Physiology-Guided Resuscitation: Monitoring and Augmenting Perfusion during Cardiopulmonary Arrest
Abstract
:1. Introduction
2. The Physiology of Cardiopulmonary Resuscitation
2.1. The Cardiac Pump Model
2.2. The Thoracic Pump Model
2.3. Microcirculation in Cardiac Arrest
2.4. Additional Physiologic Considerations
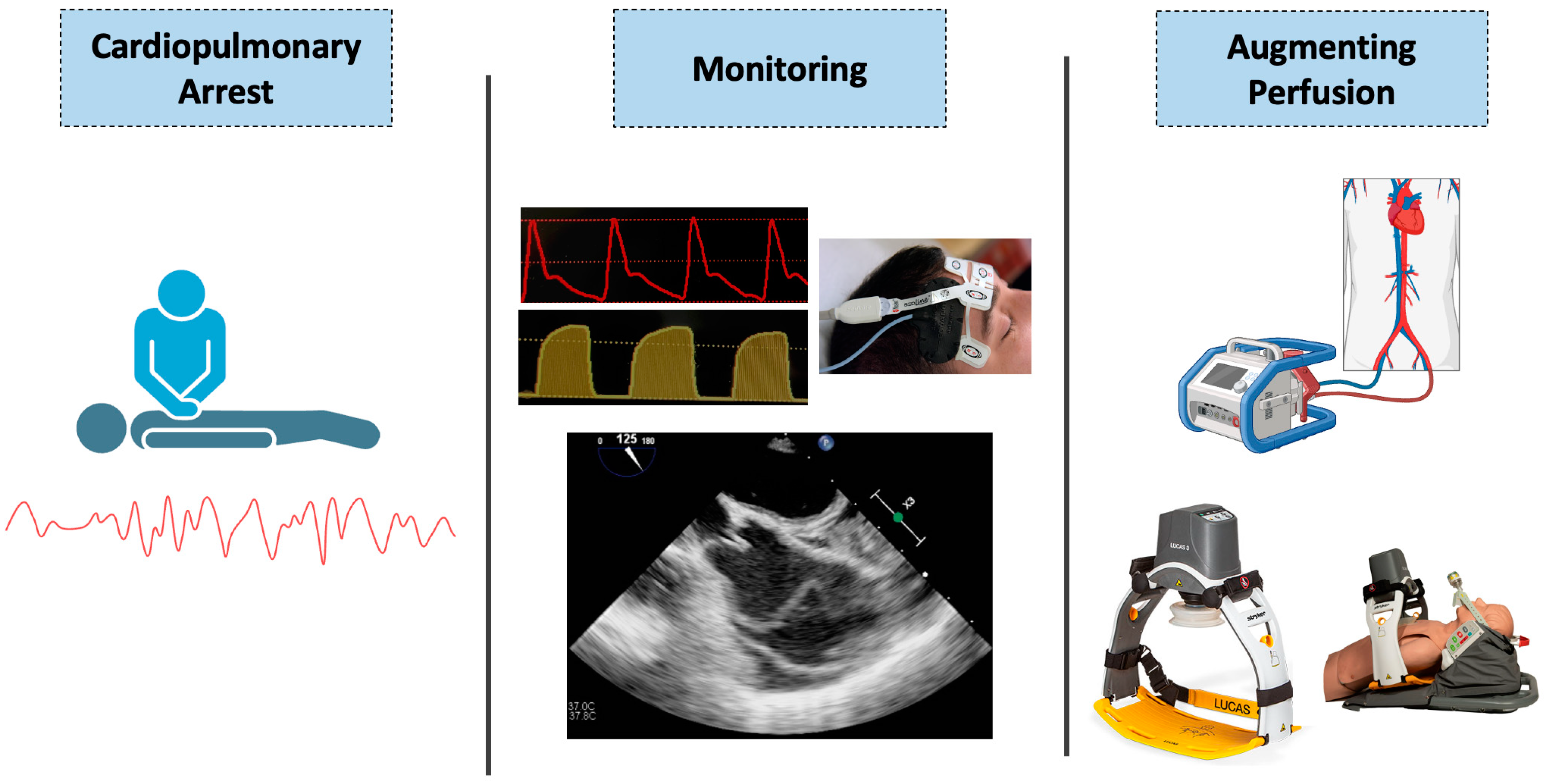
3. Monitoring Perfusion during Cardiopulmonary Resuscitation—Current Strategies
3.1. Coronary Perfusion Pressure (CPP)
3.2. End Tidal Carbon Dioxide (ETCO2)
3.3. Diastolic Blood Pressure (DBP)
3.4. Utilizing Indicators of Perfusion in Practice
4. Monitoring Perfusion during Cardiopulmonary Resuscitation—Novel Strategies
4.1. Regional Cerebral Oximetry
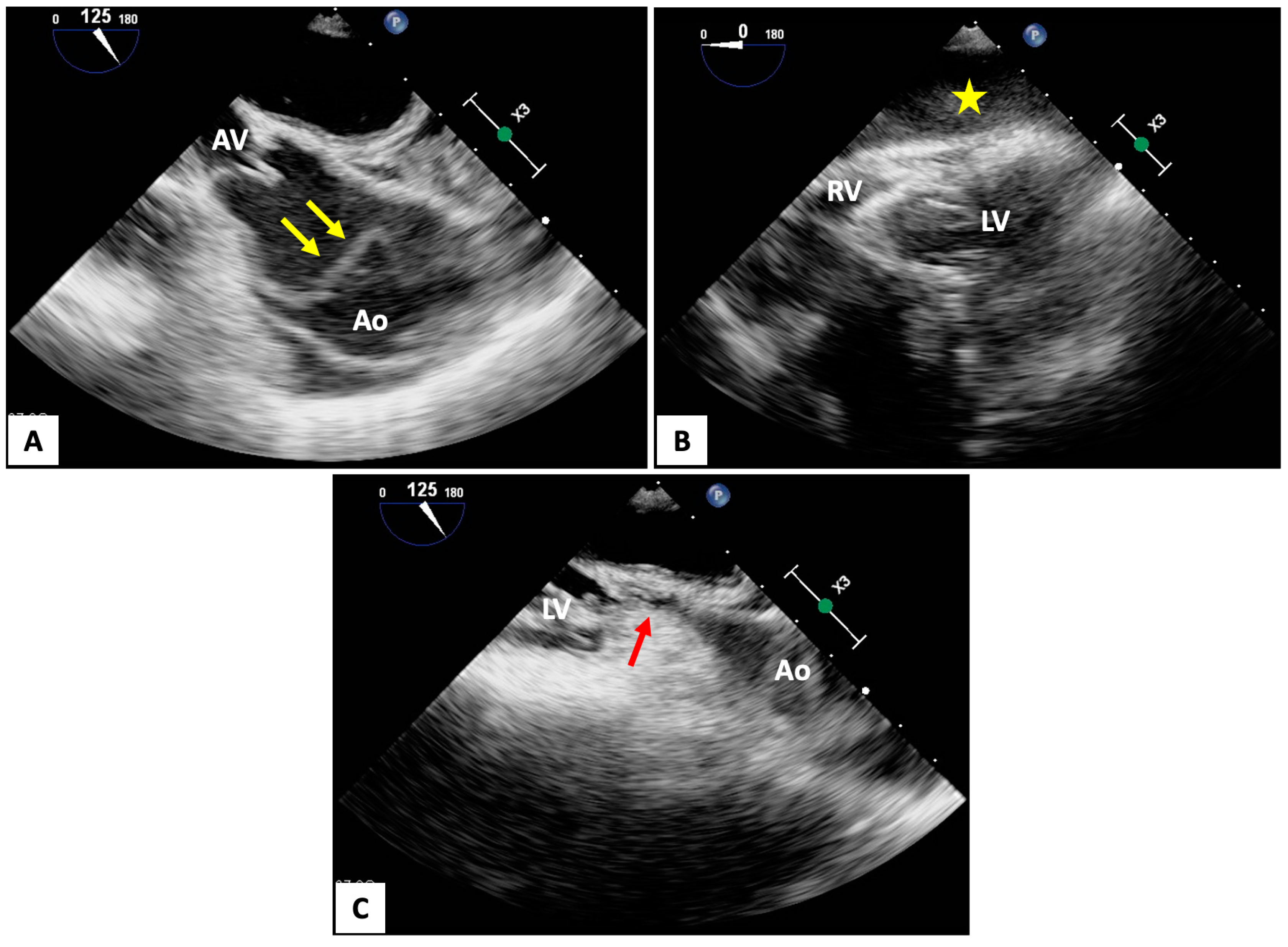
4.2. Transesophageal Echocardiography (TEE)
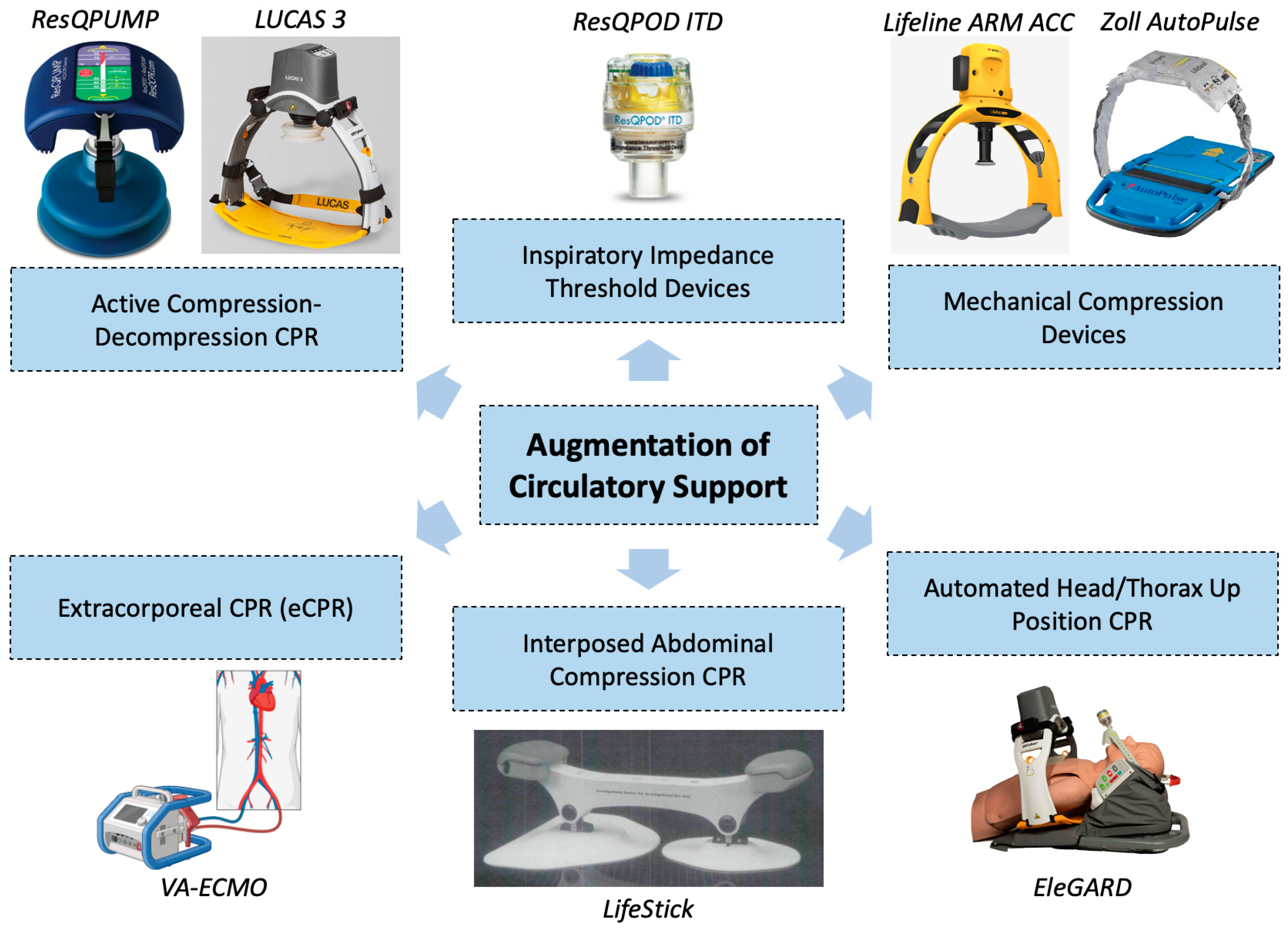
5. Augmentation of Circulatory Support
5.1. Standard Methods
5.2. Active Compression–Decompression CPR
5.3. Mechanical Compression Devices
5.4. Inspiratory Impedance Threshold Devices
5.5. Interposed Abdominal Compression CPR
5.6. Automated Head/Thorax-Up Positioning CPR
5.7. Extracorporeal Cardiopulmonary Resuscitation (eCPR)
| CPR Augmentation Strategy | Trial Design/Population | Primary Outcome | Results (Intervention versus Standard CPR) | Reference |
|---|---|---|---|---|
| Active Compression–Decompression CPR | Prospective, randomized control (n = 62) | Initial resuscitation | 62% vs. 30%, p < 0.03 | Cohen TJ et al. [95] |
| Single center | 24 h survival | 45% vs. 9%, p < 0.004 | ||
| IHCA | Hospital discharge GCS | 7% vs. 0%, p = NS | ||
| 8.0 ± 1.3 vs. 3.5 ± 0.3, p < 0.02 | ||||
| Prospective, randomized control (n = 860) | ROSC | No significant differences in any outcome | Schwab TM et al. [101] | |
| Multicenter | Hospital admission | |||
| OHCA | Survival to discharge | |||
| Neurologic function | ||||
| Prospective, randomized control | 1 h survival (IHCA) | 35.1% vs. 34.6%, p = 0.89 | Stiell IG et al. [102] | |
| Multicenter | Survival to discharge (IHCA) | 11.4% vs. 10.4%, p = 0.64 | ||
| IHCA (n = 773)/OHCA (n = 1011) | MMSE (IHCA) | 37 vs. 37 | ||
| 1 h survival (OHCA) | 16.5% vs. 18.2%, p = 0.48 | |||
| Survival to discharge (OHCA) | 3.7% vs. 4.6%, p = 0.49 | |||
| MMSE (OHCA) | 35 vs. 35 | |||
| Prospective, randomized control (n = 750) | 1-year survival | 5% vs. 2%, p = 0.03 | Plaisance P et al. [100] | |
| Multicenter | ||||
| OHCA | ||||
| Mechanical Compression Devices | Prospective, randomized control (n = 2589) | 4 h survival | 23.6% vs. 23.7%, p > 0.99 | Rubertsson S et al., LINC trial [108] |
| Multicenter | ||||
| OHCA | ||||
| Piston device | ||||
| Prospective, randomized control (n = 4231) | Survival to hospital discharge | 9.4% vs. 11.0%, OR 1.06 (95% CI 0.83–1.37) | Wik L et al., CIRC trial [109] | |
| Multicenter | ||||
| OHCA | ||||
| Band device | ||||
| Pragmatic, cluster randomized control (n = 127) | Proportion of eligible participants successfully randomized | 6% vs. 7%, OR 0.86 (95% CI 0.64–1.15) | Perkins GD et al., PARAMEDIC trial [107] | |
| Multicenter | ||||
| IHCA | ||||
| Piston device | ||||
| Impedance Threshold Devices | Prospective, randomized control (n = 400) | 24 h survival | 22% vs. 33%, p = 0.02 | Plaisance P et al. [117] |
| Multicenter | ||||
| OHCA | ||||
| Prospective, randomized control (n = 8718) | Survival to hospital discharge with modified Rankin score ≤ 3 | 5.8% vs. 6.0%, p = 0.71 | Aufderheide TP et al. [118] | |
| Multicenter | ||||
| OHCA | ||||
| Interposed Abdominal Compression CPR | Prospective, randomized control (n = 143) | ROSC | 49% vs. 28%, p = 0.01 | Sack JB et al. [124] |
| Single center | 24 h survival | 33% vs. 13%, p = 0.009 | ||
| IHCA | ||||
| Prospective, randomized control (n = 135) | ROSC | 51% vs. 27%, p = 0.007 | Sack JB et al. [123] | |
| Single center | 24 h survival | 33% vs. 13%, p = 0.02 | ||
| IHCA | Survival to hospital discharge | 25% vs. 7%, p = 0.02 | ||
| Prospective, randomized control (n = 291) | ROSC | 31% vs. 28%, p = NS | Mateer JR et al. [125] | |
| Single center | ||||
| OHCA | ||||
| Automated Head/Thorax Up Positioning CPR | Prospective, observational (n = 2322) | ROSC | 34.2% vs. 17.9%, p < 0.0001 | Pepe PE et al. [127] |
| Single center | ||||
| OHCA | ||||
| Prospective, observational (n = 227) | Survival to hospital discharge (initiated in <11 min) | OR 3.28 (95% CI 1.55–6.92) | Moore JC et al. [126] | |
| Multicenter | Survival to hospital discharge (initiated in <18 min) | OR 1.88 (95% CI 1.03, 3.44) | ||
| OHCA | ||||
| Prospective, observational (n = 706) | Survival to hospital discharge | 7.6% vs. 2.8%, OR 2.84 (95% CI 1.35, 5.96) | Bachista KM et al. [128] | |
| Multicenter | ||||
| OHCA | ||||
| Extracorporeal CPR (eCPR) | Prospective, randomized control (n = 29) | Survival to hospital discharge | 43% vs. 7%, p = 0.023 | Yannopoulos D et al., ARREST trial [116] |
| Single Center | ||||
| OHCA | ||||
| Prospective, randomized control (n = 256) | Survival with good neurologic outcome (CPC 1 or 2) at 180 days | 31.5% vs. 22.0%, p = 0.09 | Belohlavek J et al., Prague OHCA trial [114] | |
| Single Center | ||||
| OHCA | ||||
| Prospective, randomized control (n = 134) | Survival with good neurologic outcome (CPC 1 or 2) at 30 days | 20% vs. 16%, p = 0.52 | Suverein MN et al., INCEPTION trial [115] | |
| Multicenter | ||||
| OHCA |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACLS | Advanced cardiac life support. |
| ACD-CPR | Active compression–decompression cardiopulmonary resuscitation |
| ACD-ITD | Active compression–decompression—impedance threshold device. |
| AHUP-CPR | Automated head-up position—cardiopulmonary resuscitation. |
| AMC | Area of maximal compression. |
| CPR | Cardiopulmonary resuscitation. |
| CO2 | Carbon dioxide. |
| DBP | Diastolic blood pressure. |
| eCPR | Extracorporeal cardiopulmonary resuscitation. |
| ETCO2 | End-tidal carbon dioxide. |
| IAC | Interposed abdominal compressions. |
| ITD | Impedance threshold device. |
| LV | Left ventricle. |
| LVOT | Left ventricular outflow tract. |
| NIRS | Near-infrared spectroscopy. |
| OHCA | Out-of-hospital cardiac arrest. |
| ROSC | Return of spontaneous circulation. |
| rSO2 | Regional cerebral oximetry. |
| TEE | Transesophageal echocardiogram. |
| TTE | Transthoracic echocardiogram. |
| VA-ECMO | Venoarterial extracorporeal membrane oxygenation. |
| VF | Ventricular fibrillation. |
References
- DeBard, M.L. The history of cardiopulmonary resuscitation. Ann. Emerg. Med. 1980, 9, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Kouwenhoven, W.B.; Jude, J.R.; Knickerbocker, G.G. Closed-chest cardiac massage. JAMA 1960, 173, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Meaney, P.A.; Bobrow, B.J.; Mancini, M.E.; Christenson, J.; Caen, A.R.d.; Bhanji, F.; Abella, B.S.; Kleinman, M.E.; Edelson, D.P.; Berg, R.A.; et al. Cardiopulmonary Resuscitation Quality: Improving Cardiac Resuscitation Outcomes Both Inside and Outside the Hospital. Circulation 2013, 128, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.M.; Friess, S.H.; Naim, M.Y.; Lampe, J.W.; Bratinov, G.; Weiland, T.R., 3rd; Garuccio, M.; Nadkarni, V.M.; Becker, L.B.; Berg, R.A. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am. J. Respir. Crit. Care Med. 2014, 190, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Wayne, M.A.; Miller, C.C. End-Tidal Carbon Dioxide and Outcome of Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 1997, 337, 301–306. [Google Scholar] [CrossRef]
- Paradis, N.A.; Martin, G.B.; Rivers, E.P.; Goetting, M.G.; Appleton, T.J.; Feingold, M.; Nowak, R.M. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 1990, 263, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Parnia, S.; Yang, J.; Nguyen, R.; Ahn, A.; Zhu, J.; Inigo-Santiago, L.; Nasir, A.; Golder, K.; Ravishankar, S.; Bartlett, P.; et al. Cerebral Oximetry During Cardiac Arrest: A Multicenter Study of Neurologic Outcomes and Survival. Crit. Care Med. 2016, 44, 1663–1674. [Google Scholar] [CrossRef]
- Teran, F.; Dean, A.J.; Centeno, C.; Panebianco, N.L.; Zeidan, A.J.; Chan, W.; Abella, B.S. Evaluation of out-of-hospital cardiac arrest using transesophageal echocardiography in the emergency department. Resuscitation 2019, 137, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lurie, K.G.; Nemergut, E.C.; Yannopoulos, D.; Sweeney, M. The Physiology of Cardiopulmonary Resuscitation. Anesth. Analg. 2016, 122, 767–783. [Google Scholar] [CrossRef]
- Cipani, S.; Bartolozzi, C.; Ballo, P.; Sarti, A. Blood flow maintenance by cardiac massage during cardiopulmonary resuscitation: Classical theories, newer hypotheses, and clinical utility of mechanical devices. J. Intensive Care Soc. 2019, 20, 2–10. [Google Scholar] [CrossRef]
- Low, C.J.W.; Ramanathan, K.; Ling, R.R.; Ho, M.J.C.; Chen, Y.; Lorusso, R.; MacLaren, G.; Shekar, K.; Brodie, D. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with cardiac arrest: A comparative meta-analysis and trial sequential analysis. Lancet Respir. Med. 2023, 11, 883–893. [Google Scholar] [CrossRef]
- Weisfeldt, M.L.; Chandra, N. Physiology of cardiopulmonary resuscitation. Annu. Rev. Med. 1981, 32, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Feneley, M.P.; Maier, G.W.; Gaynor, J.W.; Gall, S.A.; Kisslo, J.A.; Davis, J.W.; Rankin, J.S. Sequence of mitral valve motion and transmitral blood flow during manual cardiopulmonary resuscitation in dogs. Circulation 1987, 76, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Rudikoff, M.T.; Maughan, W.L.; Effron, M.; Freund, P.; Weisfeldt, M.L. Mechanisms of blood flow during cardiopulmonary resuscitation. Circulation 1980, 61, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Redberg, R.F.; Tucker, K.J.; Cohen, T.J.; Dutton, J.P.; Callaham, M.L.; Schiller, N.B. Physiology of blood flow during cardiopulmonary resuscitation. A transesophageal echocardiographic study. Circulation 1993, 88, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Criley, J.M.; Blaufuss, A.H.; Kissel, G.L. Cough-induced cardiac compression. Self-administered from of cardiopulmonary resuscitation. JAMA 1976, 236, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.R.; Ornato, J.P.; Guard, C.S.; Roy, V.G.; Burns, C.A.; Nixon, J.V. Transesophageal echocardiography to assess mitral valve function and flow during cardiopulmonary resuscitation. Am. J. Cardiol. 1992, 70, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Merdji, H.; Levy, B.; Jung, C.; Ince, C.; Siegemund, M.; Meziani, F. Microcirculatory dysfunction in cardiogenic shock. Ann. Intensive Care 2023, 13, 38. [Google Scholar] [CrossRef]
- Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit. Care 2015, 19 (Suppl. 3), S8. [Google Scholar] [CrossRef]
- Fries, M.; Weil, M.H.; Chang, Y.T.; Castillo, C.; Tang, W. Microcirculation during cardiac arrest and resuscitation. Crit. Care Med. 2006, 34, S454–S457. [Google Scholar] [CrossRef]
- Krupickova, P.; Mlcek, M.; Huptych, M.; Mormanova, Z.; Boucek, T.; Belza, T.; Lacko, S.; Cerny, M.; Neuzil, P.; Kittnar, O.; et al. Microcirculatory blood flow during cardiac arrest and cardiopulmonary resuscitation does not correlate with global hemodynamics: An experimental study. J. Transl. Med. 2016, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Fries, M.; Tang, W.; Chang, Y.T.; Wang, J.; Castillo, C.; Weil, M.H. Microvascular blood flow during cardiopulmonary resuscitation is predictive of outcome. Resuscitation 2006, 71, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yang, Z.; Cahoon, J.; Xu, J.; Zhu, C.; Yang, M.; Hu, X.; Sun, S.; Tang, W. Post-resuscitation intestinal microcirculation: Its relationship with sublingual microcirculation and the severity of post-resuscitation syndrome. Resuscitation 2014, 85, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Omar, Y.G.; Massey, M.; Andersen, L.W.; Giberson, T.A.; Berg, K.; Cocchi, M.N.; Shapiro, N.I.; Donnino, M.W. Sublingual microcirculation is impaired in post-cardiac arrest patients. Resuscitation 2013, 84, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- van Genderen, M.E.; Lima, A.; Akkerhuis, M.; Bakker, J.; van Bommel, J. Persistent peripheral and microcirculatory perfusion alterations after out-of-hospital cardiac arrest are associated with poor survival. Crit. Care Med. 2012, 40, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Krupickova, P.; Mormanova, Z.; Boucek, T.; Belza, T.; Smalcova, J.; Belohlavek, J. Microvascular perfusion in cardiac arrest: A review of microcirculatory imaging studies. Perfusion 2018, 33, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Debaty, G.; Shin, S.D.; Metzger, A.; Kim, T.; Ryu, H.H.; Rees, J.; McKnite, S.; Matsuura, T.; Lick, M.; Yannopoulos, D.; et al. Tilting for perfusion: Head-up position during cardiopulmonary resuscitation improves brain flow in a porcine model of cardiac arrest. Resuscitation 2015, 87, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.M.; Lee, J.; Lee, Y.S.; Lee, J.H.; Lim, K.S.; Huh, J.W.; Hong, S.B.; Lim, C.M.; Koh, Y.; Kim, W.Y. Lactate Level Versus Lactate Clearance for Predicting Mortality in Patients With Septic Shock Defined by Sepsis-3. Crit. Care Med. 2018, 46, e489–e495. [Google Scholar] [CrossRef] [PubMed]
- Schork, A.; Moll, K.; Haap, M.; Riessen, R.; Wagner, R. Course of lactate, pH and base excess for prediction of mortality in medical intensive care patients. PLoS ONE 2021, 16, e0261564. [Google Scholar] [CrossRef]
- Trebuian, C.I.; Brici, O.M.; Sutoi, D.; Popa, D.I.; Chioibas, D.R.; Mederle, O.A. Lactate Levels and Clearance: Key Predictors of Prognosis for COVID-19 and Non-COVID-19 Septic Shock Patients in the Emergency Department. Clin. Pract. 2024, 14, 834–845. [Google Scholar] [CrossRef]
- Donnino, M.W.; Miller, J.; Goyal, N.; Loomba, M.; Sankey, S.S.; Dolcourt, B.; Sherwin, R.; Otero, R.; Wira, C. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation 2007, 75, 229–234. [Google Scholar] [CrossRef] [PubMed]
- During, J.; Dankiewicz, J.; Cronberg, T.; Hassager, C.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; Nielsen, N.; Pellis, T.; Stammet, P.; et al. Lactate, lactate clearance and outcome after cardiac arrest: A post-hoc analysis of the TTM-Trial. Acta Anaesthesiol. Scand. 2018, 62, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Dusik, M.; Rob, D.; Smalcova, J.; Havranek, S.; Karasek, J.; Smid, O.; Brodska, H.L.; Kavalkova, P.; Huptych, M.; Bakker, J.; et al. Serum lactate in refractory out-of-hospital cardiac arrest: Post-hoc analysis of the Prague OHCA study. Resuscitation 2023, 192, 109935. [Google Scholar] [CrossRef] [PubMed]
- Harhash, A.A.; May, T.L.; Hsu, C.H.; Agarwal, S.; Seder, D.B.; Mooney, M.R.; Patel, N.; McPherson, J.; McMullan, P.; Riker, R.; et al. Risk Stratification Among Survivors of Cardiac Arrest Considered for Coronary Angiography. J. Am. Coll. Cardiol. 2021, 77, 360–371. [Google Scholar] [CrossRef]
- Starodub, R.; Abella, B.S.; Grossestreuer, A.V.; Shofer, F.S.; Perman, S.M.; Leary, M.; Gaieski, D.F. Association of serum lactate and survival outcomes in patients undergoing therapeutic hypothermia after cardiac arrest. Resuscitation 2013, 84, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Bueter, S.; Wernly, B.; Masyuk, M.; Saeed, D.; Albert, A.; Fuernau, G.; Kelm, M.; Westenfeld, R. Lactate Clearance Predicts Good Neurological Outcomes in Cardiac Arrest Patients Treated with Extracorporeal Cardiopulmonary Resuscitation. J. Clin. Med. 2019, 8, 374. [Google Scholar] [CrossRef]
- Mizutani, T.; Umemoto, N.; Taniguchi, T.; Ishii, H.; Hiramatsu, Y.; Arata, K.; Takuya, H.; Inoue, S.; Sugiura, T.; Asai, T.; et al. The lactate clearance calculated using serum lactate level 6 h after is an important prognostic predictor after extracorporeal cardiopulmonary resuscitation: A single-center retrospective observational study. J. Intensive Care 2018, 6, 33. [Google Scholar] [CrossRef]
- Duse, D.A.; Grone, M.; Kramser, N.; Ortkemper, M.; Quast, C.; Voss, F.; Heramvand, N.; Kostev, K.; Kelm, M.; Horn, P.; et al. Elevated Initial Serum Phosphate Levels Predict Higher Mortality and Impaired Neurological Outcome in Cardiac Arrest Patients with Return of Spontaneous Circulation. Diagnostics 2023, 13, 479. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lee, B.K.; Jeung, K.W.; Youn, C.S.; Lee, D.H.; Lee, S.M.; Heo, T.; Min, Y.I. Prognostic value of serum phosphate level in adult patients resuscitated from cardiac arrest. Resuscitation 2018, 128, 56–62. [Google Scholar] [CrossRef]
- Makino, J.; Uchino, S.; Morimatsu, H.; Bellomo, R. A quantitative analysis of the acidosis of cardiac arrest: A prospective observational study. Crit. Care 2005, 9, R357–R362. [Google Scholar] [CrossRef]
- Panchal, A.R.; Bartos, J.A.; Cabanas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef] [PubMed]
- Kern, K.B. Coronary perfusion pressure during cardiopulmonary resuscitation. Best Pract. Res. Clin. Anaesthesiol. 2000, 14, 591–609. [Google Scholar] [CrossRef]
- Halperin, H.R.; Tsitlik, J.E.; Guerci, A.D.; Mellits, E.D.; Levin, H.R.; Shi, A.Y.; Chandra, N.; Weisfeldt, M.L. Determinants of blood flow to vital organs during cardiopulmonary resuscitation in dogs. Circulation 1986, 73, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Kern, K.B.; Lancaster, L.; Goldman, S.; Ewy, G.A. The effect of coronary artery lesions on the relationship between coronary perfusion pressure and myocardial blood flow during cardiopulmonary resuscitation in pigs. Am. Heart J. 1990, 120, 324–333. [Google Scholar] [CrossRef]
- Michael, J.R.; Guerci, A.D.; Koehler, R.C.; Shi, A.Y.; Tsitlik, J.; Chandra, N.; Niedermeyer, E.; Rogers, M.C.; Traystman, R.J.; Weisfeldt, M.L. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation 1984, 69, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H.; Voorhees, W.D.; Babbs, C.F. Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: Improved regional blood flow and resuscitation in dogs. Ann. Emerg. Med. 1984, 13, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.B.; Carden, D.L.; Nowak, R.M.; Lewinter, J.R.; Johnston, W.; Tomlanovich, M.C. Aortic and right atrial pressures during standard and simultaneous compression and ventilation CPR in human beings. Ann. Emerg. Med. 1986, 15, 125–130. [Google Scholar] [CrossRef]
- Paradis, N.A.; Martin, G.B.; Goetting, M.G.; Rosenberg, J.M.; Rivers, E.P.; Appleton, T.J.; Nowak, R.M. Simultaneous aortic, jugular bulb, and right atrial pressures during cardiopulmonary resuscitation in humans. Insights into mechanisms. Circulation 1989, 80, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.B.; Ogle, M.; Ewy, G.A. Coronary perfusion pressure during cardiopulmonary resuscitation. Am. J. Emerg. Med. 1985, 3, 11–14. [Google Scholar] [CrossRef]
- Anderson, C.T.; Breen, P.H. Carbon dioxide kinetics and capnography during critical care. Crit. Care 2000, 4, 207–215. [Google Scholar] [CrossRef]
- Pantazopoulos, C.; Xanthos, T.; Pantazopoulos, I.; Papalois, A.; Kouskouni, E.; Iacovidou, N. A Review of Carbon Dioxide Monitoring During Adult Cardiopulmonary Resuscitation. Heart Lung Circ. 2015, 24, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Kalenda, Z. The capnogram as a guide to the efficacy of cardiac massage. Resuscitation 1978, 6, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Bhende, M.S.; Karasic, D.G.; Menegazzi, J.J. Evaluation of an end-tidal CO2 detector during cardiopulmonary resuscitation in a canine model for pediatric cardiac arrest. Pediatr. Emerg. Care 1995, 11, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Grmec, S.; Kupnik, D. Does the Mainz Emergency Evaluation Scoring (MEES) in combination with capnometry (MEESc) help in the prognosis of outcome from cardiopulmonary resuscitation in a prehospital setting? Resuscitation 2003, 58, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Grmec, S.; Lah, K.; Tusek-Bunc, K. Difference in end-tidal CO2 between asphyxia cardiac arrest and ventricular fibrillation/pulseless ventricular tachycardia cardiac arrest in the prehospital setting. Crit. Care 2003, 7, R139–R144. [Google Scholar] [CrossRef] [PubMed]
- Falk, J.L.; Rackow, E.C.; Weil, M.H. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N. Engl. J. Med. 1988, 318, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Sheak, K.R.; Wiebe, D.J.; Leary, M.; Babaeizadeh, S.; Yuen, T.C.; Zive, D.; Owens, P.C.; Edelson, D.P.; Daya, M.R.; Idris, A.H.; et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation 2015, 89, 149–154. [Google Scholar] [CrossRef] [PubMed]
- White, R.D.; Asplin, B.R. Out-of-hospital quantitative monitoring of end-tidal carbon dioxide pressure during CPR. Ann. Emerg. Med. 1994, 23, 25–30. [Google Scholar] [CrossRef]
- Cantineau, J.P.; Lambert, Y.; Merckx, P.; Reynaud, P.; Porte, F.; Bertrand, C.; Duvaldestin, P. End-tidal carbon dioxide during cardiopulmonary resuscitation in humans presenting mostly with asystole: A predictor of outcome. Crit. Care Med. 1996, 24, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Hatch, L.; Malleck, J.; McClung, C.; Henderson, S.O. End-tidal CO2 as a predictor of survival in out-of-hospital cardiac arrest. Prehosp. Disaster Med. 2011, 26, 148–150. [Google Scholar] [CrossRef]
- Pokorná, M.; Necas, E.; Kratochvíl, J.; Skripský, R.; Andrlík, M.; Franek, O. A sudden increase in partial pressure end-tidal carbon dioxide (PETCO2) at the moment of return of spontaneous circulation. J. Emerg. Med. 2010, 38, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Wayne, M.A.; Levine, R.L.; Miller, C.C. Use of end-tidal carbon dioxide to predict outcome in prehospital cardiac arrest. Ann. Emerg. Med. 1995, 25, 762–767. [Google Scholar] [CrossRef]
- Kolar, M.; Krizmaric, M.; Klemen, P.; Grmec, S. Partial pressure of end-tidal carbon dioxide successful predicts cardiopulmonary resuscitation in the field: A prospective observational study. Crit. Care 2008, 12, R115. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.M.; Topjian, A.A.; Panchal, A.R.; Cheng, A.; Aziz, K.; Berg, K.M.; Lavonas, E.J.; Magid, D.J. Part 1: Executive Summary: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S337–S357. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.; Gravenstein, N.; Giordano, C. End-tidal carbon dioxide and ventilation during CPR in relation to the 2020 American Heart Association guidelines for cardiopulmonary resuscitation. J. Clin. Anesth. 2021, 75, 110553. [Google Scholar] [CrossRef]
- Sutton, R.M.; French, B.; Meaney, P.A.; Topjian, A.A.; Parshuram, C.S.; Edelson, D.P.; Schexnayder, S.; Abella, B.S.; Merchant, R.M.; Bembea, M.; et al. Physiologic monitoring of CPR quality during adult cardiac arrest: A propensity-matched cohort study. Resuscitation 2016, 106, 76–82. [Google Scholar] [CrossRef]
- Bullock, A.; Dodington, J.M.; Donoghue, A.J.; Langhan, M.L. Capnography Use During Intubation and Cardiopulmonary Resuscitation in the Pediatric Emergency Department. Pediatr. Emerg. Care 2017, 33, 457–461. [Google Scholar] [CrossRef]
- Berg, R.A.; Sutton, R.M.; Reeder, R.W.; Berger, J.T.; Newth, C.J.; Carcillo, J.A.; McQuillen, P.S.; Meert, K.L.; Yates, A.R.; Harrison, R.E. Association between diastolic blood pressure during pediatric in-hospital cardiopulmonary resuscitation and survival. Circulation 2018, 137, 1784–1795. [Google Scholar] [CrossRef]
- Sainio, M.; Hoppu, S.; Huhtala, H.; Eilevstjønn, J.; Olkkola, K.T.; Tenhunen, J. Simultaneous beat-to-beat assessment of arterial blood pressure and quality of cardiopulmonary resuscitation in out-of-hospital and in-hospital settings. Resuscitation 2015, 96, 163–169. [Google Scholar] [CrossRef]
- Perman, S.M.; Stanton, E.; Soar, J.; Berg, R.A.; Donnino, M.W.; Mikkelsen, M.E.; Edelson, D.P.; Churpek, M.M.; Yang, L.; Merchant, R.M.; et al. Location of In-Hospital Cardiac Arrest in the United States-Variability in Event Rate and Outcomes. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Morgan, R.W.; French, B.; Kilbaugh, T.J.; Naim, M.Y.; Wolfe, H.; Bratinov, G.; Shoap, W.; Hsieh, T.-C.; Nadkarni, V.M.; Berg, R.A.; et al. A quantitative comparison of physiologic indicators of cardiopulmonary resuscitation quality: Diastolic blood pressure versus end-tidal carbon dioxide. Resuscitation 2016, 104, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Tosh, W.; Patteril, M. Cerebral oximetry. BJA Educ. 2016, 16, 417–421. [Google Scholar] [CrossRef]
- Sandroni, C.; Parnia, S.; Nolan, J.P. Cerebral oximetry in cardiac arrest: A potential role but with limitations. Intensive Care Med. 2019, 45, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Murabito, P.; Messina, A.; Dezio, V.; Busalacchi, D.; Ristagno, G.; Cecconi, M.; Astuto, M. Cerebral regional oxygen saturation during cardiopulmonary resuscitation and return of spontaneous circulation: A systematic review and meta-analysis. Resuscitation 2021, 159, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Parnia, S.; Nasir, A.; Ahn, A.; Malik, H.; Yang, J.; Zhu, J.; Dorazi, F.; Richman, P. A feasibility study of cerebral oximetry during in-hospital mechanical and manual cardiopulmonary resuscitation. Crit. Care Med. 2014, 42, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Genbrugge, C.; Dens, J.; Meex, I.; Boer, W.; Eertmans, W.; Sabbe, M.; Jans, F.; De Deyne, C. Regional Cerebral Oximetry During Cardiopulmonary Resuscitation: Useful or Useless? J. Emerg. Med. 2016, 50, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.W., 2nd; Thomas, C.; Medado, P.; Bastani, A.; Reed, B.; Millis, S.; O’Neil, B.J. End tidal CO2 and cerebral oximetry for the prediction of return of spontaneous circulation during cardiopulmonary resuscitation. Resuscitation 2019, 139, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Chardoli, M.; Heidari, F.; Rabiee, H.; Sharif-Alhoseini, M.; Shokoohi, H.; Rahimi-Movaghar, V. Echocardiography integrated ACLS protocol versus conventional cardiopulmonary resuscitation in patients with pulseless electrical activity cardiac arrest. Chin. J. Traumatol. 2012, 15, 284–287. [Google Scholar]
- Clattenburg, E.J.; Wroe, P.; Brown, S.; Gardner, K.; Losonczy, L.; Singh, A.; Nagdev, A. Point-of-care ultrasound use in patients with cardiac arrest is associated prolonged cardiopulmonary resuscitation pauses: A prospective cohort study. Resuscitation 2018, 122, 65–68. [Google Scholar] [CrossRef]
- Huis In ‘t Veld, M.A.; Allison, M.G.; Bostick, D.S.; Fisher, K.R.; Goloubeva, O.G.; Witting, M.D.; Winters, M.E. Ultrasound use during cardiopulmonary resuscitation is associated with delays in chest compressions. Resuscitation 2017, 119, 95–98. [Google Scholar] [CrossRef]
- Fair, J., 3rd; Mallin, M.P.; Adler, A.; Ockerse, P.; Steenblik, J.; Tonna, J.; Youngquist, S.T. Transesophageal Echocardiography During Cardiopulmonary Resuscitation Is Associated With Shorter Compression Pauses Compared With Transthoracic Echocardiography. Ann. Emerg. Med. 2019, 73, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Catena, E.; Ottolina, D.; Fossali, T.; Rech, R.; Borghi, B.; Perotti, A.; Ballone, E.; Bergomi, P.; Corona, A.; Castelli, A.; et al. Association between left ventricular outflow tract opening and successful resuscitation after cardiac arrest. Resuscitation 2019, 138, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Teran, F.; Prats, M.I.; Nelson, B.P.; Kessler, R.; Blaivas, M.; Peberdy, M.A.; Shillcutt, S.K.; Arntfield, R.T.; Bahner, D. Focused Transesophageal Echocardiography During Cardiac Arrest Resuscitation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Nestaas, S.; Stensaeth, K.H.; Rosseland, V.; Kramer-Johansen, J. Radiological assessment of chest compression point and achievable compression depth in cardiac patients. Scand. J. Trauma. Resusc. Emerg. Med. 2016, 24, 54. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Rhee, J.E.; Kim, K. Is the inter-nipple line the correct hand position for effective chest compression in adult cardiopulmonary resuscitation? Resuscitation 2007, 75, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.O.; Zhao, P.G.; Choi, H.J.; Park, K.H.; Cha, K.C.; Park, S.M.; Kim, S.C.; Kim, H.; Lee, K.H. Compression of the left ventricular outflow tract during cardiopulmonary resuscitation. Acad. Emerg. Med. 2009, 16, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.L.; Fiala, K.C.; Castaneda, M.G.; Boudreau, S.M.; Arana, A.A.; Bebarta, V.S. Left ventricular compressions improve return of spontaneous circulation and hemodynamics in a swine model of traumatic cardiopulmonary arrest. J. Trauma. Acute Care Surg. 2018, 85, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.A.; Morton, J.S.; Luchkanych, A.M.S.; El Karsh, Y.; El Karsh, Z.; Morse, C.; Tomczak, C.R.; Grunau, B.E.; Olver, T.D. Left ventricle chest compression improves ETCO2, blood pressure, and cerebral blood velocity in a swine model of cardiac arrest and cardiopulmonary resuscitation. Resusc. Plus 2022, 12, 100326. [Google Scholar] [CrossRef] [PubMed]
- Teran, F.; Owyang, C.G.; Martin-Flores, M.; Lao, D.; King, A.; Palasz, J.; Araos, J.D. Hemodynamic impact of chest compression location during cardiopulmonary resuscitation guided by transesophageal echocardiography. Crit. Care 2023, 27, 319. [Google Scholar] [CrossRef]
- Lurie, K.G.; Lindner, K.H. Recent advances in cardiopulmonary resuscitation. J. Cardiovasc. Electrophysiol. 1997, 8, 584–600. [Google Scholar] [CrossRef]
- Aufderheide, T.P.; Sigurdsson, G.; Pirrallo, R.G.; Yannopoulos, D.; McKnite, S.; von Briesen, C.; Sparks, C.W.; Conrad, C.J.; Provo, T.A.; Lurie, K.G. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation 2004, 109, 1960–1965. [Google Scholar] [CrossRef]
- Abella, B.S.; Sandbo, N.; Vassilatos, P.; Alvarado, J.P.; O’Hearn, N.; Wigder, H.N.; Hoffman, P.; Tynus, K.; Vanden Hoek, T.L.; Becker, L.B. Chest compression rates during cardiopulmonary resuscitation are suboptimal: A prospective study during in-hospital cardiac arrest. Circulation 2005, 111, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Edelson, D.P.; Abella, B.S.; Kramer-Johansen, J.; Wik, L.; Myklebust, H.; Barry, A.M.; Merchant, R.M.; Hoek, T.L.; Steen, P.A.; Becker, L.B. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation 2006, 71, 137–145. [Google Scholar] [CrossRef]
- Berve, P.O.; Hardig, B.M.; Skalhegg, T.; Kongsgaard, H.; Kramer-Johansen, J.; Wik, L. Mechanical active compression-decompression versus standard mechanical cardiopulmonary resuscitation: A randomised haemodynamic out-of-hospital cardiac arrest study. Resuscitation 2022, 170, 1–10. [Google Scholar] [CrossRef]
- Cohen, T.J.; Goldner, B.G.; Maccaro, P.C.; Ardito, A.P.; Trazzera, S.; Cohen, M.B.; Dibs, S.R. A comparison of active compression-decompression cardiopulmonary resuscitation with standard cardiopulmonary resuscitation for cardiac arrests occurring in the hospital. N. Engl. J. Med. 1993, 329, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Lindner, K.H.; Pfenninger, E.G.; Lurie, K.G.; Schurmann, W.; Lindner, I.M.; Ahnefeld, F.W. Effects of active compression-decompression resuscitation on myocardial and cerebral blood flow in pigs. Circulation 1993, 88, 1254–1263. [Google Scholar] [CrossRef]
- Steinberg, M.T.; Olsen, J.A.; Eriksen, M.; Neset, A.; Norseng, P.A.; Kramer-Johansen, J.; Hardig, B.M.; Wik, L. Haemodynamic outcomes during piston-based mechanical CPR with or without active decompression in a porcine model of cardiac arrest. Scand. J. Trauma. Resusc. Emerg. Med. 2018, 26, 31. [Google Scholar] [CrossRef]
- Tucker, K.J.; Redberg, R.F.; Schiller, N.B.; Cohen, T.J. Active compression-decompression resuscitation: Analysis of transmitral flow and left ventricular volume by transesophageal echocardiography in humans. Cardiopulmonary Resuscitation Working Group. J. Am. Coll. Cardiol. 1993, 22, 1485–1493. [Google Scholar] [CrossRef]
- Lafuente-Lafuente, C.; Melero-Bascones, M. Active chest compression-decompression for cardiopulmonary resuscitation. Cochrane Database Syst. Rev. 2013, 2013, CD002751. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, P.; Lurie, K.G.; Vicaut, E.; Adnet, F.; Petit, J.L.; Epain, D.; Ecollan, P.; Gruat, R.; Cavagna, P.; Biens, J.; et al. A comparison of standard cardiopulmonary resuscitation and active compression-decompression resuscitation for out-of-hospital cardiac arrest. French Active Compression-Decompression Cardiopulmonary Resuscitation Study Group. N. Engl. J. Med. 1999, 341, 569–575. [Google Scholar] [CrossRef]
- Schwab, T.M.; Callaham, M.L.; Madsen, C.D.; Utecht, T.A. A randomized clinical trial of active compression-decompression CPR vs standard CPR in out-of-hospital cardiac arrest in two cities. JAMA 1995, 273, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Stiell, I.G.; Hebert, P.C.; Wells, G.A.; Laupacis, A.; Vandemheen, K.; Dreyer, J.F.; Eisenhauer, M.A.; Gibson, J.; Higginson, L.A.; Kirby, A.S.; et al. The Ontario trial of active compression-decompression cardiopulmonary resuscitation for in-hospital and prehospital cardiac arrest. JAMA 1996, 275, 1417–1423. [Google Scholar] [CrossRef]
- Aufderheide, T.P.; Frascone, R.J.; Wayne, M.A.; Mahoney, B.D.; Swor, R.A.; Domeier, R.M.; Olinger, M.L.; Holcomb, R.G.; Tupper, D.E.; Yannopoulos, D.; et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: A randomised trial. Lancet 2011, 377, 301–311. [Google Scholar] [CrossRef]
- Frascone, R.J.; Wayne, M.A.; Swor, R.A.; Mahoney, B.D.; Domeier, R.M.; Olinger, M.L.; Tupper, D.E.; Setum, C.M.; Burkhart, N.; Klann, L.; et al. Treatment of non-traumatic out-of-hospital cardiac arrest with active compression decompression cardiopulmonary resuscitation plus an impedance threshold device. Resuscitation 2013, 84, 1214–1222. [Google Scholar] [CrossRef]
- Wolcke, B.B.; Mauer, D.K.; Schoefmann, M.F.; Teichmann, H.; Provo, T.A.; Lindner, K.H.; Dick, W.F.; Aeppli, D.; Lurie, K.G. Comparison of standard cardiopulmonary resuscitation versus the combination of active compression-decompression cardiopulmonary resuscitation and an inspiratory impedance threshold device for out-of-hospital cardiac arrest. Circulation 2003, 108, 2201–2205. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Lone, A.N.; Talluri, S.; Khan, M.Z.; Khan, M.U.; Kaluski, E. Efficacy and safety of mechanical versus manual compression in cardiac arrest—A Bayesian network meta-analysis. Resuscitation 2018, 130, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Lall, R.; Quinn, T.; Deakin, C.D.; Cooke, M.W.; Horton, J.; Lamb, S.E.; Slowther, A.M.; Woollard, M.; Carson, A.; et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): A pragmatic, cluster randomised controlled trial. Lancet 2015, 385, 947–955. [Google Scholar] [CrossRef]
- Rubertsson, S.; Lindgren, E.; Smekal, D.; Ostlund, O.; Silfverstolpe, J.; Lichtveld, R.A.; Boomars, R.; Ahlstedt, B.; Skoog, G.; Kastberg, R.; et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest: The LINC randomized trial. JAMA 2014, 311, 53–61. [Google Scholar] [CrossRef]
- Wik, L.; Olsen, J.A.; Persse, D.; Sterz, F.; Lozano, M., Jr.; Brouwer, M.A.; Westfall, M.; Souders, C.M.; Malzer, R.; van Grunsven, P.M.; et al. Manual vs. integrated automatic load-distributing band CPR with equal survival after out of hospital cardiac arrest. The randomized CIRC trial. Resuscitation 2014, 85, 741–748. [Google Scholar] [CrossRef]
- Wroe, P.C.; Clattenburg, E.J.; Gardner, K.; Gelber, J.; Schultz, C.; Singh, A.; Nagdev, A. Emergency department use of a mechanical chest compression device frequently causes unanticipated interruptions in cardiopulmonary resuscitation. Resuscitation 2018, 133, e3–e4. [Google Scholar] [CrossRef]
- Sheraton, M.; Columbus, J.; Surani, S.; Chopra, R.; Kashyap, R. Effectiveness of Mechanical Chest Compression Devices over Manual Cardiopulmonary Resuscitation: A Systematic Review with Meta-analysis and Trial Sequential Analysis. West. J. Emerg. Med. 2021, 22, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Venturini, J.M.; Retzer, E.; Estrada, J.R.; Friant, J.; Beiser, D.; Edelson, D.; Paul, J.; Blair, J.; Nathan, S.; Shah, A.P. Mechanical chest compressions improve rate of return of spontaneous circulation and allow for initiation of percutaneous circulatory support during cardiac arrest in the cardiac catheterization laboratory. Resuscitation 2017, 115, 56–60. [Google Scholar] [CrossRef] [PubMed]
- William, P.; Rao, P.; Kanakadandi, U.B.; Asencio, A.; Kern, K.B. Mechanical Cardiopulmonary Resuscitation In and On the Way to the Cardiac Catheterization Laboratory. Circ. J. 2016, 80, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Belohlavek, J.; Smalcova, J.; Rob, D.; Franek, O.; Smid, O.; Pokorna, M.; Horak, J.; Mrazek, V.; Kovarnik, T.; Zemanek, D.; et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2022, 327, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Suverein, M.M.; Delnoij, T.S.R.; Lorusso, R.; Brandon Bravo Bruinsma, G.J.; Otterspoor, L.; Elzo Kraemer, C.V.; Vlaar, A.P.J.; van der Heijden, J.J.; Scholten, E.; den Uil, C.; et al. Early Extracorporeal CPR for Refractory Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2023, 388, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, P.; Soleil, C.; Lurie, K.G.; Vicaut, E.; Ducros, L.; Payen, D. Use of an inspiratory impedance threshold device on a facemask and endotracheal tube to reduce intrathoracic pressures during the decompression phase of active compression-decompression cardiopulmonary resuscitation. Crit. Care Med. 2005, 33, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Aufderheide, T.P.; Nichol, G.; Rea, T.D.; Brown, S.P.; Leroux, B.G.; Pepe, P.E.; Kudenchuk, P.J.; Christenson, J.; Daya, M.R.; Dorian, P.; et al. A trial of an impedance threshold device in out-of-hospital cardiac arrest. N. Engl. J. Med. 2011, 365, 798–806. [Google Scholar] [CrossRef]
- Plaisance, P.; Lurie, K.G.; Vicaut, E.; Martin, D.; Gueugniaud, P.Y.; Petit, J.L.; Payen, D. Evaluation of an impedance threshold device in patients receiving active compression-decompression cardiopulmonary resuscitation for out of hospital cardiac arrest. Resuscitation 2004, 61, 265–271. [Google Scholar] [CrossRef]
- Ward, K.R.; Sullivan, R.J.; Zelenak, R.R.; Summer, W.R. A comparison of interposed abdominal compression CPR and standard CPR by monitoring end-tidal PCO2. Ann. Emerg. Med. 1989, 18, 831–837. [Google Scholar] [CrossRef]
- Babbs, C.F. Interposed abdominal compression CPR: A comprehensive evidence based review. Resuscitation 2003, 59, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Babbs, C.F. Simplified meta-analysis of clinical trials in resuscitation. Resuscitation 2003, 57, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Sack, J.B.; Kesselbrenner, M.B.; Bregman, D. Survival from in-hospital cardiac arrest with interposed abdominal counterpulsation during cardiopulmonary resuscitation. JAMA 1992, 267, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sack, J.B.; Kesselbrenner, M.B.; Jarrad, A. Interposed abdominal compression-cardiopulmonary resuscitation and resuscitation outcome during asystole and electromechanical dissociation. Circulation 1992, 86, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Mateer, J.R.; Stueven, H.A.; Thompson, B.M.; Aprahamian, C.; Darin, J.C. Pre-hospital IAC-CPR versus standard CPR: Paramedic resuscitation of cardiac arrests. Am. J. Emerg. Med. 1985, 3, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C.; Pepe, P.E.; Scheppke, K.A.; Lick, C.; Duval, S.; Holley, J.; Salverda, B.; Jacobs, M.; Nystrom, P.; Quinn, R.; et al. Head and thorax elevation during cardiopulmonary resuscitation using circulatory adjuncts is associated with improved survival. Resuscitation 2022, 179, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Pepe, P.E.; Scheppke, K.A.; Antevy, P.M.; Crowe, R.P.; Millstone, D.; Coyle, C.; Prusansky, C.; Garay, S.; Ellis, R.; Fowler, R.L.; et al. Confirming the Clinical Safety and Feasibility of a Bundled Methodology to Improve Cardiopulmonary Resuscitation Involving a Head-Up/Torso-Up Chest Compression Technique. Crit. Care Med. 2019, 47, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Bachista, K.M.; Moore, J.C.; Labarere, J.; Crowe, R.P.; Emanuelson, L.D.; Lick, C.J.; Debaty, G.P.; Holley, J.E.; Quinn, R.P.; Scheppke, K.A.; et al. Survival for Nonshockable Cardiac Arrests Treated With Noninvasive Circulatory Adjuncts and Head/Thorax Elevation. Crit. Care Med. 2024, 52, 170–181. [Google Scholar] [CrossRef]
- Kennedy, J.H. The role of assisted circulation in cardiac resuscitation. JAMA 1966, 197, 615–618. [Google Scholar] [CrossRef]
- Available online: https://www.elso.org/registry/internationalsummaryandreports/reports.aspx (accessed on 14 May 2024).
- Ubben, J.F.H.; Heuts, S.; Delnoij, T.S.R.; Suverein, M.M.; van de Koolwijk, A.F.; van der Horst, I.C.C.; Maessen, J.G.; Bartos, J.; Kavalkova, P.; Rob, D.; et al. Extracorporeal cardiopulmonary resuscitation for refractory OHCA: Lessons from three randomized controlled trials-the trialists’ view. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 540–547. [Google Scholar] [CrossRef]
- Rob, D.; Komarek, A.; Smalcova, J.; Belohlavek, J. Effect of Intraarrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Invasive Treatment: A Post Hoc Bayesian Reanalysis of a Randomized Clinical Trial. Chest 2023. [Google Scholar] [CrossRef] [PubMed]
- Heuts, S.; van de Koolwijk, A.F.; Gabrio, A.; Ubben, J.F.H.; van der Horst, I.C.C.; Delnoij, T.S.R.; Suverein, M.M.; Maessen, J.G.; Lorusso, R.; van de Poll, M.C.G. Extracorporeal life support in cardiac arrest: A post hoc Bayesian re-analysis of the INCEPTION trial. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 191–200. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernard, S.; Pashun, R.A.; Varma, B.; Yuriditsky, E. Physiology-Guided Resuscitation: Monitoring and Augmenting Perfusion during Cardiopulmonary Arrest. J. Clin. Med. 2024, 13, 3527. https://doi.org/10.3390/jcm13123527
Bernard S, Pashun RA, Varma B, Yuriditsky E. Physiology-Guided Resuscitation: Monitoring and Augmenting Perfusion during Cardiopulmonary Arrest. Journal of Clinical Medicine. 2024; 13(12):3527. https://doi.org/10.3390/jcm13123527
Chicago/Turabian StyleBernard, Samuel, Raymond A. Pashun, Bhavya Varma, and Eugene Yuriditsky. 2024. "Physiology-Guided Resuscitation: Monitoring and Augmenting Perfusion during Cardiopulmonary Arrest" Journal of Clinical Medicine 13, no. 12: 3527. https://doi.org/10.3390/jcm13123527





