fatigue Syndrome–Activities and Participation Questionnaire (CFS-APQ). Phys. Ther. 2003, 83, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Vaes, P.; De Meirleir, K. The Chronic Fatigue Syndrome Activities and Participation Questionnaire (CFS-APQ): An overview. Occup. Ther. Int. 2005, 12, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Podell, R.; Dimmock, M.E.; Comerford, B.B. Documenting disability in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Work 2020, 66, 339–352. [Google Scholar] [CrossRef]
- Wiering, B.; de Boer, D.; Delnoij, D. Patient involvement in the development of patient-reported outcome measures: A scoping review. Health Expect. 2017, 20, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Wormgoor, M.E.A.; Rodenburg, S.C. Focus on post-exertional malaise when approaching ME/CFS in specialist healthcare improves satisfaction and reduces deterioration. Front. Neurol. 2023, 14, 1247698. [Google Scholar] [CrossRef]
- Conroy, K.; Bhatia, S.; Islam, M.; Jason, L.A. Homebound versus Bedridden Status among Those with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Healthcare 2021, 9, 106. [Google Scholar] [CrossRef]
- Cotler, J.; Holtzman, C.; Dudun, C.; Jason, L.A. A Brief Questionnaire to Assess Post-Exertional Malaise. Diagnostics 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, C.S.; Bhatia, S.; Cotler, J.; Jason, L.A. Assessment of Post-Exertional Malaise (PEM) in Patients with Myalgic Encephalomyelitis (ME) and Chronic Fatigue Syndrome (CFS): A Patient-Driven Survey. Diagnostics 2019, 9, 26. [Google Scholar] [CrossRef]
- Brage, S.; Fleten, N.; Knutsrod, O.G.; Reiso, H.; Ryen, A. Norsk Funksjonsskjema—Et nytt instrument ved sykmelding og uførhetsvurdering. Tidsskr. Nor. Lægeforen. 2004, 124, 2472–2474. [Google Scholar]
- World Health Organization. The International Classification of Functioning, Disability and Health (ICF). 2001. Available online: http://www.who.int/classifications/icf/en/ (accessed on 1 May 2024).
- CDC. The ICF: An Overview. Available online: https://www.cdc.gov/nchs/data/icd/icfoverview_finalforwho10sept.pdf (accessed on 1 May 2024).
- Sommerfelt, K.; Schei, T.; Angelsen, A. Severe and Very Severe Myalgic Encephalopathy/Chronic Fatigue Syndrome ME/CFS in Norway: Symptom Burden and Access to Care. J. Clin. Med. 2023, 12, 1487. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mokkink, L.B.; de Vet, H.C.W.; Prinsen, C.A.C.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; Terwee, C.B. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual. Life Res. 2018, 27, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Lewis, I.; Pairman, J.; Spickett, G.; Newton, J.L. Is chronic fatigue syndrome in older patients a different disease?—A clinical cohort study. Eur. J. Clin. Investig. 2013, 43, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gulledge, C.M.; Smith, D.G.; Ziedas, A.; Muh, S.J.; Moutzouros, V.; Makhni, E.C. Floor and Ceiling Effects, Time to Completion, and Question Burden of PROMIS CAT Domains Among Shoulder and Knee Patients Undergoing Nonoperative and Operative Treatment. JBJS Open Access 2019, 4, e0015. [Google Scholar] [CrossRef] [PubMed]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W.; Terwee, C.B. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Bileviciute-Ljungar, I.; Schult, M.L.; Borg, K.; Ekholm, J. Preliminary ICF core set for patients with myalgic encephalomyelitis/chronic fatigue syndrome in rehabilitation medicine. J. Rehabil. Med. 2020, 52, jrm00074. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.; Rowe, P.C.; Visser, F.C. Validation of the Severity of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome by Other Measures than History: Activity Bracelet, Cardiopulmonary Exercise Testing and a Validated Activity Questionnaire: SF-36. Healthcare 2020, 8, 273. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged: The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Graf, C. The Lawton instrumental activities of daily living scale. J. Am. J. Nurs. 2008, 108, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Holtzman, C.S.; Sunnquist, M.; Cotler, J. The development of an instrument to assess post-exertional malaise in patients with myalgic encephalomyelitis and chronic fatigue syndrome. J. Health Psychol. 2021, 26, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Murdock, K.W.; Wang, X.S.; Shi, Q.; Cleeland, C.S.; Fagundes, C.P.; Vernon, S.D. The utility of patient-reported outcome measures among patients with myalgic encephalomyelitis/chronic fatigue syndrome. Qual. Life Res. 2017, 26, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.T.; Cormier, L.E.; Pontus, C.; Bergman, A.; Webley, W. Long COVID’s Impact on Patients, Workers, & Society: A review. Medicine 2024, 103, e37502. [Google Scholar] [CrossRef]
- Bonilla, H.; Quach, T.C.; Tiwari, A.; Bonilla, A.E.; Miglis, M.; Yang, P.C.; Eggert, L.E.; Sharifi, H.; Horomanski, A.; Subramanian, A.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): Results from a post-COVID-19 multidisciplinary clinic. Front. Neurol. 2023, 14, 1090747. [Google Scholar] [CrossRef]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P.; Translation, I.T.F.F.; Cultural, A. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Pendergrast, T.; Brown, A.; Sunnquist, M.; Jantke, R.; Newton, J.L.; Strand, E.B.; Jason, L.A. Housebound versus nonhousebound patients with myalgic encephalomyelitis and chronic fatigue syndrome. Chronic Illn. 2016, 12, 292–307. [Google Scholar] [CrossRef]
- Magaziner, J.; Zimmerman, S.I.; Gruber-Baldini, A.L.; Hebel, J.R.; Fox, K.M. Proxy Reporting in Five Areas of Functional Status. Am. J. Epidemiol. 1997, 146, 418–428. [Google Scholar] [CrossRef]
| Round 1 | Round 2 | Round 3 | Round 4 | Round 5 | Round 6 | |
|---|---|---|---|---|---|---|
| Total respondents | 290 | 435 | 536 | 1945 1 | 1463 1 | 353 1 |
| Very severe | 9/256 (4) | 7/431 (2) | 50 (2.6) | 22 (1.5) | 1 (0.3) | |
| Severe | 40/256 (16) | 58/431 (13) | 395 (20) | 155 (11) | 11 (26) | |
| Moderate | 160/256 (63) | 274//431 (64) | 1062 (55) | 847 (58) | 200 (57) | |
| Mild | 47/256 (19) | 92/431 (21) | 367 (19) | 425 (29) | 51 (14) | |
| Better than mild | 66 (3.4) | 16 (1.3) | 11 (3) | |||
| Female | 231/253 (92) | 385/428 (90) | 476/536 (89) | 1614/1915 (84) | 1269/1450 (88) | 318/353 (90) |
| Male | 21/253 (8) | 43/428 (10) | 60/536 (11) | 301/1915 (16) | 181/1450 (12) | 35/353 (10) |
| Age under 60 years | 237/253 (94) | 401/429 (93) | 511/536 (95) | 1387/1940 (71) | 1263/1463 (86) | 301/353 (10) |
| ME/CFS Round 5 (n = 1263) | HC Round 5 (n = 178) | ME/CFS Round 4 (n = 1387) | |
|---|---|---|---|
| Female/Male 1 | 1102/149 (88) | 118/56 (68) | 1157/209 (85/15) |
| Very severe ME/CFS | 19 (2) | 44 (3.2) | |
| Severe ME/CFS | 136 (11) | 299 (22) | |
| Moderate ME/CFS | 733 (58) | 752 (54) | |
| Mild ME/CFS | 360 (29) | 255 (18) | |
| Better than mild ME/CFS | 15 (1) | 37 (2.7) | |
| Age < 19 years | 48 (4) | 3 (2) | 15 (1.1) |
| Age 20–39 years | 420 (33) | 67 (38) | 440 (32) |
| Age 40+ years | 795 (63) | 108 (61) | 932 (67) |
| Onset < 15 years | 194 (15) | 169 (12) | |
| Onset 16–29 years | 424 (29) | 533 (38) | |
| Onset 30+ years | 646 (45) | 684 (49) | |
| Duration < 5 years | 143 (12) | 289 (21) | |
| Duration 6–15 years | 666 (56) | 582 (42) | |
| Duration 16+ years | 372 (31) | 516 (37) | |
| Disability benefits | 1038 (82) | 5 (3) | |
| Parttime < 50% work/education | 120 (10) | 4 (2) | |
| Parttime 50–100% work/education | 33 (3) | 11 (6) | |
| Full time work/education | 30 (2) | 149 (84) | |
| Stay at home | - | 6 (3) | |
| None of the above | 42 (3) |
| Round 5 | Round 4 | ||||||
|---|---|---|---|---|---|---|---|
| ME | HC | HC-ME | ME | ||||
| Items | Mean | SD | Mean | SD | Mean Diff. | Mean | SD |
| A1 (1) Using toilet (not bedpan or bedside commode) | 5.3 | 0.9 | 6.0 | 0.1 | 0.7 | 5.1 | 1.1 |
| A2 Brushing your teeth without assistance | 5.2 | 1.0 | 6.0 | 0.0 | 0.8 | 5.2 | 1.0 |
| A3 Showering seated, with assistance | 4.6 | 1.6 | 6.0 | 0.5 | 1.4 | 4.4 | 1.6 |
| A4 Showering seated, without assistance | 4.2 | 1.6 | 6.0 | 0.1 | 1.8 | 4.1 | 1.7 |
| A5 (2) Showering standing up | 3.4 | 1.8 | 6.0 | 0.2 | 2.6 | 3.3 | 2.0 |
| A6 Getting up and staying out of bed for approx. 1 h | 4.5 | 1.4 | 6.0 | 0.1 | 1.5 | 4.2 | 1.6 |
| A7 (3) Getting dressed in regular clothes | 4.8 | 1.2 | 6.0 | 0.1 | 1.2 | 4.7 | 1.2 |
| B8 (4) Walking a short distance indoors, from one room to another | 4.9 | 1.1 | 6.0 | 0.1 | 1.1 | 4.6 | 1.3 |
| B9 Walking a short continuous distance, approx. 100 m (length of a football field), in- or outdoors | 4.0 | 1.6 | 6.0 | 1.0 | 2.0 | 3.7 | 1.8 |
| B10 (5) Walking between approx. 100 m and 1 km on level ground (length of 1 to 10 football fields) | 3.0 | 1.7 | 6.0 | 0.2 | 3.0 | 2.6 | 1.9 |
| B11 Going for a longer walk. Approx. 1 km (0.6 mile), mostly level ground | 2.4 | 1.7 | 5.9 | 0.3 | 3.5 | 1.9 | 1.8 |
| B12 Going for a longer walk. Approx. 1 km (0.6 mile), hilly or varied terrain | 1.8 | 1.6 | 5.8 | 0.5 | 4.0 | 1.3 | 1.6 |
| B13 (6) Physical activity with increased heart rate, for approx. 15 min | 1.4 | 1.4 | 5.7 | 0.8 | 4.3 | 1.5 | 1.6 |
| B14 Physical activity with increased heart rate, for approx. ½ h | 0.7 | 1.2 | 5.4 | 1.1 | 4.7 | 0.9 | 1.4 |
| C15 (7) Sitting in bed for approx. 30 min | 5.2 | 1.2 | 6.0 | 0.1 | 0.8 | 5.0 | 1.4 |
| C16 Physical activity with increased heart rate, for approx. ½ h | 4.8 | 1.4 | 6.0 | 0.4 | 1.2 | 4.5 | 1.6 |
| C17 (8) Sitting in an upright chair (dining chair) with feet on floor for approx. 2 h | 3.2 | 1.9 | 5.9 | 0.5 | 2.7 | 2.8 | 2.1 |
| C18 (9) Standing up for approx. 5 min, e.g., while queuing or while cooking | 3.9 | 1.6 | 6.0 | 0.1 | 2.0 | 3.9 | 1.8 |
| C19 Standing up for a long time—approx. ½ h | 2.5 | 1.8 | 5.9 | 0.4 | 3.4 | 2.2 | 1.9 |
| D20 Light housework (dusting, tidying etc.) for approx. ½ h continuously | 2.9 | 1.6 | 5.9 | 0.4 | 3.0 | 2.7 | 1.8 |
| D21 (10) Heavier housework (washing floors, vacuuming etc.) for approx. ½ h continuously | 1.9 | 1.5 | 5.7 | 0.6 | 3.8 | 1.8 | 1.7 |
| D22 Laundry (sorting, hanging up to dry and folding) | 3.3 | 1.5 | 5.9 | 0.3 | 2.6 | 3.0 | 1.7 |
| D23 Making a simple cold meal, such as a sandwich or cereal | 4.8 | 1.3 | 6.0 | 0.1 | 1.2 | 3.3 | 1.8 |
| D24 Cooking a simple hot meal | 4.1 | 1.4 | 5.9 | 0.2 | 1.8 | 4.3 | 1.5 |
| D25 (11) Cooking a complicated meal from scratch, approx. 1 h of preparation | 2.6 | 1.6 | 5.8 | 0.5 | 3.2 | 1.9 | 1.7 |
| E26 Speaking a few words | 5.6 | 0.8 | 6.0 | 0.1 | 0.4 | 5.5 | 0.9 |
| E27 (12) Having a conversation for approx. 5 min | 5.2 | 1.0 | 6.0 | 0.1 | 0.8 | 5.0 | 1.1 |
| E28 Having a conversation for approx. ½ h | 4.0 | 1.3 | 5.9 | 0.3 | 1.9 | 3.9 | 1.4 |
| E29 Writing a short message by hand | 5.3 | 1.0 | 6.0 | 0.1 | 0.7 | 5.2 | 1.0 |
| E30 (13) Participating in a conversation with three people for approx. ½ h | 3.1 | 1.5 | 5.9 | 0.4 | 2.7 | 3.1 | 1.6 |
| E31 Socializing with friends for approx. 1 h | 2.7 | 1.4 | 5.8 | 0.5 | 3.1 | 2.3 | 1.6 |
| E32 (14) Participating in a dinner party, party or family event | 1.7 | 1.2 | 5.6 | 0.8 | 3.9 | 1.8 | 1.4 |
| F33 (15) Stepping right outside your home | 4.5 | 1.5 | 6.0 | 0.1 | 1.5 | 4.2 | 1.8 |
| F34 Going on a necessary errand, such as a doctor’s appointment | 3.0 | 1.4 | 5.9 | 0.4 | 2.9 | 2.9 | 1.5 |
| F35 (16) Going to a shop for groceries | 2.9 | 1.5 | 5.9 | 0.4 | 3.0 | 2.2 | 1.7 |
| F36 Doing enjoyable leisure activities, such as going to a café, non-essential shopping etc. | 2.5 | 1.5 | 5.9 | 0.3 | 3.4 | 2.4 | 1.6 |
| F37 Riding as a passenger in a car for approx. 15 min | 4.3 | 1.5 | 6.0 | 0.2 | 1.7 | 3.9 | 1.7 |
| F38 (17) Using public transport (bus or train) | 2.4 | 1.8 | 5.9 | 0.4 | 3.5 | 2.1 | 1.9 |
| F39 (18) Participating in organized leisure activities such as classes, sports etc. | 1.0 | 1.4 | 5.6 | 0.8 | 4.6 | 1.1 | 1.5 |
| G40 Staying in a room with dim lighting for approx. 1/2 h | 5.4 | 0.9 | 6.0 | 0.1 | 0.6 | 5.5 | 1.0 |
| G41 (19) Staying in a room with normal lighting, without sunglasses, for approx. 1 h | 5.0 | 1.4 | 6.0 | 0.1 | 1.0 | 4.9 | 1.5 |
| G42 (20) Staying outdoors in daylight without sunglasses for approx. 2 h | 3.2 | 1.9 | 5.8 | 0.5 | 2.6 | 3.4 | 2.1 |
| G43 Staying in an environment with the sound of a few people in quiet conversation for approx. 1 h | 3.9 | 1.4 | 6.0 | 0.2 | 2.1 | 4.2 | 1.6 |
| G44 (21) Staying in a noisy environment, (shopping mall, café or open plan office) for approx. 1 h | 2.2 | 1.4 | 5.5 | 0.7 | 3.4 | 2.6 | 1.8 |
| G45 Going to a cinema, concert etc. with high noise levels | 1.6 | 1.4 | 5.6 | 0.8 | 4.0 | 1.7 | 1.7 |
| H46 (22) Reading a short text, such as a mobile phone text message | 5.4 | 0.9 | 6.0 | 0.1 | 0.6 | 5.4 | 0.9 |
| H47 Reading fiction/light reading | 3.3 | 2.0 | 5.9 | 0.3 | 2.6 | 3.5 | 2.0 |
| H48 (23) Reading and understanding a non-fiction text, such as an official document one A4 page long | 3.1 | 1.7 | 5.8 | 0.5 | 2.7 | 4.0 | 1.6 |
| H49 Performing simple mental arithmetic | 4.1 | 1.7 | 5.9 | 0.5 | 1.8 | 4.2 | 1.7 |
| H50 Writing short messages on a smartphone or tablet | 5.2 | 0.9 | 6.0 | 0.2 | 0.8 | 5.0 | 1.0 |
| H51 (24) Using social media to stay in touch with others | 4.6 | 1.2 | 5.9 | 0.3 | 1.4 | 4.6 | 1.3 |
| H52 Watching TV (series, news) | 4.5 | 1.2 | 6.0 | 0.2 | 1.5 | 4.5 | 1.4 |
| H53 (25) Focusing on a task for approx. 10 min continuously | 3.8 | 1.5 | 5.9 | 0.3 | 2.1 | 4.3 | 1.3 |
| H54 (26) Focusing on a task for approx. 2 h continuously | 1.9 | 1.6 | 5.6 | 0.8 | 3.7 | 2.4 | 1.8 |
| H55 (27) Managing a full working day (non-physical work such as office work, classes, or lectures) | 0.6 | 1.1 | 5.5 | 0.9 | 4.8 | 0.8 | 1.4 |
| Strongly Agree | Agree | Disagree | Strongly Disagree | |
|---|---|---|---|---|
| Easy to understand | 501 (40) | 740 (59) | 20 (2) | 2 (0.2) |
| Easy to know what to answer to the questions | 283 (22) | 808 (64) | 164 (13) | 8 (0.6) |
| Gave a correct picture of my illness-situation | 292 (23) | 877 (69) | 91 (7) | 3 (0.2) |
| Needed help from others to answer | 66 (5) | 79 (6) | 394 (31) | 724 (57) |
| Needed several breaks when filling out | 69 (6) | 323 (26) | 528 (42) | 343 (27) |
| FUNCAP55 A to H domains | ME/CFS (n = 1263) | HC (n = 178) | Mean Difference 95% CI |
| A Personal hygiene/basic functions | 4.6 (1.1) | 6.0 (0.1) | −1.4 (−1.6 to −1.2) |
| B Walking/moving around | 2.3 (1.1) | 5.1 (0.3) | −2.8 (−3.0 to −3.6) |
| C Being upright | 3.9 (1.4) | 5.9 (0.2) | −2.0 (−2.2 to −1.8) |
| D Activities in the home | 3.3 (1.3) | 5.9 (0.3) | −2.6 (−2.8 to −2.4) |
| E Communication | 3.9 (1.0) | 5.9 (0.3) | −1.9 (−2.1 to −1.8) |
| F Activities outside your home | 3.0 (1.3) | 5.9 (0.3) | −2.9 (−3.1 to −2.7) |
| G Reactions to light and sound | 3.5 (1.1) | 5.8 (0.3) | −2.3 (−2.4 to −2.1) |
| H Concentration | 3.7 (1.0) | 5.8 (0.3) | −2.2 (−2.3 to −2.0) |
| Total score (mean of A–H sub-scores) | 3.5 (1.0) | 5.8 (0.2) | −2.3 (−2.4 to −2.1) |
| FUNCAP27 A to H domains | ME/CFS (n = 1263) | HC (n = 178) | Mean Difference 95% CI |
| 4.5 (1.1) | 6.0 (0.1) | −1.5 (−1.6 to −1.3) | |
| B Walking/moving around | 3.1 (1.2) | 5.9 (0.3) | −2.8 (−2.9 to −2.6) |
| C Being upright | 4.1 (1.4) | 5.9 (0.2) | −1.8 (−2.0 to −1.6) |
| D Activities in the home | 2.2 (1.4) | 5.7 (0.5) | −3.5 (−3.7 to −3.3) |
| E Communication | 3.3 (1.0) | 5.8 (0.4) | −2.5 (−2.6 to −2.3) |
| F Activities outside your home | 2.7 (1.3) | 5.8 (0.4) | −3.1 (−3.3 to −2.9) |
| G Reactions to light and sound | 3.4 (1.3) | 5.8 (0.4) | −2.3 (−2.5 to −2.1) |
| H Concentration | 3.2 (1.0) | 5.8 (0.4) | −2.5 (−2.7 to −2.4) |
| Total score | 3.3 (1.1) | 5.8 (0.3) | −2.5 (−2.7 to −2.3) |
| Range | FUNCAP55 Floor | Ceiling | Range | FUNCAP27 Floor | Ceiling | |
|---|---|---|---|---|---|---|
| A Personal hygiene/Basic functions | 0–6 | 0.2 | 6.0 | 0–6 | 0.6 | 7.1 |
| B Walking/Moving around | 0–5.3 | 1.0 | 0 | 0–6 | 1.0 | 0.4 |
| C Being upright | 0–6 | 1.4 | 1.5 | 0–6 | 1.5 | 4.2 |
| D Activities in the home | 0–6 | 2.9 | 0.2 | 0–6 | 14.6 | 0.2 |
| E Communication | 0–6 | 0.2 | 0.3 | 0–6 | 0.6 | 0.3 |
| F Activities outside your home | 0–6 | 2.3 | 0.1 | 0–6 | 3.6 | 0.1 |
| G Reactions to light and sound | 0–6 | 0.6 | 0.5 | 0–6 | 2.2 | 0.6 |
| H Concentration | 0–6 | 0.2 | 0.2 | 0–6 | 0.2 | 0.2 |
| Total Score | 0–5.9 | 0.1 | 0 | 0–6 | 0.1 | 0.1 |
| Round 5. Norwegian (n = 1263): | |||||||||
| Domains, ME/CFS respondents | A | B | C | D | E | F | G | H | TS |
| A. Personal hygiene/basic functions | 1 | 0.76 | 0.82 | 0.82 | 0.74 | 0.78 | 0.72 | 0.64 | 0.88 |
| B. Walking/moving around | 1 | 0.77 | 0.82 | 0.72 | 0.83 | 0.70 | 0.65 | 0.88 | |
| C. Being upright | 1 | 0.84 | 0.79 | 0.80 | 0.75 | 0.68 | 0.91 | ||
| D. Activities in home | 1 | 0.79 | 0.86 | 0.76 | 0.71 | 0.93 | |||
| E. Communication | 1 | 0.81 | 0.81 | 0.80 | 0.90 | ||||
| F. Activities outside your home | 1 | 0.81 | 0.73 | 0.93 | |||||
| G. Reactions to light and sound | 1 | 0.74 | 0.88 | ||||||
| H. Concentration | 1 | 0.82 | |||||||
| TS. Total score (mean of A-H sub-scores) | 1 | ||||||||
| Round 4. International/English (n = 1387): | |||||||||
| Domains, ME/CFS respondents | A | B | C | D | E | F | G | H | TS |
| A. Personal hygiene/basic functions | 1 | 0.73 | 0.82 | 0.80 | 0.72 | 0.77 | 0.71 | 0.65 | 0.88 |
| B. Walking/moving around | 1 | 0.76 | 0.81 | 0.68 | 0.83 | 0.66 | 0.58 | 0.86 | |
| C. Being upright | 1 | 0.72 | 0.76 | 0.80 | 0.73 | 0.66 | 0.91 | ||
| D. Activities in home | 1 | 0.76 | 0.86 | 0.73 | 0.69 | 0.92 | |||
| E. Communication | 1 | 0.82 | 0.79 | 0.82 | 0.88 | ||||
| F. Activities outside your home | 1 | 0.78 | 0.70 | 0.93 | |||||
| G. Reactions to light and sound | 1 | 0.73 | 0.86 | ||||||
| H. Concentration | 1 | 0.81 | |||||||
| TS. Total score (mean of A-H sub-scores) | 1 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommerfelt, K.; Schei, T.; Seton, K.A.; Carding, S.R. Assessing Functional Capacity in Myalgic Encephalopathy/Chronic Fatigue Syndrome: A Patient-Informed Questionnaire. J. Clin. Med. 2024, 13, 3486. https://doi.org/10.3390/jcm13123486
Sommerfelt K, Schei T, Seton KA, Carding SR. Assessing Functional Capacity in Myalgic Encephalopathy/Chronic Fatigue Syndrome: A Patient-Informed Questionnaire. Journal of Clinical Medicine. 2024; 13(12):3486. https://doi.org/10.3390/jcm13123486
Chicago/Turabian StyleSommerfelt, Kristian, Trude Schei, Katharine A. Seton, and Simon R. Carding. 2024. "Assessing Functional Capacity in Myalgic Encephalopathy/Chronic Fatigue Syndrome: A Patient-Informed Questionnaire" Journal of Clinical Medicine 13, no. 12: 3486. https://doi.org/10.3390/jcm13123486






