Should Physicians Be Aware of Rhythm Disturbances in Adults with Systemic Autoimmune Diseases and Anti-Ro52 Antibodies? A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Type of Study
2.2. Patients
2.3. Variables
2.4. Statistical Analysis
3. Results
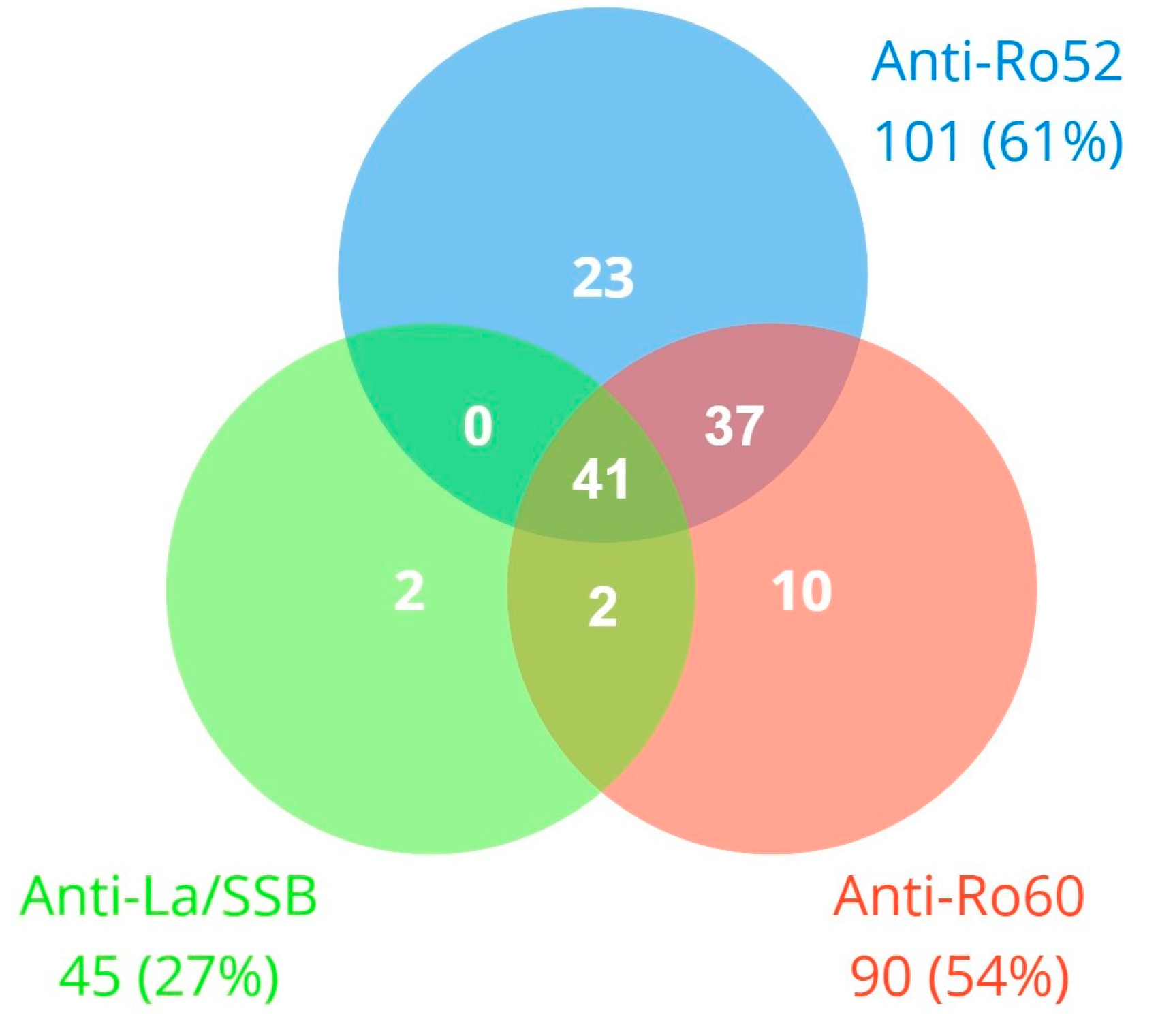
3.1. Study Population
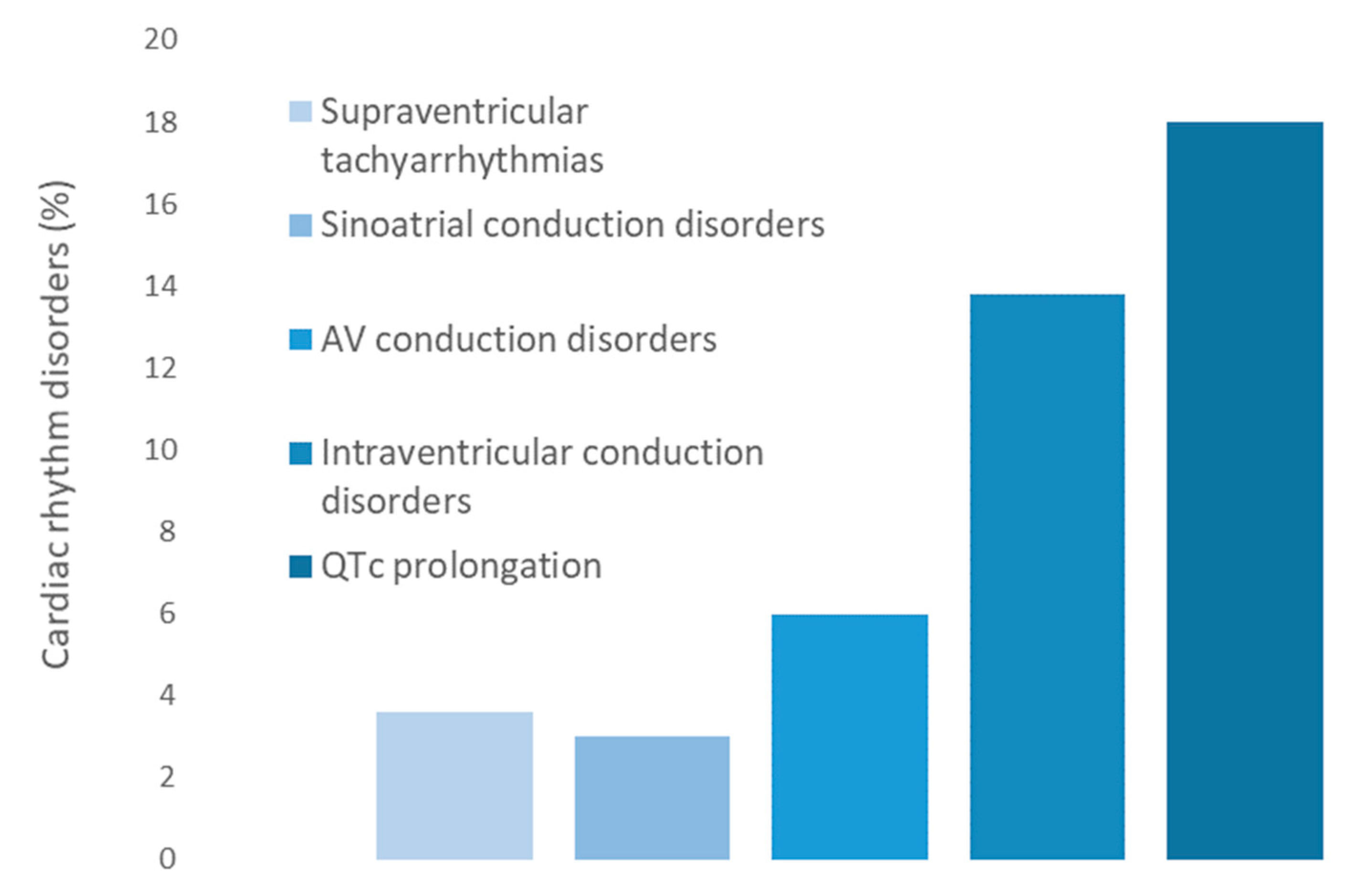
3.2. Cardiac Rhythm Disorders
3.3. AV Conduction Disorders
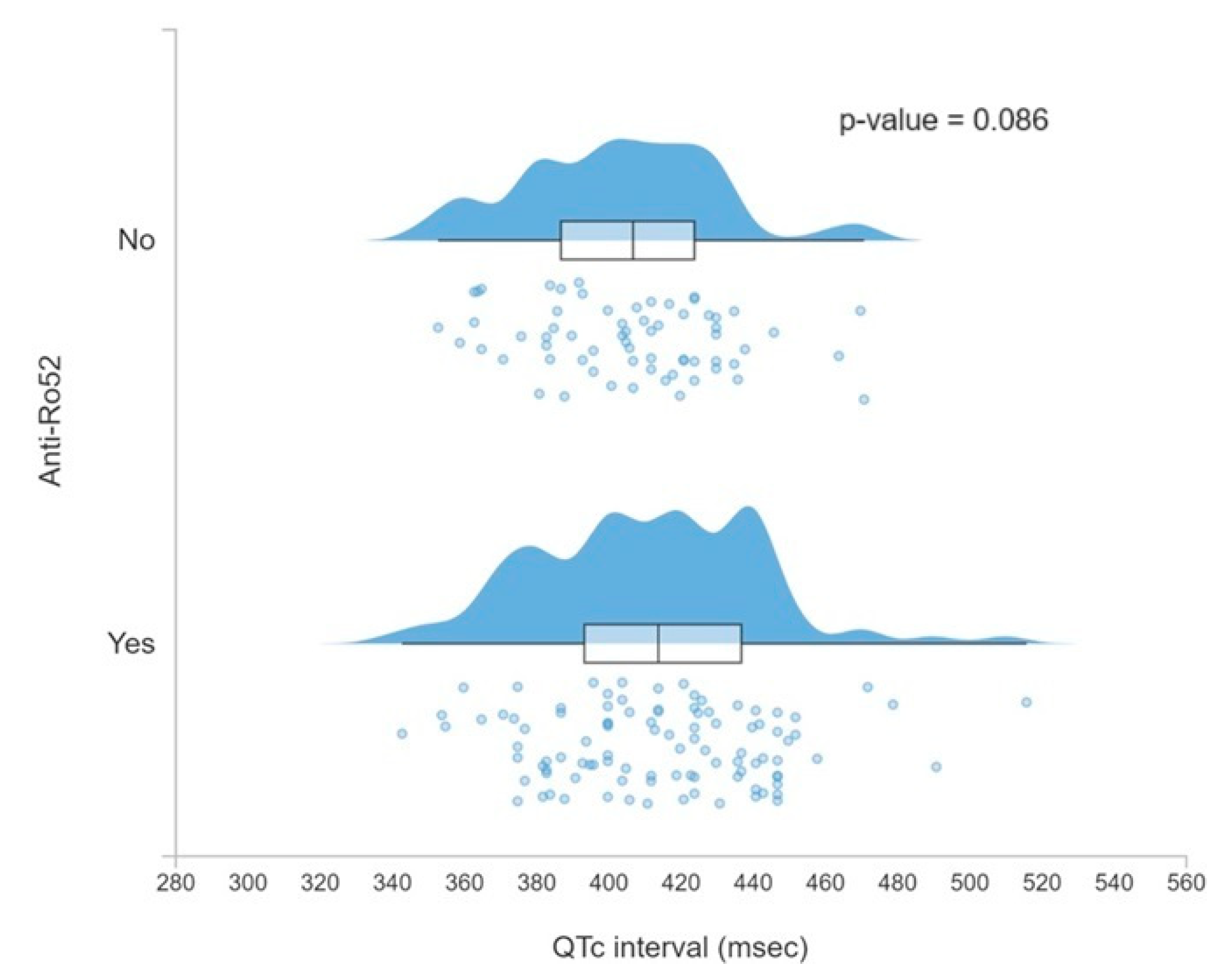
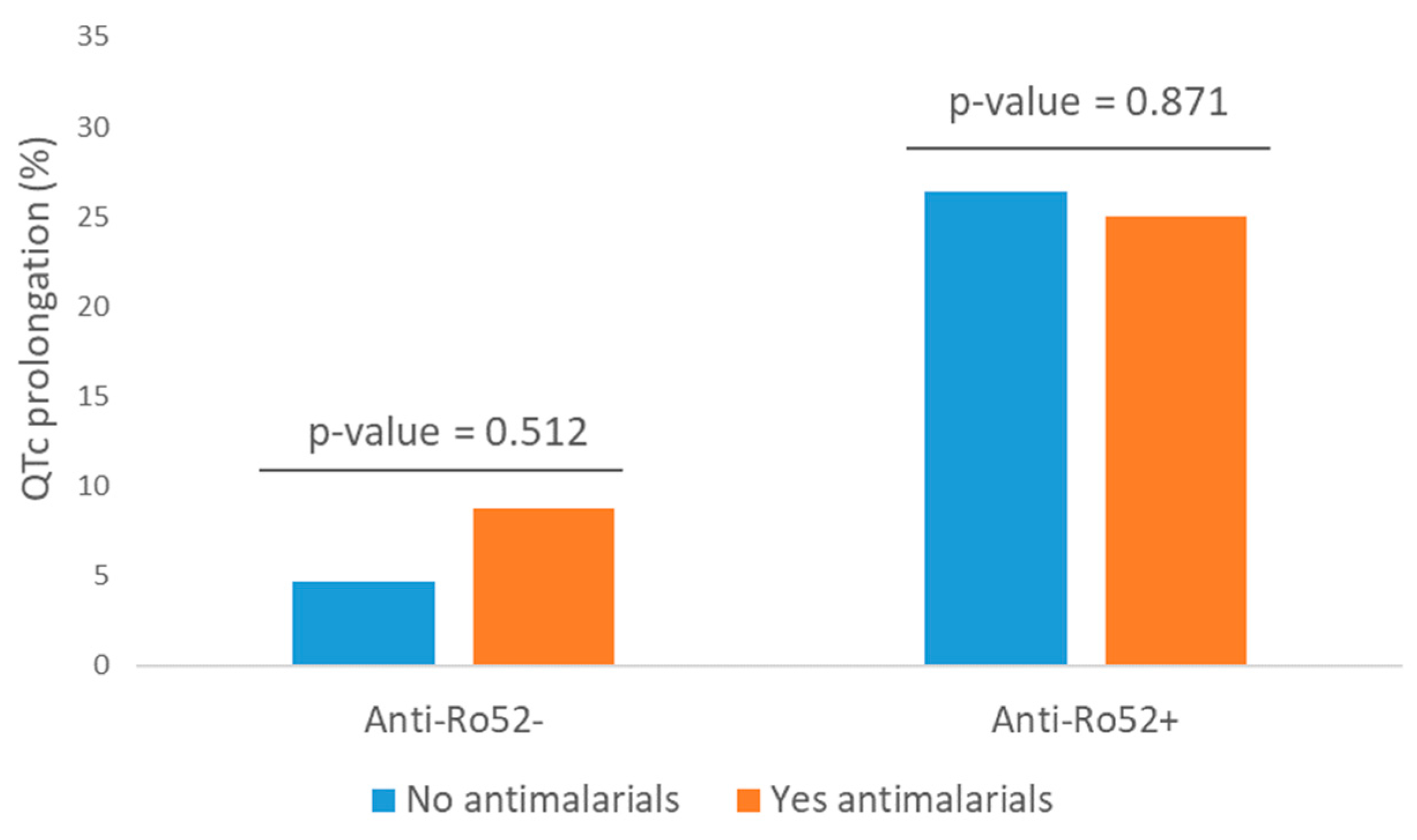
3.4. QTc Prolongation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation List
References
- Hayashi, N.; Koshiba, M.; Nishimura, K.; Sugiyama, D.; Nakamura, T.; Morinobu, S.; Kawano, S.; Kumagai, S. Prevalence of Disease-Specific Antinuclear Antibodies in General Population: Estimates from Annual Physical Examinations of Residents of a Small Town over a 5-Year Period. Mod. Rheumatol. 2008, 18, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-P.; Wang, C.-G.; Liu, X.; Huang, Y.-Q.; Guo, D.-L.; Jing, X.-Z.; Yuan, C.-G.; Yang, S.; Liu, J.-M.; Han, M.-S.; et al. The Prevalence of Antinuclear Antibodies in the General Population of China: A Cross-Sectional Study. Curr. Ther. Res. 2014, 76, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Cevenini, G.; Qu, Y.S.; Fabris, F.; El-Sherif, N.; Acampa, M.; Cartocci, A.; Laghi-Pasini, F.; Capecchi, P.L.; Boutjdir, M.; et al. Risk of QTc Interval Prolongation Associated with Circulating Anti-Ro/SSA Antibodies Among US Veterans: An Observational Cohort Study. J. Am. Heart Assoc. 2021, 10, e018735. [Google Scholar] [CrossRef] [PubMed]
- McCuistion, C.; Schoch, E. Possible Discoid Lupus Erythematosus in Newborn Infant: Report of a Case with Subsequent Development of Acute Systemic Lupus Erythematosus in Mother. AMA Arch. Derm. Syphilol. 1954, 70, 782. [Google Scholar] [CrossRef] [PubMed]
- Brucato, A.; Frassi, M.; Franceschini, F.; Cimaz, R.; Faden, D.; Pisoni, M.P.; Muscar, M.; Vignati, G.; Stramba-Badiale, M.; Catelli, L.; et al. Risk of Congenital Complete Heart Block in Newborns of Mothers with Anti-Ro/SSA Antibodies Detected by Counterimmunoelectrophoresis: A Prospective Study of 100 Women. Arthritis Rheum. 2001, 44, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, E.T.; Hamilton, R.M.; Silverman, E.D.; Zamora, S.A.; Hornberger, L.K. Outcome of Children with Fetal, Neonatal or Childhood Diagnosis of Isolated Congenital Atrioventricular Block. J. Am. Coll. Cardiol. 2002, 39, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Buyon, J.P.; Clancy, R.M.; Friedman, D.M. Cardiac Manifestations of Neonatal Lupus Erythematosus: Guidelines to Management, Integrating Clues from the Bench and Bedside. Nat. Rev. Rheumatol. 2009, 5, 139–148. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Izmirly, P.M.; Ramos-Casals, M.; Buyon, J.P.; Khamashta, M.A. The Clinical Spectrum of Autoimmune Congenital Heart Block. Nat. Rev. Rheumatol. 2015, 11, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Qu, Y.; Yue, Y.; Boutjdir, M. Functional Basis of Sinus Bradycardia in Congenital Heart Block. Circ. Res. 2004, 94, e32–e38. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Laghi-Pasini, F.; Boutjdir, M.; Capecchi, P.L. Cardioimmunology of Arrhythmias: The Role of Autoimmune and Inflammatory Cardiac Channelopathies. Nat. Rev. Immunol. 2019, 19, 63–64. [Google Scholar] [CrossRef]
- Qu, Y.; Xiao, G.-Q.; Chen, L.; Boutjdir, M. Autoantibodies from Mothers of Children with Congenital Heart Block Downregulate Cardiac L-Type Ca Channels. J. Mol. Cell. Cardiol. 2001, 33, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.M. Impaired Clearance of Apoptotic Cardiocytes Is Linked to Anti-SSA/Ro and -SSB/La Antibodies in the Pathogenesis of Congenital Heart Block. J. Clin. Investig. 2006, 116, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Carús, M.-E.; Askanase, A.D.; Clancy, R.M.; Di Donato, F.; Chou, T.-M.; Libera, M.R.; Chan, E.K.L.; Buyon, J.P. Anti-SSA/Ro and Anti-SSB/La Autoantibodies Bind the Surface of Apoptotic Fetal Cardiocytes and Promote Secretion of TNF-α by Macrophages. J. Immunol. 2000, 165, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.-Q.; Hu, K.; Boutjdir, M. Direct Inhibition of Expressed Cardiac L- and T-Type Calcium Channels by IgG from Mothers Whose Children Have Congenital Heart Block. Circulation 2001, 103, 1599–1604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jobling, K.; Rajabally, H.; Ng, W.-F. Anti-Ro Antibodies and Complete Heart Block in Adults with Sjögren’s Syndrome. Eur. J. Rheumatol. 2018, 5, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Anti-Ro/SSA Antibodies and Cardiac Arrhythmias in the Adult: Facts and Hypotheses. Scand. J. Immunol. 2010, 72, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Brucato, A.; Capecchi, P.L.; Baldi, L.; Bacarelli, M.R.; Nucci, C.; Moscadelli, V.; Morozzi, G.; Boutjdir, M.; Laghi-Pasini, F. Isolated Atrioventricular Block of Unknown Origin in the Adult and Autoimmunity: Diagnostic and Therapeutic Considerations Exemplified by 3 Anti-Ro/SSA–Associated Cases. Heart Case Rep. 2015, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Villuendas, R.; Olivé, A.; Juncà, G.; Salvador, I.; Martínez-Morillo, M.; Santos-Pardo, I.; Pereferrer, D.; Zamora, E.; Bayes-Genis, A. Autoimmunity and Atrioventricular Block of Unknown Etiology in Adults. J. Am. Coll. Cardiol. 2014, 63, 1335–1336. [Google Scholar] [CrossRef] [PubMed]
- Akuka, A.; Ben-Shabat, N.; Watad, A.; Tsur, A.M.; Ehrenberg, S.; McGonagle, D.; Comaneshter, D.; Beinart, R.; Cohen, A.D.; Amital, H. Association of Anti-Ro Seropositivity with Cardiac Rhythm and Conduction Disturbances. Eur. Heart J. 2022, 43, 4912–4919. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Boutjdir, M.; Capecchi, P.L. Anti-Ro/SSA-Antibodies and Heart Rhythm Disturbances in the General Population: The ‘Dark Side of the Immune’. Eur. Heart J. 2022, 43, 4920–4922. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Murthy Ginjupalli, V.K.; Srivastava, U.; Bertolozzi, I.; Bacarelli, M.R.; Verrengia, D.; Salvini, V.; Accioli, R.; Carbone, S.F.; Santoro, A.; et al. Anti-Ro/SSA Antibodies Blocking Calcium Channels as a Potentially Reversible Cause of Atrioventricular Block in Adults. JACC Clin. Electrophysiol. 2023, 9, 1631–1648. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Castrichini, M.; Srivastava, U.; Fabris, F.; Shah, K.; Li, Z.; Qu, Y.; El-Sherif, N.; Zhou, Z.; January, C.; et al. Pathogenesis of the Novel Autoimmune-Associated Long-QT Syndrome. Circulation 2015, 132, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Katayama, Y.; Kusano, K.F.; Haraoka, K.; Tani, Y.; Nagase, S.; Morita, H.; Miura, D.; Fujimoto, Y.; Furukawa, T.; et al. Anti-KCNH2 Antibody-Induced Long QT Syndrome. J. Am. Coll. Cardiol. 2007, 50, 1808–1809. [Google Scholar] [CrossRef] [PubMed]
- Szendrey, J.; Lamothe, S.M.; Vanner, S.; Guo, J.; Yang, T.; Li, W.; Davis, J.; Joneja, M.; Baranchuk, A.; Zhang, S. Anti-Ro52 Antibody Acts on the S5-Pore Linker of HERG to Chronically Reduce Channel Expression. Cardiovasc. Res. 2018, 115, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Bourré-Tessier, J.; Clarke, A.E.; Huynh, T.; Bernatsky, S.; Joseph, L.; Belisle, P.; Pineau, C.A. Prolonged Corrected QT Interval in Anti-Ro/SSA-Positive Adults with Systemic Lupus Erythematosus. Arthritis Care Res 2011, 63, 1031–1037. [Google Scholar] [CrossRef]
- Villuendas, R.; Martínez-Morillo, M.; Juncà, G.; Teniente-Serra, A.; Diez, C.; Heredia, S.; Riveros-Frutos, A.; Bayés-Genís, A.; Olivé, A. Usefulness of Cardiac Screening in Patients with Systemic Lupus Erythematosus and Anti-Ro/SSA Antibodies. Lupus 2021, 30, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Massie, C.; Hudson, M.; Tatibouet, S.; Steele, R.; Huynh, T.; Fritzler, M.J.; Baron, M.; Pineau, C.A. Absence of an Association between Anti-Ro Antibodies and Prolonged QTc Interval in Systemic Sclerosis: A Multicenter Study of 689 Patients. Semin. Arthritis Rheum. 2014, 44, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Bergfeldt, L. HLA-B27-Associated Cardiac Disease. Ann. Intern. Med. 1997, 127, 621. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhyth. Circulation 2019, 140, e382–e482. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.-P.; et al. 2019 ESC Guidelines for the Management of Patients with Supraventricular TachycardiaThe Task Force for the Management of Patients with Supraventricular Tachycardia of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 655–720. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J. Prolonged QT-Interval Syndromes. JAMA J. Am. Med. Assoc. 1986, 256, 2985. [Google Scholar] [CrossRef]
- Haeusler, I.L.; Chan, X.H.S.; Guérin, P.J.; White, N.J. The Arrhythmogenic Cardiotoxicity of the Quinoline and Structurally Related Antimalarial Drugs: A Systematic Review. BMC Med. 2018, 16, 200. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Acampa, M.; Morozzi, G.; Bellisai, F.; Bacarelli, M.R.; Dragoni, S.; Fineschi, I.; Simpatico, A.; Galeazzi, M.; et al. Anti-Ro/SSA-Associated Corrected QT Interval Prolongation in Adults: The Role of Antibody Level and Specificity. Arthritis Care Res. 2011, 63, 1463–1470. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Liu, J.; Liu, Y.; Kowey, P.R.; Zhang, Y.; Chen, Y.; Wei, Y.; Gao, L.; Li, H.; et al. Acquired Long QT Syndrome in Hospitalized Patients. Heart Rhythm. 2017, 14, 974–978. [Google Scholar] [CrossRef]
- Veglio, M.; Borra, M.; Stevens, L.K.; Fuller, J.H.; Perin, P.C. The Relation between QTc Interval Prolongation and Diabetic Complications. The EURODIAB IDDM Complication Study Group. Diabetologia 1999, 42, 68–75. [Google Scholar] [CrossRef]
- Tisdale, J.E.; Chung, M.K.; Campbell, K.B.; Hammadah, M.; Joglar, J.A.; Leclerc, J.; Rajagopalan, B. Drug-Induced Arrhythmias: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e214–e233. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.S.; Maddison, P.J.; Taylor, P.V.; Esscher, E.; Scott, O.; Skinner, R.P. Connective-Tissue Disease, Antibodies to Ribonucleoprotein, and Congenital Heart Block. N. Engl. J. Med. 1983, 309, 209–212. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Salvini, V.; Srivastava, U.; Ginjupalli, V.K.M.; Santoro, A.; Bertolozzi, I.; Accioli, R.; Laghi-Pasini, F.; Boutjdir, M.; Capecchi, P.L. Anti-Ca v 1.2 Antibody–Induced Atrioventricular Block as a Novel Form in the Adult: Long-Term Pacemaker-Sparing Activity of Hydroxychloroquine. Circ. Arrhythm. Electrophysiol. 2022, 15, 7. [Google Scholar] [CrossRef]
- Tam, W.K. Association of Anti-Ro/Sjögren’s Syndrome Type A Antibodies and Complete Atrioventricular Block in an Adult With Sjögren’s Syndrome. Arch. Rheumatol. 2018, 33, 225–229. [Google Scholar] [CrossRef]
- Saribayev, M.; Tufan, F.; Oz, F.; Erer, B.; Ozpolat, T.; Ozturk, G.B.; Akin, S.; Saka, B.; Erten, N.; Tascioglu, C.; et al. Corticosteroid Treatment Normalizes QTc Prolongation and Improves Heart Block in an Elderly Patient with Anti-Ro-Positive Systemic Lupus Erythematosus. Aging Clin. Exp. Res. 2014, 26, 337–339. [Google Scholar] [CrossRef]
- Santos-Pardo, I.; Martínez-Morillo, M.; Villuendas, R.; Bayes-Genis, A. Anti-Ro Antibodies and Reversible Atrioventricular Block. New Engl. J. Med. 2013, 368, 2335–2337. [Google Scholar] [CrossRef]
- Sung, M.J.; Park, S.-H.; Kim, S.-K.; Lee, Y.-S.; Park, C.-Y.; Choe, J.-Y. Complete Atrioventricular Block in Adult Sjögren’s Syndrome with Anti-Ro Autoantibody. Korean J. Intern. Med. 2011, 26, 213. [Google Scholar] [CrossRef] [PubMed]
- Adami, M.; Nardin, M.; Morello, E.; Crivellaro, C.; Wiedermann, C.J. Fatal Cardiac Arrest in an Adult Patient with Euthyroid Anti-SSA/Ro-Positive Connective Tissue Disease: A Case Report. Int. Arch. Med. 2009, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Arce-Salinas, C.A.; Carmona-Escamilla, M.A.; Rodríguez-García, F. Complete Atrioventricular Block as Initial Manifestation of Systemic Lupus Erythematosus. Clin. Exp. Rheumatol. 2009, 27, 344–346. [Google Scholar]
- Yavasoglu, I.; Kadikoylu, G.; Bolaman, Z. Adult Systemic Lupus Erythematosus and Secondary Atrioventricular Block. J. Electrocardiol. 2007, 40, S26–S27. [Google Scholar] [CrossRef]
- Lim, L.; Joshua, F. Resolution of Complete Heart Block after Prednisolone in a Patient with Systemic Lupus Erythematosus. Lupus 2005, 14, 561–563. [Google Scholar] [CrossRef]
- Liautaud, S.; Khan, A.J.; Nalamasu, S.R.; Tan, I.J.; Onwuanyi, A.E. Variable Atrioventricular Block in Systemic Lupus Erythematosus. Clin. Rheumatol. 2005, 24, 162–165. [Google Scholar] [CrossRef]
- Lodde, B.M.; Sankar, V.; Kok, M.R.; Leakan, R.A.; Tak, P.P.; Pillemer, S.R. Adult Heart Block Is Associated with Disease Activity in Primary Sjögren’s Syndrome. Scand. J. Rheumatol. 2005, 34, 383–386. [Google Scholar] [CrossRef]
- Comín-Colet, J.; Sánchez-Corral, M.A.; Alegre-Sancho, J.J.; Valverde, J.; López-Gómez, D.; Sabaté, X.; Juan-Mas, A.; Esplugas, E. Complete Heart Block in an Adult with Systemic Lupus Erythematosus and Recent Onset of Hydroxychloroquine Therapy. Lupus 2001, 10, 59–62. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Gerl, H.; Dorner, T. Complete Heart Block Caused by Primary Sjogren’s Syndrome and Hypopituitarism. Ann. Rheum. Dis. 1998, 57, 635. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.A.; Pickrell, M.B.; Reichlin, M. Development of Complete Heart Block in an Adult Patient with Sjögren’s Syndrome and Anti-Ro/SS-A Autoantibodies. Arthritis Rheum. 1996, 39, 1427–1429. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, D.; Raz, E.; Shalev, O.; Steiner, I.; Ben-Chetrit, E. Complete Heart Block and Seizures in an Adult with Systemic Lupus Erythematosus: A Possible Pathophysiologic Role for Anti-SS-A/Ro and Anti-SS-B/La Autoantibodies. Arthritis Rheum. 1993, 36, 259–262. [Google Scholar] [CrossRef]
- Martinez-Costa, X.; Ordi, J.; Barberá, J.; Selva, A.; Bosch, J.; Vilardell, M. High Grade Atrioventricular Heart Block in 2 Adults with Systemic Lupus Erythematosus. J. Rheumatol. 1991, 18, 1926–1928. [Google Scholar]
- Logar, D.; Kveder, T.; Rozman, B.; Dobovisek, J. Possible Association between Anti-Ro Antibodies and Myocarditis or Cardiac Conduction Defects in Adultswith Systemic Lupus Erythematosus. Ann. Rheum. Dis. 1990, 49, 627–629. [Google Scholar] [CrossRef]
- Bilazarian, S.D.; Taylor, A.J.; Brezinski, D.; Hochberg, M.C.; Guarnieri, T.; Provost, T.T. High-Grade Atrioventricular Heart Block in an Adult with Systemic Lupus Erythematosus: The Association of Nuclear RNP (U1 RNP) Antibodies, a Case Report, and Review of the Literature. Arthritis Rheum. 1989, 32, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Maier, W.P. Complete Heart Block as the Initial Manifestation of Systemic Lupus Erythematosus. Arch. Intern. Med. 1987, 147, 170. [Google Scholar] [CrossRef]
- Behan, W.M.; Aitchison, M.; Behan, P.O. Pathogenesis of Heart Block in a Fatal Case of Dermatomyositis. Br. Heart J. 1986, 56, 479–482. [Google Scholar] [CrossRef][Green Version]
- Hu, Z.; Wu, L.; Lin, Z.; Liu, X.; Zhao, C.; Wu, Z. Prevalence and Associated Factors of Electrocardiogram Abnormalities in Patients With Systemic Lupus Erythematosus: A Machine Learning Study. Arthritis Care Res. (Hoboken) 2022, 74, 1640–1648. [Google Scholar] [CrossRef]
- Mostafavi, A.A.; Taassoarian, B.; Khadir, V.; Abbaszadeh, S.; Sanatkar, S.A.; Rafiei, M. Assessment of the Relationship Between Dose and Number of Effective Used Drugs on on QT Interval in Patients with Lupus. Shiraz E Med. J. 2020, 21, e83710. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Yue, Y.; Srivastava, U.; Fabris, F.; Capecchi, P.L.; Bertolozzi, I.; Bacarelli, M.R.; Morozzi, G.; Acampa, M.; Natale, M.; et al. Arrhythmogenicity of Anti-Ro/SSA Antibodies in Patients with Torsades de Pointes. Circ. Arrhythm. Electrophysiol. 2016, 9, e003419. [Google Scholar] [CrossRef] [PubMed]
- Perez-García, L.F.; Estevez-García, I.O.; Moreno-Ramírez, M.; Félix, J.L.; Marquez-Velasco, R.; Iturralde, P.; Silveira, L.H.; Amezcua-Guerra, L.M. Anti-Ro52/TRIM21 Antibodies Are Associated with QT Interval Prolongation in Patients with Systemic Lupus Erythematosus. Arthritis Rheumatol. 2016, 68. [Google Scholar]
- Tufan, A.N.; Sag, S.; Oksuz, M.F.; Ermurat, S.; Coskun, B.N.; Gullulu, M.; Budak, F.; Baran, I.; Pehlivan, Y.; Dalkilic, E. Prolonged Tpeak–Tend Interval in Anti-Ro52 Antibody-Positive Connective Tissue Diseases. Rheumatol. Int. 2017, 37, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, C.N.; Reina, S.; Arakaki, D.; Eimon, A.; Carrizo, C.; Borda, E. Elevated IL-1β Levels in Anti-Ro/SSA Connective Tissue Diseases Patients with Prolonged Corrected QTc Interval. Clin. Exp. Rheumatol. 2015, 33, 715–720. [Google Scholar]
- Sham, S.; Madheshwaran, M.; Tamilselvam, T.N.; Rajeswari, S. Correlation of QT Interval with Disease Activity in Newly Detected SLE Patients at Baseline and during Flare. Indian J. Rheumatol. 2015, 10, 121–124. [Google Scholar] [CrossRef]
- Nomura, A.; Kishimoto, M.; Takahashi, O.; Deshpande, G.A.; Yamaguchi, K.; Okada, M. Prolongation of Heart Rate-Corrected QT Interval Is a Predictor of Cardiac Autonomic Dysfunction in Patients with Systemic Lupus Erythematosus. Rheumatol. Int. 2014, 34, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Capecchi, P.L.; Guideri, F.; Bellisai, F.; Selvi, E.; Acampa, M.; Costa, A.; Maggio, R.; Garcia-Gonzalez, E.; Bisogno, S.; et al. Comparison of Frequency of Complex Ventricular Arrhythmias in Patients with Positive versus Negative Anti-Ro/SSA and Connective Tissue Disease. Am. J. Cardiol. 2007, 100, 1029–1034. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Acampa, M.; Guideri, F.; Capecchi, P.L.; Campanella, V.; Morozzi, G.; Galeazzi, M.; Marcolongo, R.; Laghi-Pasini, F. Prolongation of the Corrected QT Interval in Adult Patients with Anti-Ro/SSA–Positive Connective Tissue Diseases. Arthritis Rheum. 2004, 50, 1248–1252. [Google Scholar] [CrossRef]
- Behan, W.M.; Behan, P.O.; Gairns, J. Cardiac Damage in Polymyositis Associated with Antibodies to Tissue Ribonucleoproteins. Heart 1987, 57, 176–180. [Google Scholar] [CrossRef]
| Overall Series (n = 167) | Anti-Ro52+ (n = 101) | Anti-Ro52− (n = 66) | p-Value | |
|---|---|---|---|---|
| Age years (SD) | 59.19 (12.8) | 60.21 (13.4) | 57.62 (11.7) | 0.202 |
| Female sex n (%) | 140 (83.8) | 89 (88.1) | 51 (77.3) | 0.063 |
| Smoker (active or former) n (%) | 60 (35.9) | 33 (32.7) | 27 (40.9) | 0.278 |
| Hypertension n (%) | 53 (31.7) | 31 (30.7) | 22 (33.3) | 0.720 |
| Diabetes mellitus n (%) | 14 (8.4) | 7 (6.9) | 7 (10.6) | 0.402 |
| Dyslipidemia n (%) | 56 (33.5) | 27 (26.7) | 29 (43.9) | 0.021 * |
| Heart disease n (%) | 16 (9.6) | 9 (8.9) | 7 (10.6) | 0.716 |
| Respiratory disease n (%) | 33 (19.8) | 19 (18.8) | 14 (21.4) | 0.703 |
| CKD n (%) | 12 (7.2) | 11 (10.9) | 1 (1.5) | 0.022 * |
| Electrolyte disorders n (%) | 4 (2.4) | 4 (4.0) | 0 (0.0) | 0.102 |
| Thyroid disease n (%) | 41 (24.6) | 27 (26.7) | 14 (21.2) | 0.418 |
| Β-blockers n (%) | 15 (9.0) | 11 (10.9) | 4 (6.1) | 0.286 |
| Non-dihydropyridine calcium antagonists n (%) | 3 (1.8) | 1 (1.0) | 2 (3.0) | 0.332 |
| Other drugs studied n (%) | 15 (9.0) | 7 (6.9) | 8 (12.1) | 0.251 |
SAD n (%)
| 58 (34.7) 41 (24.6) 38 (22.8) 10 (6.0) 39 (23.4) | 52 (51.5) 25 (24.8) 12 (11.9) 7 (6.9) 21 (20.8) | 6 (9.1) 16 (24.2) 26 (39.4) 3 (4.5) 18 (27.3) | <0.001 * 0.940 <0.001 * 0.525 0.333 |
| Time from diagnosis years (IQR) | 7 (11) | 6 (11) | 10 (13) | 0.141 |
| Corticosteroids n (%) | 50 (29.9) | 23 (22.8) | 27 (40.9) | 0.012 * |
| Antimalarials n (%) | 71 (42.5) | 48 (47.5) | 23 (34.8) | 0.105 |
| Immunosuppressants n (%) | 56 (33.5) | 22 (21.8) | 34 (51.5) | <0.001 * |
| Biologic drugs n (%) | 12 (7.2) | 5 (5.0) | 7 (10.6) | 0.166 |
| Rhythm disorders n (%) | 57 (34.1) | 43 (42.6) | 14 (21.2) | 0.004 * |
| 5 (3.0) | 4 (4.0) | 1 (1.5) | 0.365 |
| 10 (6.0) | 7 (6.9) | 3 (4.5) | 0.525 |
| 23 (13.8) | 14 (13.9) | 9 (13.6) | 0.967 |
| 30 (18.0) | 26 (25.7) | 4 (6.1) | 0.001 * |
| 6 (3.6) | 3 (3.0) | 3 (4.5) | 0.593 |
| 0 (0) | 0 (0) | 0 (0) | |
| RR interval ms (SD) | 860 (157) | 843 (140) | 881 (178) | 0.145 |
| PQ interval ms (IQR) | 162 (40) | 162 (40) | 163 (40) | 0.890 |
| QRS interval ms (IQR) | 80 (30) | 79 (20) | 82 (20) | 0.217 |
| QTc interval ms (SD) | 412 (29) | 415 (31) | 407 (26) | 0.086 |
| Bivariate Analysis (p-Value) | Specific Cardiac Rhythm Disorder (p-Value) | Multivariate Regression (p-Value) | Multivariate Regression OR (CI 95%) | |
|---|---|---|---|---|
| Age | 0.010 | Intraventricular (0.001) QTc prolongation (0.009) | 0.516 | 1.01 (0.98–1.04) |
| HTN | 0.017 | Intraventricular (0.001) Tachyarrhythmias (0.026) | 0.289 | 1.55 (0.68–3.53) |
| DM | 0.005 | Intraventricular (0.020) QTc prolongation (0.017) | 0.009 * | 6.00 (1.56–23.11) |
| Chronic respiratory disease | 0.049 | Intraventricular (0.015) | 0.043 * | 2.45 (1.03–5.84) |
| CKD | 0.021 | AV disorder (0.001) QTc prolongation (0.036) | 0.227 | 2.32 (0.59–9.11) |
| Anti-Ro52+ | 0.005 | QTc prolongation (0.003) | 0.004 * | 3.19 (1.43–7.13) |
| Bivariate Analysis (p-Value) | Multivariate Regression (p-Value) | Multivariate Regression OR (CI 95%) | |
|---|---|---|---|
| AV conduction disorders | |||
| CKD | 0.001 | 0.019 * | 8.82 (1.42–54.67) |
| β-blockers | 0.029 | 0.377 | 2.47 (0.33–18.40) |
| SLE | 0.046 | 0.044 * | 5.04 (1.05–24.27) |
| Biologic drugs | 0.011 | 0.004 * | 15.08 (2.32–97.89) |
| Antimalarials | 0.085 | ||
| QTc prolongation | |||
| Age | 0.009 | 0.083 | 1.03 (1.00–1.07) |
| DM | 0.017 | 0.035 * | 4.48 (1.11–17.12) |
| CKD | 0.036 | 0.522 | 1.56 (0.40–6.12) |
| Anti-Ro52+ | 0.003 | 0.036 * | 6.05 (1.11–20.16) |
| Anti-Ro52 strong-positivity | 0.005 | 0.542 | 1.40 (0.48–4.05) |
| Anti-Ro60+ | 0.022 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamazo-Herrero, J.; Medina-Luezas, J.A.; Cusacovich, I.; Martín-Asenjo, M.; González-Montagut-Gómez, C.; Sánchez-González, M.D.; Aramburu-Muñoz, F.; Janta, I.; García-Morán, E.; Veras-Burgos, C.M.; et al. Should Physicians Be Aware of Rhythm Disturbances in Adults with Systemic Autoimmune Diseases and Anti-Ro52 Antibodies? A Cross-Sectional Study. J. Clin. Med. 2024, 13, 3510. https://doi.org/10.3390/jcm13123510
Gamazo-Herrero J, Medina-Luezas JA, Cusacovich I, Martín-Asenjo M, González-Montagut-Gómez C, Sánchez-González MD, Aramburu-Muñoz F, Janta I, García-Morán E, Veras-Burgos CM, et al. Should Physicians Be Aware of Rhythm Disturbances in Adults with Systemic Autoimmune Diseases and Anti-Ro52 Antibodies? A Cross-Sectional Study. Journal of Clinical Medicine. 2024; 13(12):3510. https://doi.org/10.3390/jcm13123510
Chicago/Turabian StyleGamazo-Herrero, Javier, Julio Antonio Medina-Luezas, Ivan Cusacovich, Miguel Martín-Asenjo, Carmen González-Montagut-Gómez, María Dolores Sánchez-González, Francisco Aramburu-Muñoz, Iustina Janta, Emilio García-Morán, Carlos Miguel Veras-Burgos, and et al. 2024. "Should Physicians Be Aware of Rhythm Disturbances in Adults with Systemic Autoimmune Diseases and Anti-Ro52 Antibodies? A Cross-Sectional Study" Journal of Clinical Medicine 13, no. 12: 3510. https://doi.org/10.3390/jcm13123510
APA StyleGamazo-Herrero, J., Medina-Luezas, J. A., Cusacovich, I., Martín-Asenjo, M., González-Montagut-Gómez, C., Sánchez-González, M. D., Aramburu-Muñoz, F., Janta, I., García-Morán, E., Veras-Burgos, C. M., Corral-Gudino, L., Abad-Molina, C., & González-Fuentes, R. (2024). Should Physicians Be Aware of Rhythm Disturbances in Adults with Systemic Autoimmune Diseases and Anti-Ro52 Antibodies? A Cross-Sectional Study. Journal of Clinical Medicine, 13(12), 3510. https://doi.org/10.3390/jcm13123510






