Comparison of Nefopam-Based Patient-Controlled Analgesia with Opioid-Based Patient-Controlled Analgesia for Postoperative Pain Management in Immediate Breast Reconstruction Surgery: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Randomization and Intervention
2.3. General Anesthesia and Postoperative Management
2.4. Outcome Measurement
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vadivelu, N.; Schreck, M.; Lopez, J.; Kodumudi, G.; Narayan, D. Pain after mastectomy and breast reconstruction. Am. Surg. 2008, 74, 285–296. [Google Scholar] [CrossRef]
- Stadler, M.; Bardiau, F.; Seidel, L.; Albert, A.; Boogaerts Jean, G. Difference in Risk Factors for Postoperative Nausea and Vomiting. Anesthesiology 2003, 98, 46–52. [Google Scholar] [CrossRef]
- Kharasch, E.D.; Clark, J.D. Persistent Postoperative Opioid Use: Perception, Progress, and Promise. Anesthesiology 2020, 132, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.; Chauvin, M.; Verleye, M. Nefopam analgesia and its role in multimodal analgesia: A review of preclinical and clinical studies. Clin. Exp. Pharmacol. Physiol. 2016, 43, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.S.; Lysakowski, C.; Tramèr, M.R. Nefopam for the prevention of postoperative pain: Quantitative systematic review. Br. J. Anaesth. 2008, 101, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S.; Kim, H.; Oh, C.; Park, S.; Kim, Y.; Hong, B.; Yoon, S.; Noh, C.; Ko, Y. The Analgesic Efficacy of Nefopam in Patient-Controlled Analgesia after Laparoscopic Gynecologic Surgery: A Randomized, Double-Blind, Non-Inferiority Study. J. Clin. Med. 2021, 10, 1043. [Google Scholar] [CrossRef]
- Jung, K.T.; So, K.Y.; Kim, S.C.; Kim, S.H. Effect of Nefopam-Based Patient-Controlled Analgesia with and without Fentanyl on Postoperative Pain Intensity in Patients Following Laparoscopic Cholecystectomy: A Prospective, Randomized, Controlled, Double-Blind Non-Inferiority Trial. Medicina 2021, 57, 316. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.S.; Kim, Y.C.; Yoo, Y.; Lee, C.; Cho, C.W.; Kim, W.J. Opioid sparing effect and safety of nefopam in patient controlled analgesia after laparotomy: A randomized, double blind study. J. Int. Med. Res. 2016, 44, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Huh, K.H.; Roh, Y.H.; Oh, Y.J.; Park, J.; Choi, Y.S. Nefopam as an adjunct to intravenous patient-controlled analgesia after renal transplantation: A randomised trial. Acta Anaesthesiol. Scand. 2015, 59, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, W.J.; Choi, D.K.; Lee, Y.K.; Choi, I.C.; Sim, J.Y. The analgesic efficacy and safety of nefopam in patient-controlled analgesia after cardiac surgery: A randomized, double-blind, prospective study. J. Int. Med. Res. 2014, 42, 684–692. [Google Scholar] [CrossRef]
- Tigerstedt, I.; Sipponen, J.; Tammisto, T.; Turunen, M. Comparison of nefopam and pethidine in postoperative pain. Br. J. Anaesth. 1977, 49, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, A.; Laska, E. Nefopam and morphine in man. Clin. Pharmacol. Ther. 1975, 18 Pt 1, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Karm, M.H.; So, E.; Choi, Y.J.; Park, S.; Oh, Y.; Yun, H.J.; Kim, H.J.; Seo, K.S. Effects on postoperative nausea and vomiting of nefopam versus fentanyl following bimaxillary orthognathic surgery: A prospective double-blind randomized controlled trial. J. Dent. Anesth. Pain Med. 2019, 19, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Heel, R.C.; Brogden, R.N.; Pakes, G.E.; Speight, T.M.; Avery, G.S. Nefopam: A review of its pharmacological properties and therapeutic efficacy. Drugs 1980, 19, 249–267. [Google Scholar] [CrossRef]
- Son, J.-S.; Doo, A.; Kwon, Y.-J.; Han, Y.-J.; Ko, S. A comparison between ketorolac and nefopam as adjuvant analgesics for postoperative patient-controlled analgesia: A randomized, double-blind, prospective study. Korean J. Anesthesiol. 2017, 70, 612–618. [Google Scholar] [CrossRef]
- Beverly, A.; Kaye, A.D.; Ljungqvist, O.; Urman, R.D. Essential Elements of Multimodal Analgesia in Enhanced Recovery after Surgery (ERAS) Guidelines. Anesthesiol. Clin. 2017, 35, e115–e143. [Google Scholar] [CrossRef]
- Chiu, C.; Aleshi, P.; Esserman, L.J.; Inglis-Arkell, C.; Yap, E.; Whitlock, E.L.; Harbell, M.W. Improved analgesia and reduced post-operative nausea and vomiting after implementation of an enhanced recovery after surgery (ERAS) pathway for total mastectomy. BMC Anesthesiol. 2018, 18, 41. [Google Scholar] [CrossRef]
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid pharmacology. Pain Physician 2008, 11 (Suppl. S2), S133–S153. [Google Scholar] [CrossRef]
- Alfonsi, P.; Adam, F.; Passard, A.; Guignard, B.; Sessler, D.I.; Chauvin, M. Nefopam, a nonsedative benzoxazocine analgesic, selectively reduces the shivering threshold in unanesthetized subjects. Anesthesiology 2004, 100, 37–43. [Google Scholar] [CrossRef]
- Tigerstedt, I.; Tammisto, T.; Leander, P. Comparison of the analgesic dose-effect relationships of nefopam and oxycodone in postoperative pain. Acta Anaesthesiol. Scand. 1979, 23, 555–560. [Google Scholar] [CrossRef]
- Mulita, F.; Karpetas, G.; Liolis, E.; Vailas, M.; Tchabashvili, L.; Maroulis, I. Comparison of analgesic efficacy of acetaminophen monotherapy versus acetaminophen combinations with either pethidine or parecoxib in patients undergoing laparoscopic cholecystectomy: A randomized prospective study. Med. Glas. 2021, 18, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S.; European Palliative Care Research Collaborative. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: A systematic literature review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar]
- Bahreini, M.; Jalili, M.; Moradi-Lakeh, M. A comparison of three self-report pain scales in adults with acute pain. J. Emerg. Med. 2015, 48, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef]


| Group | F | FN | N | p |
|---|---|---|---|---|
| (N = 38) | (N = 38) | (N = 38) | ||
| Age (yr) | 49.6 ± 7.8 | 46.9 ± 8.6 | 46.8 ± 7.7 | 0.246 |
| Height (cm) | 157.8 ± 0.7 | 160.7 ± 4.4 | 159.1 ± 5.6 | 0.069 |
| Weight (kg) | 59.0 ± 7.7 | 61.1 ± 7.5 | 57.3 ± 5.7 | 0.076 |
| ASA | 0.261 | |||
| 1 | 19 (50.0%) | 18 (47.4%) | 24 (63.2%) | |
| 2 | 19 (50.0%) | 20 (52.6%) | 13 (34.2%) | |
| 3 | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) | |
| Anesthesia time (min) | 288.9 ± 90.7 | 304.4 ± 115.6 | 272.4 ± 82.1 | 0.381 |
| Operation time (min) | 248.5 ± 89.2 | 261.5 ± 109.4 | 236.5 ± 80.4 | 0.531 |
| Total infused propofol dose (mg) | 2086.8 ± 838.4 | 2376.9 ± 1094.9 | 1928.1 ± 658.9 | 0.096 |
| Total infused remifentanil dose (mcg) | 1954.3 ± 546.0 | 2214.0 ± 882.6 | 1911.2 ± 639.2 | 0.143 |
| Type of breast reconstruction | 0.417 | |||
| Implant | 30 (78.9%) | 27 (71.1%) | 26 (68.4%) | |
| DIEP flap | 7 (18.4%) | 7 (18.4%) | 6 (15.8%) | |
| LD flap | 1 (2.6%) | 4 (10.5%) | 6 (15.8%) | |
| Surgical location | 0.855 | |||
| Rt | 19 (50.0%) | 18 (47.4%) | 18 (47.4%) | |
| Lt | 16 (42.1%) | 17 (44.7%) | 19 (50.0%) | |
| Both | 3 (7.9%) | 3 (7.9%) | 1 (2.6%) | |
| PCA | 602.9 ± 105.0 | 312.2 ± 68.1 | N/A | |
| Fentanyl (mcg) | ||||
| Nefopam (mg) | N/A | 58.7 ± 8.9 | 110.0 ± 9.1 | |
| Group F | Group FN | Group N | p | Difference between Group F and Group FN | Difference between Group F and Group N | |
|---|---|---|---|---|---|---|
| (N = 38) | (N = 38) | (N = 38) | ||||
| VASr 24 | 2.9 ± 1.0 | 3.1 ± 1.2 | 2.8 ± 0.9 | 0.501 | −0.2 | −0.1 |
| (−0.278, 0.678) | (−0.499, 0.299) | |||||
| VASm 24 | 4.1 ± 1.2 | 4.4 ± 1.3 | 4.1 ± 1.2 | 0.515 | 0.3 | 0 |
| (−0.184, 0.784) | (−0.478, 0.478) |
| Group | F | FN | N | p |
|---|---|---|---|---|
| (N = 38) | (N = 38) | (N = 38) | ||
| Pain intensity level (mild/moderate/severe) VASr | ||||
| PACU | 18/19/1 | 17/17/4 | 18/16/4 | 0.691 |
| 6 h | 18/17/3 | 17/20/1 | 19/17/2 | 0.885 |
| 12 h | 25/12/1 | 24/14/0 | 25/13/0 | 0.968 |
| 24 h | 29/9/0 | 26/12/0 | 27/11/0 | 0.810 |
| VASm | ||||
| PACU | 12/21/5 | 15/16/7 | 17/12/9 | 0.356 |
| 6 h | 8/24/6 | 7/23/8 | 8/25/5 | 0.936 |
| 12 h | 8/29/1 | 7/24/7 | 9/25/4 | 0.254 |
| 24 h | 14/23/1 | 12/24/2 | 13/23/2 | 0.988 |
| Length of stay in PACU (min) | 47.3 ± 9.7 | 58.3 ± 5.9 | 49.8 ± 4.6 | 0.456 |
| Time to 1st rescue drug administration (min) | 8.2 ± 12.6 | 5.0 ± 8.9 | 7.1 ± 10.4 | 0.836 |
| Cumulative PCA consumption (mL) | ||||
| PACU | 3.6 ± 1.2 | 2.9 ± 1.2 | 3.3 ± 0.9 | 0.026 |
| 6 h | 13.0 ± 5.4 | 10.8 ± 4.3 | 9.4 ± 3.6 | 0.078 |
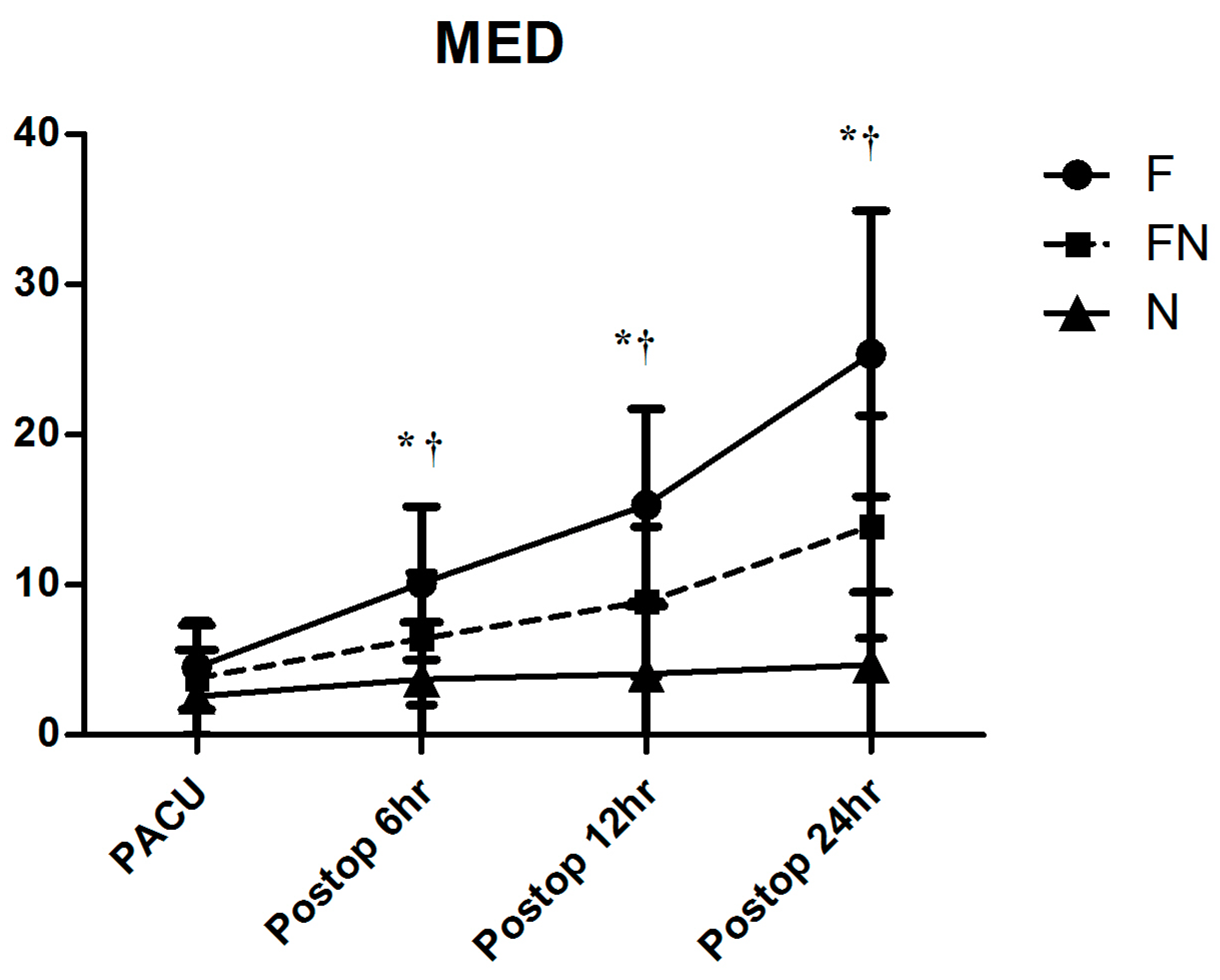
| 12 h | 21.3 ± 7.6 | 16.5 ± 4.5 | 15.0 ± 3.8 | 0.001 |
| 24 h | 35.9 ± 10.2 | 31.1 ± 9.4 | 30.6 ± 7.9 | 0.026 |
| Rescue analgesic requirement (Yes) | ||||
| PACU | 18 (47.4%) | 16 (42.1%) | 18 (47.4%) | 0.540 |
| 24 h | 13 (34.2%) | 15 (39.5%) | 13 (34.2%) | 0.911 |
| No. of rescue drugs administered (1st/2nd/3rd) | ||||
| PACU | 17/1/0 | 13/3/0 | 16/2/0 | 0.797 |
| 24 h | 11/ 1/1 | 12/3/0 | 10/2/1 | 0.929 |
| Group | F | FN | N | p |
|---|---|---|---|---|
| (N = 38) | (N = 38) | (N = 38) | ||
| Side effects (Y) | ||||
| PONV | 4 (10.5%) | 5 (13.2%) | 1 (2.6%) | 0.337 |
| Dizziness | 5 (13.2%) | 4 (10.5%) | 2 (5.3%) | 0.619 |
| Ileus | 3 (7.9%) | 1 (2.6%) | 1 (2.6%) | 0.616 |
| Urinary retention | 1 (2.6%) | 1 (2.6%) | 1 (2.6%) | 1.000 |
| Respiratory depression | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Hypotension | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| PCA withdrawal d/t PONV | 1 (2.6%) | 1 (2.6%) | 1 (2.6%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, J.; Lee, N.; Kim, M.; Choi, H.; Oh, D.Y.; Choi, J.; Hwang, W. Comparison of Nefopam-Based Patient-Controlled Analgesia with Opioid-Based Patient-Controlled Analgesia for Postoperative Pain Management in Immediate Breast Reconstruction Surgery: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 3490. https://doi.org/10.3390/jcm13123490
Huh J, Lee N, Kim M, Choi H, Oh DY, Choi J, Hwang W. Comparison of Nefopam-Based Patient-Controlled Analgesia with Opioid-Based Patient-Controlled Analgesia for Postoperative Pain Management in Immediate Breast Reconstruction Surgery: A Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(12):3490. https://doi.org/10.3390/jcm13123490
Chicago/Turabian StyleHuh, Jaewon, Noori Lee, Minju Kim, Hoon Choi, Deuk Young Oh, Jangyoun Choi, and Wonjung Hwang. 2024. "Comparison of Nefopam-Based Patient-Controlled Analgesia with Opioid-Based Patient-Controlled Analgesia for Postoperative Pain Management in Immediate Breast Reconstruction Surgery: A Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 12: 3490. https://doi.org/10.3390/jcm13123490
APA StyleHuh, J., Lee, N., Kim, M., Choi, H., Oh, D. Y., Choi, J., & Hwang, W. (2024). Comparison of Nefopam-Based Patient-Controlled Analgesia with Opioid-Based Patient-Controlled Analgesia for Postoperative Pain Management in Immediate Breast Reconstruction Surgery: A Randomized Controlled Trial. Journal of Clinical Medicine, 13(12), 3490. https://doi.org/10.3390/jcm13123490







