The Impact of Cardiovascular Antecedents on the Prognosis of COVID-19 Critically Ill Patients
Abstract
1. Introduction
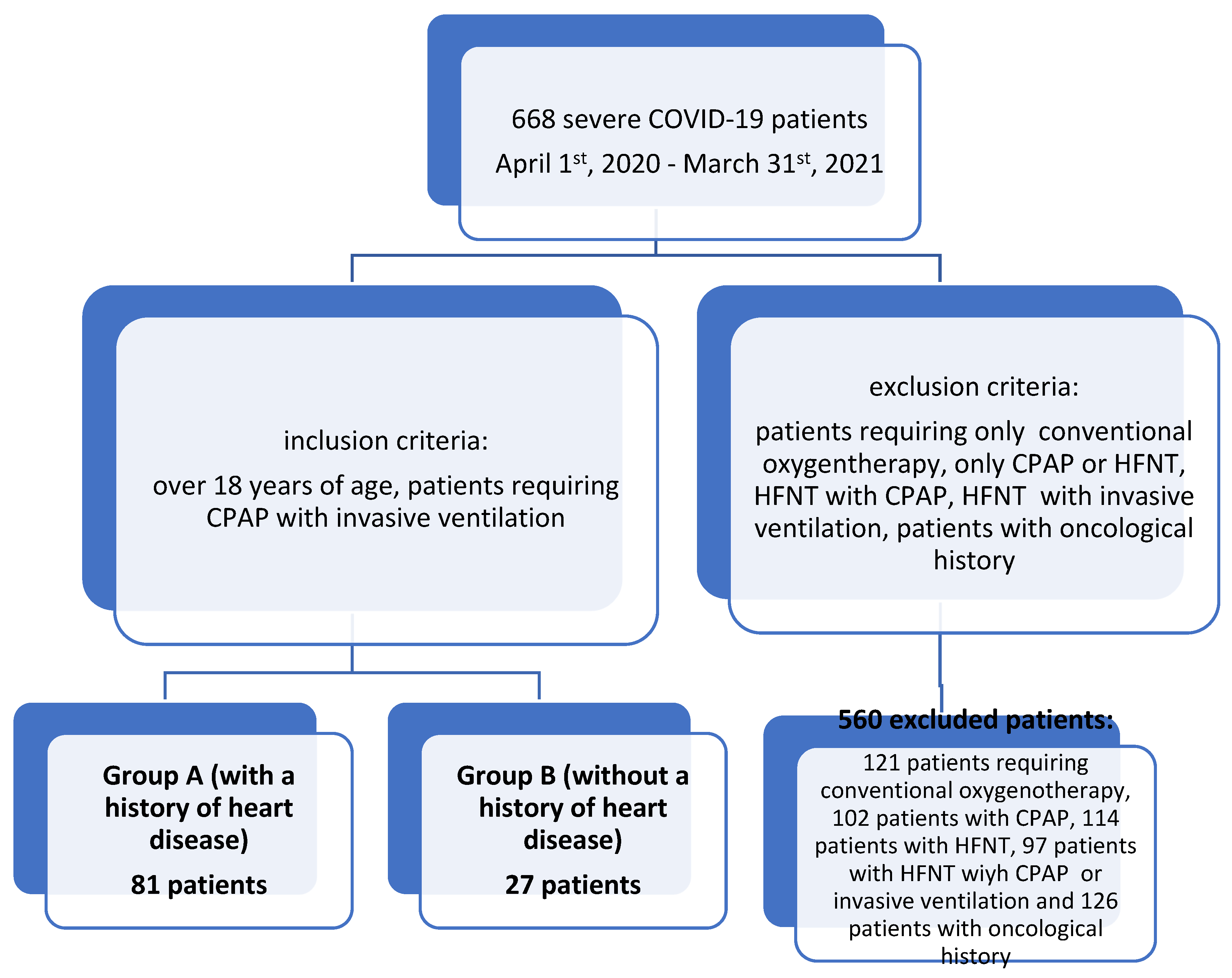
2. Materials and Methods
3. Results
- -
- Regarding the sex of the patients in the study sample, the predominance of male subjects will be noted, regardless of the classification in the two study groups. This variable is one without statistical significance according to the chi-square test (p = 0.5).
- -
- Regarding radiological features, the comparative analysis presents the following conclusions.
- -
- Patients with pathological antecedents of cardiovascular diseases presented radiological changes such as lung opacities (58% versus 37%) and changes such as accentuated pulmonary interstitium (30.9% versus 25.9%), compared to patients without pathological antecedents of cardiovascular disease, which is statistically significant (p = 0.003).
- -
- We specify that for three patients (11.1%) from group B the lung X-ray revealed pleurisy. Also, no radiological changes were observed in nine patients (11.1%), from group A and in seven patients (25.9%) from group B.
- -
- They have a higher risk of death.
- -
- They have a younger average age and a shorter duration of hospitalization, which actually implies forms with rapidly severe evolutions in younger patients.
- -
- The inflammatory profile, especially the C-reactive protein, is higher upon admission to the ICU.
- -
- LDH and serum creatine kinase on admission to the ICU have higher mean values.
4. Discussion
- A.
- Direct viral aggression
- B.
- Hyperactivation of the immune system-cytokine storm
- C.
- Immunological disorders and autoimmunity
- D.
- Hypoxia
- E.
- Blood clotting disorders
- F.
- Dyselectrolytemias
- G.
- Drug toxicity
- H.
- Neurohormonal activation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. COVID-19 Weekly Epidemiological Update. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 10 April 2024).
- Xiong, T.Y.; Redwood, S.; Prendergast, B.; Chen, M. Coronaviruses, and the cardiovascular system: Acute and long-term implications. Eur. Heart J. 2020, 41, 1798–1800. [Google Scholar] [CrossRef] [PubMed]
- Sartor, G.; Del Riccio, M.; Dal Poz, I.; Bonanni, P.; Bonaccorsi, G. COVID-19 in Italy: Considerations on official data. Int. J. Infect. Dis. 2020, 98, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Facts. Clinical Features and Sequelae. Available online: https://www.ecdc.europa.eu/en/infectious-disease-topics/z-disease-list/covid-19/facts/clinical-features-and-sequelae (accessed on 20 April 2024).
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.H.; Li, T.Y.; He, Z.C.; Ping, Y.F.; Liu, H.W.; Yu, S.C.; Mou, H.M.; Wang, L.H.; Zhang, H.R.; Fu, W.J. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li XueZaZhi 2020, 49, 411–417. [Google Scholar]
- Nappi, F.; Avtaar Singh, S.S. SARS-CoV-2-Induced Myocarditis: A State-of-the-Art Review. Viruses 2023, 15, 916. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ma, F.; Wei, X.; Fang, Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur. Heart J. 2021, 42, 206. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020, 5, 819–824. [Google Scholar] [CrossRef]
- Manole, C.; Dediu-Anghel, M.; Baroiu, L.; Ștefanopol, I.A.; Nechifor, A.; Niculet, E.; Mihailov, R.; Moroianu, L.A.; Voinescu, D.C.; Firescu, D. Efficiency of continuous positive airway pressure and high-flow nasal oxygen therapy in critically ill patients with COVID-19. J. Int. Med. Res. 2024, 52, 03000605231222151. [Google Scholar] [CrossRef]
- Manole, C.; Baroiu, L.; Nechita, A.; Voinescu, D.C.; Ciubara, A.; Debita, M.; Tatu, A.L.; Ciubara, A.B.; Stefanopol, I.A.; Anghel, L.; et al. Comparative Evaluation of the Clinical Severity of COVID-19 of Vaccinated and Unvaccinated Patients in Southeastern Romania in the First 6 Months of 2022, during the Omicron Wave. Healthcare 2023, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, K.U. Pathogenesis of SARS-CoV-2 induced cardiac injury from the perspective of the virus. J. Mol. Cell Cardiol. 2020, 147, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Cirulis, M.M.; Beesley, S.J.; Wilson, E.L.; Stubben, C.; Olsen, T.D.; Hirshberg, E.L.; Smith, L.M.; Lanspa, M.J.; Abraham, T.P.; Grissom, C.K. The peripheral blood transcriptome in septic cardiomyopathy: An observational, pilot study. Intensive Care Med. Exp. 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [PubMed]
- Ramasamy, A.; Wang, C.; Brode, W.M.; Verduzco-Gutierrez, M.; Melamed, E. Immunologic and Autoimmune-Related Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: Clinical Symptoms and Mechanisms of Disease. Phys. Med. Rehabil. Clin. 2023, 34, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Castilla, J.; Stebliankin, V.; Baral, P.; Balbin, C.A.; Sobhan, M.; Cickovski, T.; Mondal, A.M.; Narasimhan, G.; Chapagain, P.; Mathee, K. Potential Autoimmunity Resulting from Molecular Mimicry between SARS-CoV-2 Spike and Human Proteins. Viruses 2022, 14, 1415. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, X.; Tong, Q.; Li, W.; Wang, B.; Sutter, K.; Trilling, M.; Lu, M.; Dittmer, U.; Yang, D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020, 92, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Baroiu, L.; Dumitru, C.; Iancu, A.; Leșe, A.C.; Drăgănescu, M.; Baroiu, N.; Anghel, L. COVID-19 impact on the liver. World J. Clin. Cases 2021, 9, 3814–3825. [Google Scholar] [CrossRef] [PubMed]
- Kaiafa, G.; Savopoulos, C.; Karlafti, E.; Pantazi, K.; Paramythiotis, D.; Thomaidou, E.; Daios, S.; Ztriva, E.; Gionis, M.; Fyntanidou, V. Coagulation Profile of COVID-19 Patients. Life 2022, 12, 1658. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef]
- Passi, R.; Brittan, M.; Baker, A.H. The role of the endothelium in severe acute respiratory syndrome coronavirus 2 infection and pathogenesis. Curr. Opin. Physiol. 2023, 34, 100670. [Google Scholar] [CrossRef] [PubMed]
- Dettlaff-Pokora, A.; Swierczynski, J. Dysregulation of the Renin-Angiotensin-Aldosterone System (RAA) in Patients Infected with SARS-CoV-2-Possible Clinical Consequences. Int. J. Mol. Sci. 2021, 22, 4503. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ye, Q. Kidney involvement in COVID-19 and its treatments. J. Med. Virol. 2021, 93, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Pourfridoni, M.; Abbasnia, S.M.; Shafaei, F.; Razaviyan, J.; Heidari-Soureshjani, R. Fluid and Electrolyte Disturbances in COVID-19 and Their Complications. Biomed. Res. Int. 2021, 2021, 6667047. [Google Scholar] [CrossRef] [PubMed]
- Rokkas, T. Gastrointestinal involvement in COVID-19: A systematic review and meta-analysis. Ann. Gastroenterol. 2020, 33, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; Bakry, E.H.; Zaky, M.Y. Insights into COVID-19 and Its Potential Implications for Kidney Dysfunction. Int. J. Transl. Med. 2023, 3, 255–273. [Google Scholar] [CrossRef]
- Lippi, G.; South, A.M.; Henry, B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann. Clin. Biochem. 2020, 57, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.anm.ro/_/_RCP/RCP_6397_30.04.14.pdf (accessed on 11 April 2024).
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2017, 14, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Borovac, J.A.; D’Amario, D.; Bozic, J.; Glavas, D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J. Cardiol. 2020, 12, 373–408. [Google Scholar] [CrossRef]
- Fox, S.E.; Heide, R.S.V. COVID-19: The Heart of the Matter-Pathological Changes and a Proposed Mechanism. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 217–224. [Google Scholar] [CrossRef]
- Vrints, C.J.M.; Krychtiuk, K.A.; Van Craenenbroeck, E.M.; Segers, V.F.; Price, S.; Heidbuchel, H. Endothelialitis plays a central role in the pathophysiology of severe COVID-19 and its cardiovascular complications. Acta Cardiol. 2021, 76, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Robinson, F.A.; Mihealsick, R.P.; Wagener, B.M.; Hanna, P.; Poston, M.D.; Efimov, I.R.; Shivkumar, K.; Hoover, D.B. Role of angiotensin-converting enzyme 2 and pericytes in cardiac complications of COVID-19 infection. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1059–H1068. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.C.; Garg, S.; George, M.G.; Patel, K.; Jackson, S.L.; Loustalot, F.; Wortham, J.M.; Taylor, C.A.; Whitaker, M.; Reingold, A. COVID-19-Associated Hospitalization Surveillance Network. Acute Cardiac Events During COVID-19-Associated Hospitalizations. J. Am. Coll. Cardiol 2023, 81, 557–569. [Google Scholar]
- Baroiu, L.; Beznea, A.; Condratovici, C.P.; Onisor, C.; Grigore, C.A.; Topor, G.; Rugina, S. Comparative Effectiveness of Vancomycin and Metronidazole for the Initial Episode of Nonsevere Clostridium Difficle Infection. Rev. Chim. 2019, 70, 3741–3745. [Google Scholar] [CrossRef]
- Stefanopol, I.A.; Miulescu, M.; Baroiu, L.; Anghele, A.D.; Danila, D.M.; Tiron, Z. An Unusual Case of Meckel Diverticulitis Misdiagnosed as an Infected Urachal Cyst. Medicina 2021, 57, 495. [Google Scholar] [CrossRef] [PubMed]
- Pelin, A.M.; Balan, G.; Stefanescu, C.; Rosca, S.; Busila, C. New Criteria in Defining the Metabolic Syndrome in Children?—An Analysis of the Relationship Between the Hepatic Enzymes and the Insulin Resistance, HOMA-IR And Lipid Parameters in Obese Children. Prog. Nutr. 2021, 23, e2021316. [Google Scholar]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- National Health Commission of People’s Republic of China Diagnosis and Treatment of Pneumonia Caused by Novel Coronavirus (Trial Version 4). In Chinese. Published 2020. Available online: http://www.nhc.gov.cn/yzygj/s7653p/202001/4294563ed35b43209b31739bd0785e67/files/7a9309111267475a99d4306962c8bf78.pdf (accessed on 20 April 2024).
- Madjid, M.; Vela, D.; Khalili-Tabrizi, H.; Casscells, S.W.; Litovsky, S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: Clues to the triggering effect of acute infections on acute coronary syndromes. Tex. Heart Inst. J. 2007, 34, 11–18. [Google Scholar] [PubMed]
- Corrales-Medina, V.F.; Musher, D.M.; Shachkina, S.; Chirinos, J.A. Acute pneumonia and the cardiovascular system. Lancet 2013, 381, 496–505. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef]
- Giustino, G.; Croft, L.B.; Stefanini, G.G.; Bragato, R.; Silbiger, J.J.; Vicenzi, M.; Danilov, T.; Kukar, N.; Shaban, N.; Kini, A. Characterization of Myocardial Injury in Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Bois, M.C.; Boire, N.A.; Layman, A.J.; Aubry, M.C.; Alexander, M.P.; Roden, A.C.; Hagen, C.E.; Quinton, R.A.; Larsen, C.; Erben, Y. COVID-19-Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation 2021, 143, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.H.; Regueiro, M.; Sandhu, D.S. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J. Gastroenterol. 2020, 26, 2323–2332. [Google Scholar] [CrossRef]
- Baroiu, L.; Lese, A.C.; Stefanopol, I.A.; Iancu, A.; Dumitru, C.; Ciubara, A.B.; Bujoreanu, F.C.; Baroiu, N.; Ciubara, A.; Nechifor, A.; et al. The Role of D-Dimers in the Initial Evaluation of COVID-19. Ther. Clin. Risk Manag. 2022, 18, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Bozada-Gutiérrez, K.; Trejo-Ávila, M.; Moreno-Portillo, M. Postoperative complications and predictors of mortality in patients with COVID-19. Cirugía Y Cir. 2023, 91, 344–353. [Google Scholar] [CrossRef]
- Kanyangarara, N.M.; Kumar, R. A Simplified Approach to COVID-19 Pneumonia Classification. Acad. J. Health Sci. 2023, 38, 117–121. [Google Scholar]
- Iturbide-Mauricio, L.; Palacios-Vaca, J.; Calleja-López, J.R.; Pérez-Navarro, M.; García-García, J.A.; Espinosa-García, A.M. The role of corticosteroids in patients hospitalized for COVID-19 at Mexico general Hospital. Cirugía Y Cir. 2023, 91, 233–239. [Google Scholar] [CrossRef]
- Sandoval-Bedolla, K.L.; Elizalde-Barrera, C.I.; García-López, V.H.; Huerta-Ramírez, S.; Martínez-Cardozo, D. Índice de inmunidad-inflamaciónsistémica (IIS) comomarcadorpronóstico de mortalidadenpacientes con COVID-19. Cirugía Y Cir. 2023, 91, 361–367. [Google Scholar]
- Dou, Q.; Wei, X.; Zhou, K.; Yang, S.; Jia, P. Cardiovascular manifestations and mechanisms in patients with COVID-19. Trends Endocrinol. Metab. 2020, 31, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Modin, D.; Claggett, B.; Sindet-Pedersen, C.; Lassen, M.C.H.; Skaarup, K.G.; Jensen, J.U.S.; Fralick, M.; Schou, M.; Lamberts, M.; Gerds, T. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation 2020, 142, 2080–2082. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Visco, V.; Gambardella, J.; Ferruzzi, G.J.; Rispoli, A.; Rusciano, M.R.; Toni, A.L.; Virtuoso, N.; Carrizzo, A.; Di Pietro, P. Cardiovascular Implications of microRNAs in Coronavirus Disease 2019. J. Pharmacol. Exp. Ther. 2023, 384, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Guarracino, A.; Ballesio, F.; Parca, L.; Ausiello, G.; Helmer-Citterich, M. Evaluation of potential miRNA sponge effects of SARS genomes in human. Non-Coding RNA Res. 2022, 7, 48–53. [Google Scholar] [CrossRef]
- Li, C.; Wang, R.; Wu, A.; Yuan, T.; Song, K.; Bai, Y.; Liu, X. SARS-COV-2 as potential microRNA sponge in COVID-19 patients. BMC Med. Genom. 2022, 15 (Suppl. 2), 94. [Google Scholar] [CrossRef]

| Severe COVID-19 with a History of Cardiovascular Disease Group A (N 1 = 81) | Severe COVID-19 with No History of Cardiovascular Disease Group B (N 2 = 27) | p (T-t) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Min; Max | Mean | SD | Min; Max | ||
| Age (years old) | 68.57 | 13.23 | 21; 99 | 69.44 | 17.69 | 20; 91 | <0.001 |
| Intensive care unit Length of stay (days) | 4.98 | 4.57 | 1; 25 | 7.78 | 6.25 | 1; 25 | <0.001 |
| SpO2 upon admission (%) | 71.32 | 65.43 | 48; 90 | 76.82 | 71.54 | 61; 90 | 0.712 |
| Leucocytes (× 109/L) | 12.74 | 7.49 | 0; 48.05 | 13.86 | 8.85 | 2.81; 40.49 | 0.527 |
| ESR (mm/1 h) | 80.06 | 38.57 | 3; 178 | 70.18 | 42.14 | 10; 140 | 0.264 |
| C-reactive protein mg/L | 127.15 | 84.87 | 3; 425 | 69.01 | 43.25 | 6; 192 | <0.001 |
| LDH μmol/sl | 1510.43 | 885.77 | 2; 58,855 | 1038.62 | 983.80 | 4; 3529 | 0.022 |
| D-dimer mcg/mL | 3.74 | 1.39 | 0; 7.76 | 3.34 | 0.78 | 2.34; 4.78 | 0.064 |
| Activated partial thromboplastin time—aPTT seconds | 34.70 | 14.51 | 0; 114.40 | 42.17 | 21.23 | 19.10; 110 | 0.098 |
| Serum glucose mg/dL | 167.35 | 94.04 | 37; 516 | 186.14 | 106.21 | 52; 432 | 0.386 |
| Serum urea mg/dL | 97.19 | 79.22 | 20; 400 | 81.59 | 57.80 | 28; 232 | 0.348 |
| Serum creatinine mg/dL | 1.96 | 1.91 | 0.64; 11.6 | 1.68 | 1.65 | 0.65; 7.7 | 0.497 |
| ALT U/L | 45.16 | 234 | 5; 2321 | 30.87 | 24.77 | 7; 162.98 | 0.361 |
| AST U/L | 53.45 | 136 | 15; 1328 | 30.97 | 17.31 | 14; 130.98 | 0.081 |
| Serum total bilirubin mg/dL | 1.04 | 1.65 | 0.24; 14.16 | 0.68 | 0.44 | 0.22; 2.20 | 0.262 |
| Serum direct bilirubin mg/dL | 0.51 | 1.15 | 0.08; 9.84 | 0.36 | 0.23 | 0.11; 1.23 | 0.503 |
| Serum albumin g/dL | 4.04 | 1.01 | 1.8; 5.9 | 4.78 | 0.73 | 2.99; 6 | 0.007 |
| Serum amylase U/L | 70.83 | 42.25 | 11; 200 | 66.18 | 56.68 | 8; 280 | 0.651 |
| Serum creatine kinase U/L | 533.82 | 520.21 | 37; 7869 | 201.40 | 265.99 | 43; 1318 | 0.016 |
| Serum troponin ng/dL | 0.29 | 0.58 | 0.02; 2.49 | 0.34 | 0.63 | 0.05; 2.99 | 0.706 |
| Serum sodium mmol/L | 141.01 | 9.44 | 111; 117 | 140.18 | 6.12 | 129; 157 | 0.602 |
| Serum potassium mmol/L | 4.74 | 4.80 | 2.2; 47 | 3.99 | 0.60 | 2.60; 5.80 | 0.425 |
| Serum chlorine mmol/L | 103.85 | 12.05 | 28.2; 136.9 | 102.90 | 6.39 | 89.2; 114.3 | 0.695 |
| Severe COVID-19 with a History of Cardiovascular Disease (N 1 = 81) | Severe COVID-19 with No History of Cardiovascular Disease (N 2 = 27) | Significance Level Χ2 Test p | |||
|---|---|---|---|---|---|
| N | % | n | % | ||
| Sex Female Male | 33 48 | 40.7 59.3 | 13 14 | 48.1 51.9 | 0.653 |
| Metabolic comorbidities | 47 | 58.02 | 13 | 48.14 | 0.502 |
| Neurological comorbidities | 15 | 18.51 | 8 | 29.62 | 0.342 |
| Renal and hepatic comorbidities | 2 | 2.46 | 5 | 18.51 | 0.013 |
| Pulmonary comorbidities | 5 | 6.17 | 3 | 11.11 | 0.671 |
| Deaths | 58 | 71.6 | 13 | 48.1 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechita, L.C.; Ignat, M.D.; Balta, A.A.S.; Barbu, R.E.; Baroiu, L.; Voinescu, D.C.; Nechita, A.; Debita, M.; Busila, C.; Stefanopol, I.A. The Impact of Cardiovascular Antecedents on the Prognosis of COVID-19 Critically Ill Patients. J. Clin. Med. 2024, 13, 3518. https://doi.org/10.3390/jcm13123518
Nechita LC, Ignat MD, Balta AAS, Barbu RE, Baroiu L, Voinescu DC, Nechita A, Debita M, Busila C, Stefanopol IA. The Impact of Cardiovascular Antecedents on the Prognosis of COVID-19 Critically Ill Patients. Journal of Clinical Medicine. 2024; 13(12):3518. https://doi.org/10.3390/jcm13123518
Chicago/Turabian StyleNechita, Luiza Camelia, Mariana Daniela Ignat, Alexia Anastasia Stefania Balta, Raisa Eloise Barbu, Liliana Baroiu, Doina Carina Voinescu, Aurel Nechita, Mihaela Debita, Camelia Busila, and Ioana Anca Stefanopol. 2024. "The Impact of Cardiovascular Antecedents on the Prognosis of COVID-19 Critically Ill Patients" Journal of Clinical Medicine 13, no. 12: 3518. https://doi.org/10.3390/jcm13123518
APA StyleNechita, L. C., Ignat, M. D., Balta, A. A. S., Barbu, R. E., Baroiu, L., Voinescu, D. C., Nechita, A., Debita, M., Busila, C., & Stefanopol, I. A. (2024). The Impact of Cardiovascular Antecedents on the Prognosis of COVID-19 Critically Ill Patients. Journal of Clinical Medicine, 13(12), 3518. https://doi.org/10.3390/jcm13123518







