Hypothalamic Hemangioma-like Pilocytic Astrocytoma in an Adult Patient: A Systematic Review with a Focus on Differential Diagnosis and Neurological Presentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection Criteria
- Articles published in the English language.
- Investigated hypothalamic pilocytic astrocytoma in adult patients.
- Provided detailed information on diagnostic methods, differential diagnosis, clinical presentation, treatment strategies, and outcomes.
- Included original research articles, case reports, case series, or review articles.
- Not written in the English language.
- Did not specifically focus on hypothalamic pilocytic astrocytoma in adult patients.
- Lack of detailed information on diagnostic methods, differential diagnosis, clinical presentation, treatment strategies, or outcomes.
- Animal studies, editorials, letters to the editor, commentaries, conference abstracts, or reviews without original data.
- Duplicate publications or overlapping data from the same study population.
- Studies with insufficient data or inadequate reporting that precluded any meaningful extraction of relevant information.
- Studies conducted exclusively in pediatric populations or patients with other types of brain tumors not directly related to hypothalamic pilocytic astrocytoma in adults.
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis
3. Results
3.1. Patients’ Demographics
3.2. Illustrative Case
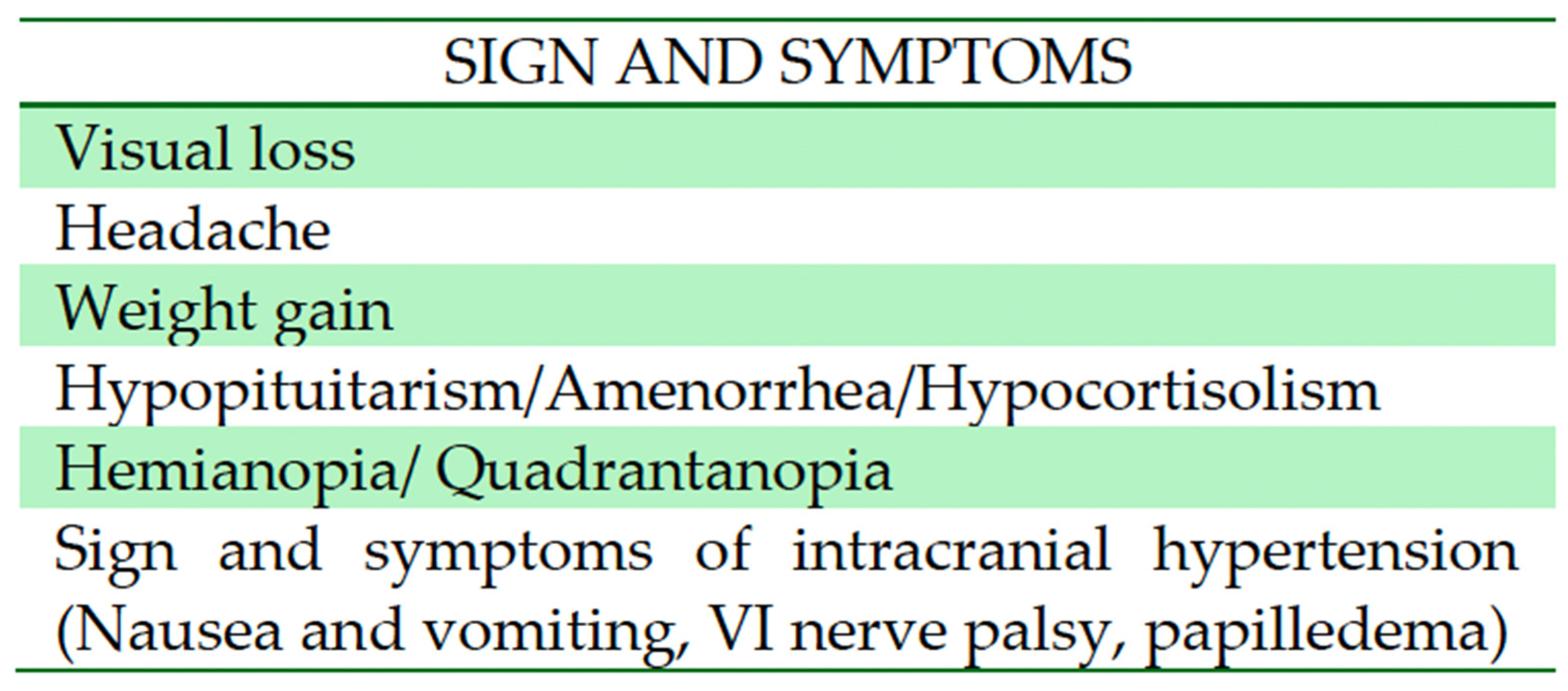
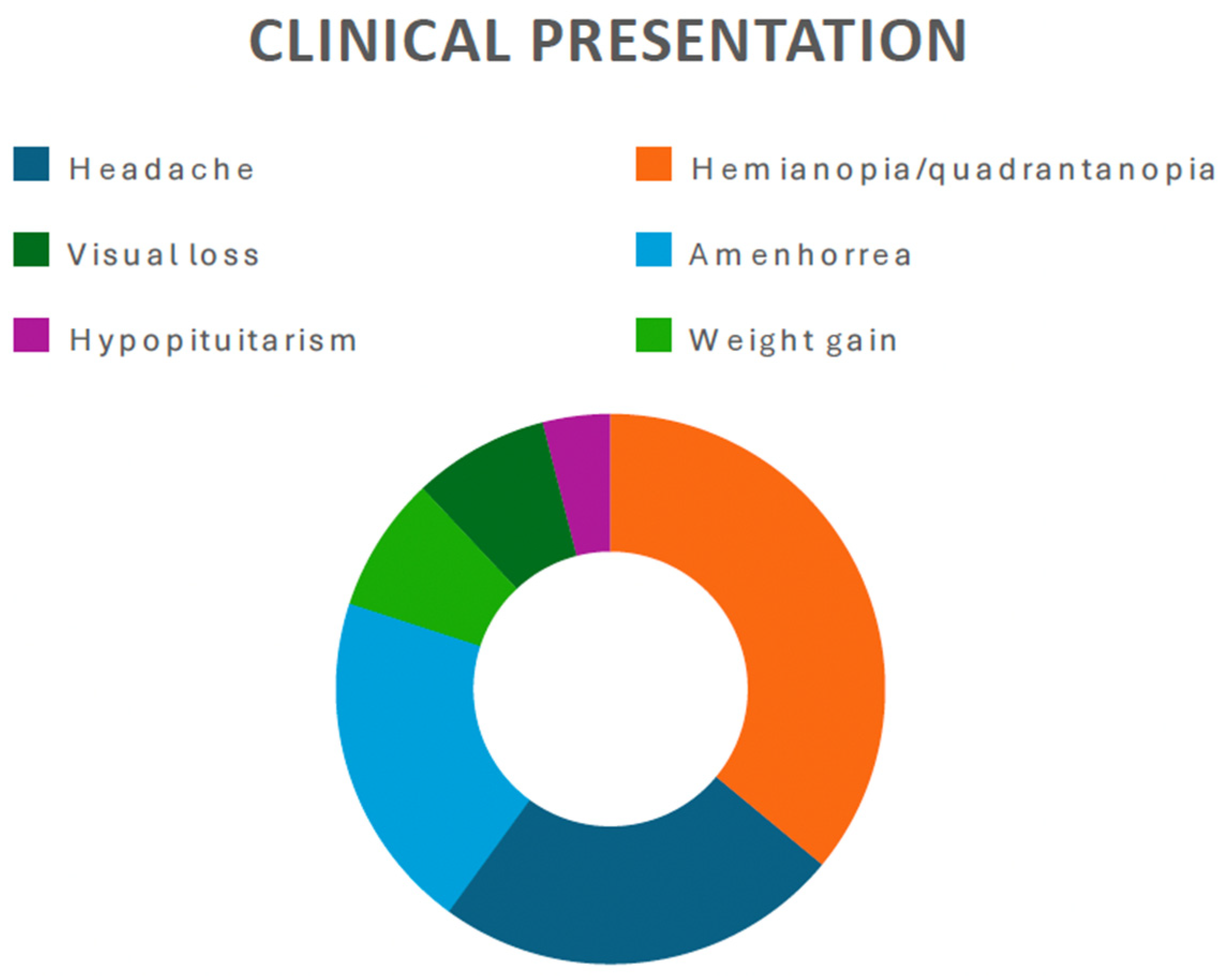
3.2.1. Clinical Presentation
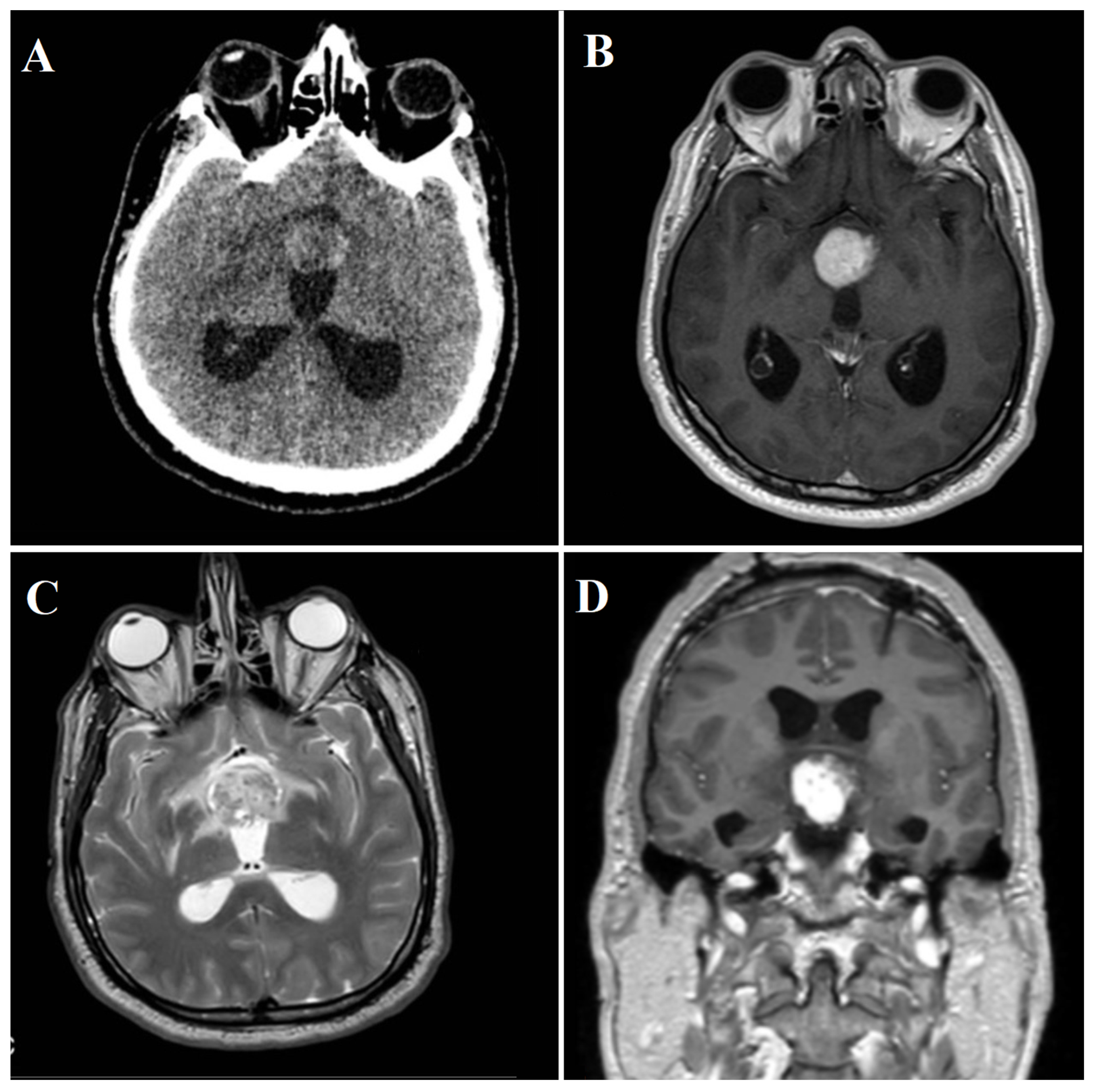
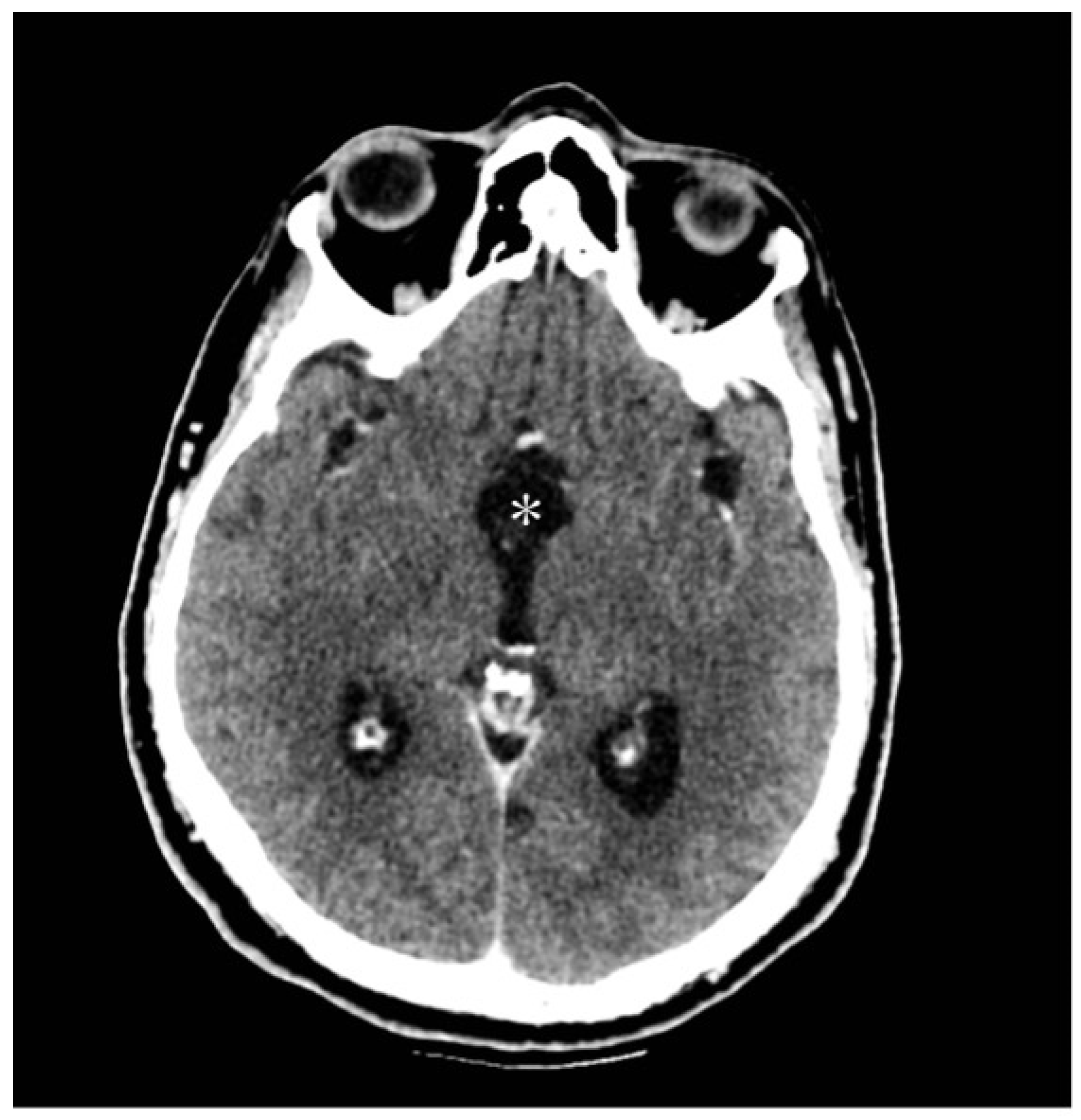
3.2.2. Radiological Findings
3.2.3. Diagnostic Workup
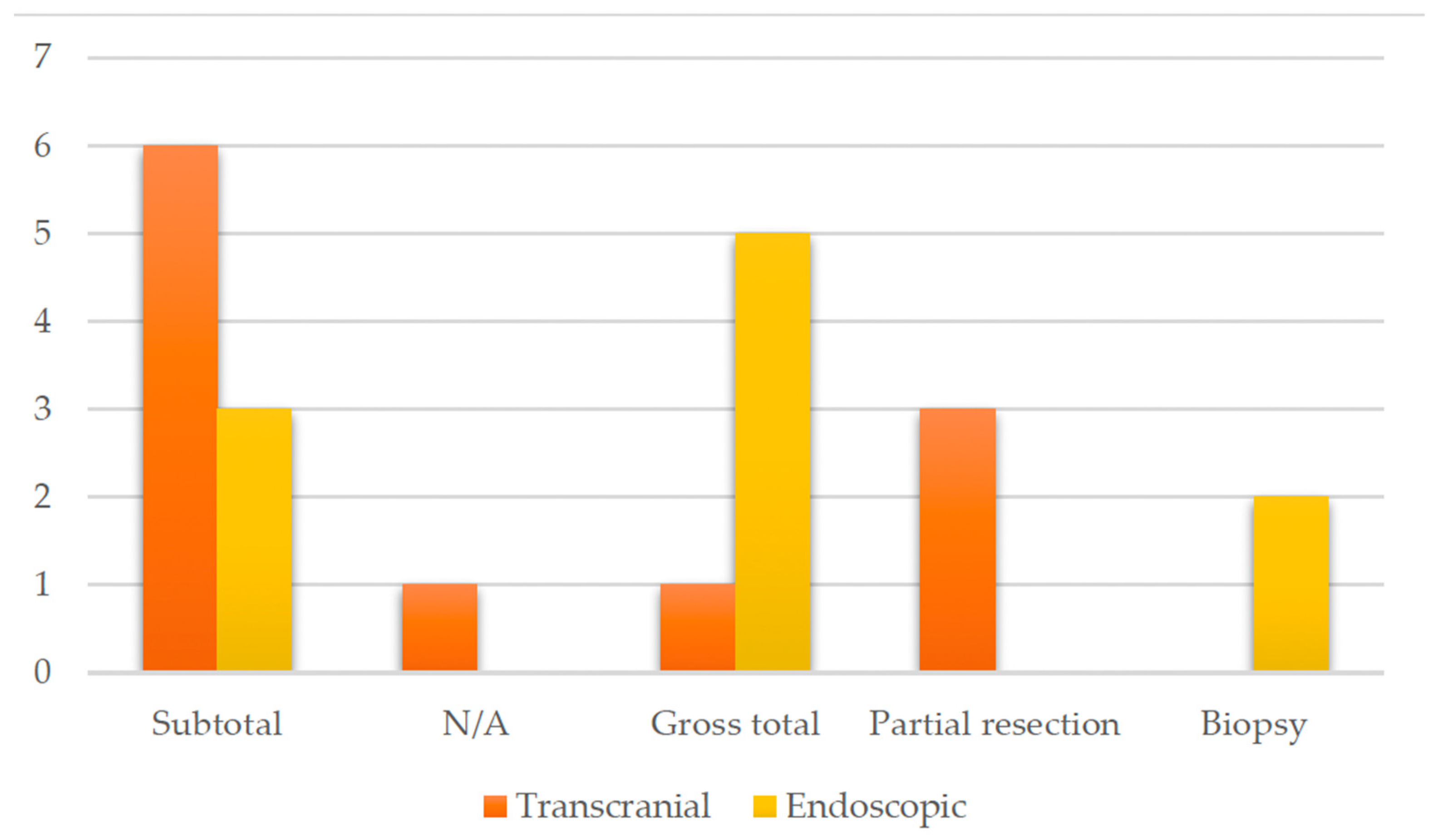
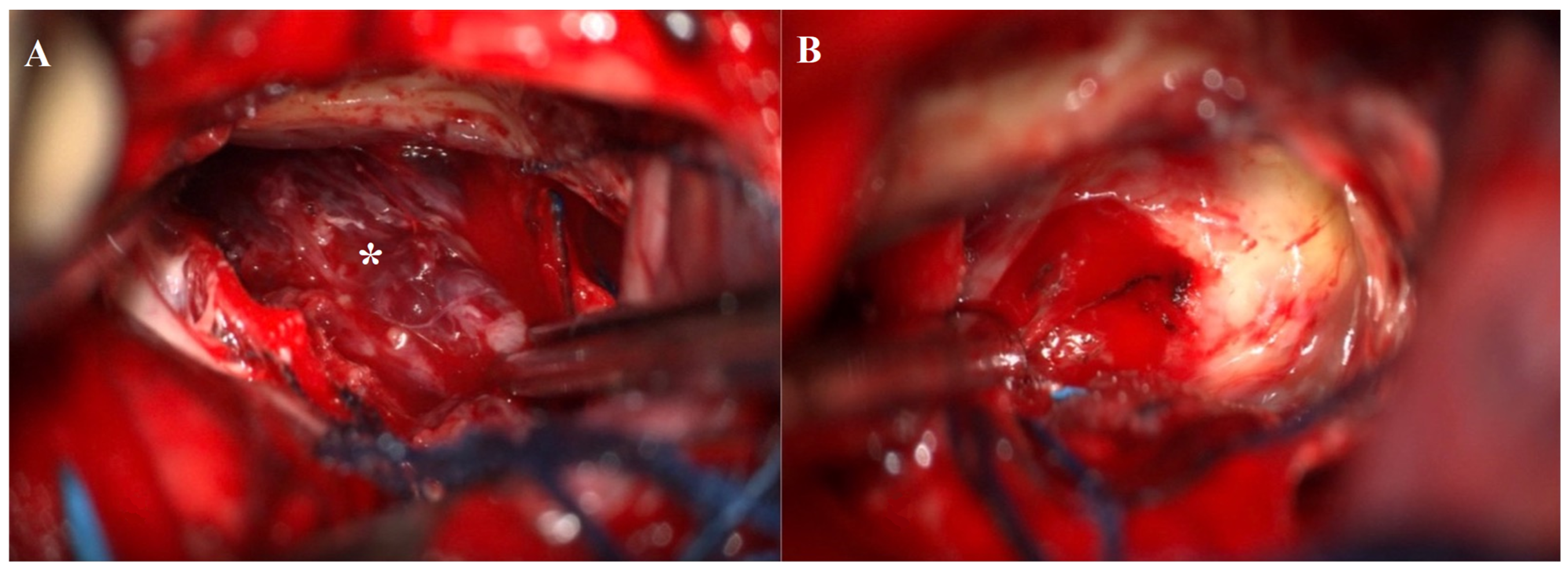
3.2.4. Treatment and Surgical Intervention
4. Discussion
4.1. Epidemiology and Clinical Presentation
4.2. Diagnostic Challenges and Imaging Modalities
4.3. Treatment Strategies
5. Limitations
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014, 16 (Suppl. S4), iv1–iv63. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Chitnavis, B.P.; Al-Sarraj, S.; Connor, S.; Sharr, M.M.; Gullan, R.W. Pilocytic astrocytoma of the adult–clinical features, radiological features, and management. Br. J. Neurosurg. 2004, 18, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.; De Jesus, O. Pilocytic Astrocytoma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Collins, V.P.; Jones, D.T.; Giannini, C. Pilocytic astrocytoma: Pathology, molecular mechanisms, and markers. Acta Neuropathol. 2015, 129, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Bond, K.M.; Hughes, J.D.; Porter, A.L.; Orina, J.; Fang, S.; Parney, I.F. Adult Pilocytic Astrocytoma: An Institutional Series and Systematic Literature Review for Extent of Resection and Recurrence. World Neurosurg. 2018, 110, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Gregory, T.A.; Chumbley, L.B.; Henson, J.W.; Wheeler, B.J. Adult pilocytic astrocytoma in the molecular era: A comprehensive review. CNS Oncol. 2021, 10, CNS68. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, M.; Frappaz, D.; Packer, R.J. Pilocytic astrocytomas. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 134, pp. 329–344. [Google Scholar]
- Kikuchi, K.; Hiwatashi, A.; Togao, O.; Yamashita, K.; Kamei, R.; Kitajima, M.; Kanoto, M.; Takahashi, H.; Uchiyama, Y.; Harada, M.; et al. Usefulness of perfusion- and diffusion-weighted imaging to differentiate between pilocytic astrocytomas and high-grade gliomas: A multicenter study in Japan. Neuroradiology 2018, 60, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Fulham, M.J.; Melisi, J.W.; Nishimiya, J.; Dwyer, A.J.; Di Chiro, G. Neuroimaging of juvenile pilocytic astrocytomas: An enigma. Radiology 1993, 189, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Snilstveit, B.; Oliver, S.; Vojtkova, M. Narrative approaches to systematic review and synthesis of evidence for international development policy and practice. J. Dev. Eff. 2012, 4, 409–429. [Google Scholar] [CrossRef]
- Valdueza, J.M.; Lohmann, F.; Dammann, O.; Hagel, C.; Eckert, B.; Freckmann, N. Analysis of 20 primarily surgically treated chiasmatic/hypothalamic pilocytic astrocytomas. Acta Neurochir. 1994, 126, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Akagi, K.; Abekura, M.; Maeda, Y.; Kitagawa, M.; Ryujin, H.; Iwasa, N. Hypothalamic Pilocytic Astrocytoma Presenting with Intratumoral and Subarachnoid Hemorrhage. Neurol. Med.-Chir. 1997, 37, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Balkhoyor, K.B.; Bernstein, M. Involution of diencephalic pilocytic astrocytoma after partial resection. Report of two cases in adults. J. Neurosurg. 2000, 93, 484–486. [Google Scholar] [CrossRef] [PubMed]
- de Divitiis, E.; Cavallo, L.M.; Cappabianca, P.; Esposito, F. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: Part 2. Neurosurgery 2007, 60, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Arbolay, O.L.; González, J.G.; González, R.H.; Gálvez, Y.H. Extended Endoscopic Endonasal Approach to the Skull Base. MIN-Minim. Invasive Neurosurg. 2009, 52, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Paluzzi, A.; Fernandez-Miranda, J.C.; Pinheiro-Neto, C.; Alcocer-Barradas, V.; Lopez-Alvarez, B.; Gardner, P.; Snyderman, C. Endoscopic Endonasal Infrasellar Approach to the Sellar and Suprasellar Regions: Technical Note. Skull Base 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Mazzatenta, D.; Valluzzi, A.; Marucci, G.; Acciarri, N.; Pasquini, E.; Frank, G. Expanding indications for the extended endoscopic endonasal approach to hypothalamic gliomas: Preliminary report. Neurosurg. Focus 2014, 37, E11. [Google Scholar] [CrossRef]
- Al-Shaar, H.A.; Raheja, A.; Palmer, C.A.; Schmidt, M.H.; Couldwell, W.T. Hypothalamic–Optochiasmatic Pilocytic Astrocytoma Associated with Occipital and Sacral Spinal Cavernomas: A Mere Coincidence or a True Association? World Neurosurg. 2016, 90, 707.e17–707.e21. [Google Scholar] [CrossRef]
- Hidalgo, E.T.; McQuinn, M.W.; Wisoff, J.H. Regression after subtotal resection of an optic pathway glioma in an adult without adjuvant therapy: Case report. J. Neurosurg. 2018, 130, 2005–2008. [Google Scholar] [CrossRef]
- Bin Abdulqader, S.; Al-Ajlan, Z.; Albakr, A.; Issawi, W.; Al-Bar, M.; Recinos, P.F.; Alsaleh, S.; Ajlan, A. Endoscopic transnasal resection of optic pathway pilocytic astrocytoma. Child’s Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2019, 35, 73–81. [Google Scholar] [CrossRef]
- Shoji, T.; Kanamori, M.; Saito, R.; Watanabe, Y.; Watanabe, M.; Fujimura, M.; Ogawa, Y.; Sonoda, Y.; Kumabe, T.; Kure, S.; et al. Frequent Clinical and Radiological Progression of Optic Pathway/Hypothalamic Pilocytic Astrocytoma in Adolescents and Young Adults. Neurol. Med.-Chir. 2020, 60, 277–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Z.-Y.; Wang, X.-S.; Gong, Y.; Musyafar, O.L.A.; Yu, J.-J.; Huo, G.; Mou, J.-M.; Yang, G. Treatment with endoscopic transnasal resection of hypothalamic pilocytic astrocytomas: A single-center experience. BMC Surg. 2021, 21, 103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mair, M.J.; Wöhrer, A.; Furtner, J.; Simonovska, A.; Kiesel, B.; Oberndorfer, S.; Ungersböck, K.; Marosi, C.; Sahm, F.; Hainfellner, J.A.; et al. Clinical characteristics and prognostic factors of adult patients with pilocytic astrocytoma. J. Neuro-Oncol. 2020, 148, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Salles, D.; Laviola, G.; Malinverni, A.C.d.M.; Stávale, J.N. Pilocytic Astrocytoma: A Review of General, Clinical, and Molecular Characteristics. J. Child Neurol. 2020, 35, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Matyja, E.; Grajkowska, W.; Stępień, K.; Naganska, E. Heterogeneity of histopathological presentation of pilocytic astrocytoma–diagnostic pitfalls. A review. Folia Neuropathol. 2016, 3, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Murali, S.H.; Venkat, E.H.; Poyuran, R. Endoscopic Endonasal Transcavernous Posterior Clinoidectomy with Interdural Pituitary Transposition for a Suprasellar Optic Pathway Pilocytic Astrocytoma: 2-Dimensional Operative Video. Oper. Neurosurg. 2023, 24, e380. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Moreira, D.C.; Bag, A.K.; Qaddoumi, I.; Acharya, S.; Chiang, J. The clinical and molecular characteristics of progressive hypothalamic/optic pathway pilocytic astrocytoma. Neuro-Oncology 2023, 25, 750–760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaha, M.; Bouzayen, F.; Limam, Y.; Mokni, M.; Jemni-Gharbi, H.; Tlili-Graiess, K. Pilocytic astrocytoma mimicking cavernous angioma: Imaging features and histological characteristics. Neurochirurgie 2017, 63, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Lehman, N.L.; Sauvageau, E. A Pilocytic Astrocytoma Mimicking a Clinoidal Meningioma. Case Rep. Radiol. 2014, 2014, 524574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skipworth, J.R.; Hill, C.S.; Jones, T.; Foster, J.; Chopra, I.; Powell, M. Pilocytic astrocytoma mimicking craniopharyngioma: A case series. Ann. R. Coll. Surg. Engl. 2012, 94, e125–e128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dutta, G.; Singh, D.; Singh, H.; Sachdeva, D.; Kumar, V.; Chaturvedi, A. Pilocytic astrocytoma of the cerebellopontine angle mimicking vestibular schwannoma: Report of a rare entity. Br. J. Neurosurg. 2020, 34, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, S.; Martucci, M.; Russo, R.; Visconti, E.; Gangemi, E.; D’Argento, F.; Verdolotti, T.; Lauriola, L.; Colosimo, C. MR imaging of pilocytic brain astrocytoma: Beyond the stereotype of benign astrocytoma. Child’s Nerv. Syst. 2017, 33, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.J.; Leeds, N.E.; Kumar, V.A.; Fuller, G.N.; Lang, F.F.; Milas, Z.; Weinberg, J.S.; Ater, J.L.; Sawaya, R. Magnetic Resonance Imaging Features of Pilocytic Astrocytoma of the Brain Mimicking High-Grade Gliomas. J. Comput. Assist. Tomogr. 2010, 34, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Yamamoto, J.; Takahashi, M.; Soejima, Y.; Akiba, D.; Kitagawa, T.; Ueta, K.; Miyaoka, R.; Umemura, T.; Nishizawa, S. Pilocytic astrocytoma presenting with atypical features on magnetic resonance imaging. J. Neuroradiol. 2015, 42, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Li, Q.; Xu, D.; Xiu, W.; Zeng, Q.; Zhu, X.; Xu, F.; Jiang, B.; Zhang, M. Differentiation between pilocytic astrocytoma and glioblastoma: A decision tree model using contrast-enhanced magnetic resonance imaging-derived quantitative radiomic features. Eur. Radiol. 2019, 29, 3968–3975. [Google Scholar] [CrossRef] [PubMed]
- Vats, N.; Sengupta, A.; Gupta, R.K.; Patir, R.; Vaishya, S.; Ahlawat, S.; Saini, J.; Agarwal, S.; Singh, A. Differentiation of Pilocytic Astrocytoma from Glioblastoma using a Machine-Learning framework based upon quantitative T1 perfusion MRI. Magn. Reson. Imaging 2023, 98, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Bartels, U.; Hawkins, C.; Ma, J.; Ho, M.; Dirks, P.; Rutka, J.; Stephens, D.; Bouffet, E. Vascularity and angiogenesis as predictors of growth in optic pathway/hypothalamic gliomas. J. Neurosurg. Pediatr. 2006, 104, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Qin, X.; Jin, Z.; Jiang, Y.; Wang, Y. Epidemiology and Survival of Patients with Optic Pathway Gliomas: A Population-Based Analysis. Front. Oncol. 2022, 12, 789856. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Kim, D.; Eom, J.; Ahn, S.S.; Moon, J.H.; Kim, E.H.; Kang, S.G.; Chang, J.H.; Kim, S.H.; Lee, S.K. A diagnostic tree for differentiation of adult pilocytic astrocytomas from high-grade gliomas. Eur. J. Radiol. 2021, 143, 109946. [Google Scholar] [CrossRef] [PubMed]


| Author and Year | Study Design | Age | Sex | Neurological Presentation | Treatment | Diagnostic Method | Outcome | Follow Up | Techique |
|---|---|---|---|---|---|---|---|---|---|
| Valdueza et al., 1994 [13] | Original article | N/A | N/A | N/A | N/A | T1-weighted images show low-intensity masses with marked enhancement after administration of gadolinium | N/A | 4 year | Transcranial |
| Matsumoto et al., 1997 [14] | Case report | 45 | Male | Headache, bitemporal hemianopia | Subtotal | T1-isointense mass, high intensity center suggesting subacute hemorrhage, irregular contrast enhancement | Improved vision | 6 months (residual tumor stable) | Transcranial |
| Bakhoyor et al., 2000 [15] | Case report | 19 | Female | Headache, dizziness, lightheadedness | Partial resection | Densely and uniformly enhancing lesion in the right frontal horn of the lateral ventricle | None | 6 months (involution of the tumor) | Transcranial |
| Bakhoyor et al., 2000 [15] | Case report | 21 | Female | Right homonymous hemianopia | Partial resection | Heterogenous enhancing mass above the optic chiasm in the region of the hypothalamus | Improvement in visual deficit | 6 months (involution of the tumor) | Transcranial |
| De Divitiis et al., 2007 [16] | Original article Case series | 43 | Female | N/A | Subtotal | N/A | None | N/A | Endoscopic |
| Arbolay et al., 2009 [17] | Original article, case series | 42 | Male | Headache | Biopsy | N/A | Died due to meningitis | N/A | Endoscopic |
| Paluzzi et al., 2011 [18] | Technical note | 44 | Male | Headache, amenhorrea | Subtotal | Pituitary lesion attached to the pituitary stalk | Adrenal insufficiency and DI | N/A | Endoscopic |
| Zoli et al., 2014 [19] | Original article, case series | 38 | Male | Progressive visual loss, hypopituitarism | Gross total | Mixed and solid cystic mass extending within the ventricle and displacing the optic chiasm | CSF leak, DI, improvement in visual deficit | 17 months | Endoscopic |
| Zoli et al., 2014 [19] | Original article, case series | 42 | Male | Homonymous hemianopia | Subtotal | N/A | Improved vision, hypopituitarism, and DI | 81 months | Endoscopic |
| Zoli et al., 2014 [19] | Original article, case series | 23 | Female | Bitemporal hemianopia and acuity deficit | Biopsy+ Radiotherapy | Highly suggestive of high-grade glioma | Vision normalized, DI | 45 months | Endoscopic |
| Abou Al-Shaar et al., 2016 [20] | Case report | 30 | Male | Hypocortisolism, visual defect, | Partial Resection | Sellar– suprasellar solid and cystic lesions which displace the infundibulum | None | 6 months | Transcranial |
| Hidalgo et al., 2018 [21] | Case report | 50 | Female | Personality change, weight gain, incomplete right homonymous hemianopia | Gross total | Enhancing lesion along the optic tract with a small cystic component | Transient DI, improvement in visual field | 20 year | Transcranial |
| Bin Abdulquader et al., 2018 [22] | Original article, case series | 32 | Female | Hallucinations, nausea and vomiting | Subtotal | N/A | Transient DI, improvement in visual field | 7 months | Endoscopic |
| Shoji et al., 2020 [23] | Original article, case series | 18 | Male | Right hemianopia | Subtotal | N/A | None | 11 year | Transcranial |
| Shoji et al., 2020 [23] | Original article, case series | 19 | Female | Right upper quadrantanopia | Subtotal | N/A | None | 10 year | Transcranial |
| Shoji et al., 2020 [23] | Original article, case series | 26 | Female | Right lower quadrantanopia | Subtotal | Enhanced lesion in the optic chiasma extending to the third ventricle | None | 14 year | Transcranial |
| Shoji et al., 2020 [23] | Original article, case series | 36 | Female | None | Subtotal | N/A | None | 1 year | Transcranial |
| Zhou et al., 2021 [24] | Original article, case series | 20 | Female | Headache, amenhorrea, weight gain | Gross total | Suprasellar tumor with solid and cystic portions | Hyperprolactinemia, improved vision | 1 year | Endoscopic |
| Zhou et al., 2021 [24] | Original article, case series | 41 | Female | Amenhorrea, dizziness, memory deterioration | Gross total | Enhanced suprasellar, interpeduncular, and prepontine cistern lesion | Hypopituitarism, improved vision | 1 year | Endoscopic |
| Zhou et al., 2021 [24] | Original article, case series | 22 | Female | Menstrual disorder | Gross total | N/A | Hypopituitarism, no change in vision | 1 year | Endoscopic |
| Zhou et al., 2021 [24] | Original article, case series | 46 | Female | Bilateral visual disturbance, headache, amenhorrea | Gross total | Giant hypothalamic tumor with no clear margin between the tumor and hypothalamic structure | Dead (due to hypothalamus reaction) | 1 year | Endoscopic |
| Present case | / | 48 | Male | Third nerve palsy, stuporous state | Gross total | SWI_sequences with intralesional bleeding, microcalcification. Post-contrast administration revealed intense enhancement and increased perfusion indexes | Transient DI, hypopituitarism | 1 year | Transcranial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanzo, R.; Rosetti, V.; Tomassini, A.; Fuschillo, D.; Lofrese, G.; Iacopino, D.G.; Tosatto, L.; D’Andrea, M. Hypothalamic Hemangioma-like Pilocytic Astrocytoma in an Adult Patient: A Systematic Review with a Focus on Differential Diagnosis and Neurological Presentation. J. Clin. Med. 2024, 13, 3536. https://doi.org/10.3390/jcm13123536
Costanzo R, Rosetti V, Tomassini A, Fuschillo D, Lofrese G, Iacopino DG, Tosatto L, D’Andrea M. Hypothalamic Hemangioma-like Pilocytic Astrocytoma in an Adult Patient: A Systematic Review with a Focus on Differential Diagnosis and Neurological Presentation. Journal of Clinical Medicine. 2024; 13(12):3536. https://doi.org/10.3390/jcm13123536
Chicago/Turabian StyleCostanzo, Roberta, Vittoria Rosetti, Alessia Tomassini, Dalila Fuschillo, Giorgio Lofrese, Domenico Gerardo Iacopino, Luigino Tosatto, and Marcello D’Andrea. 2024. "Hypothalamic Hemangioma-like Pilocytic Astrocytoma in an Adult Patient: A Systematic Review with a Focus on Differential Diagnosis and Neurological Presentation" Journal of Clinical Medicine 13, no. 12: 3536. https://doi.org/10.3390/jcm13123536
APA StyleCostanzo, R., Rosetti, V., Tomassini, A., Fuschillo, D., Lofrese, G., Iacopino, D. G., Tosatto, L., & D’Andrea, M. (2024). Hypothalamic Hemangioma-like Pilocytic Astrocytoma in an Adult Patient: A Systematic Review with a Focus on Differential Diagnosis and Neurological Presentation. Journal of Clinical Medicine, 13(12), 3536. https://doi.org/10.3390/jcm13123536






