Aortic Stenosis and the Evolution of Cardiac Damage after Transcatheter Aortic Valve Replacement
Abstract
1. Introduction
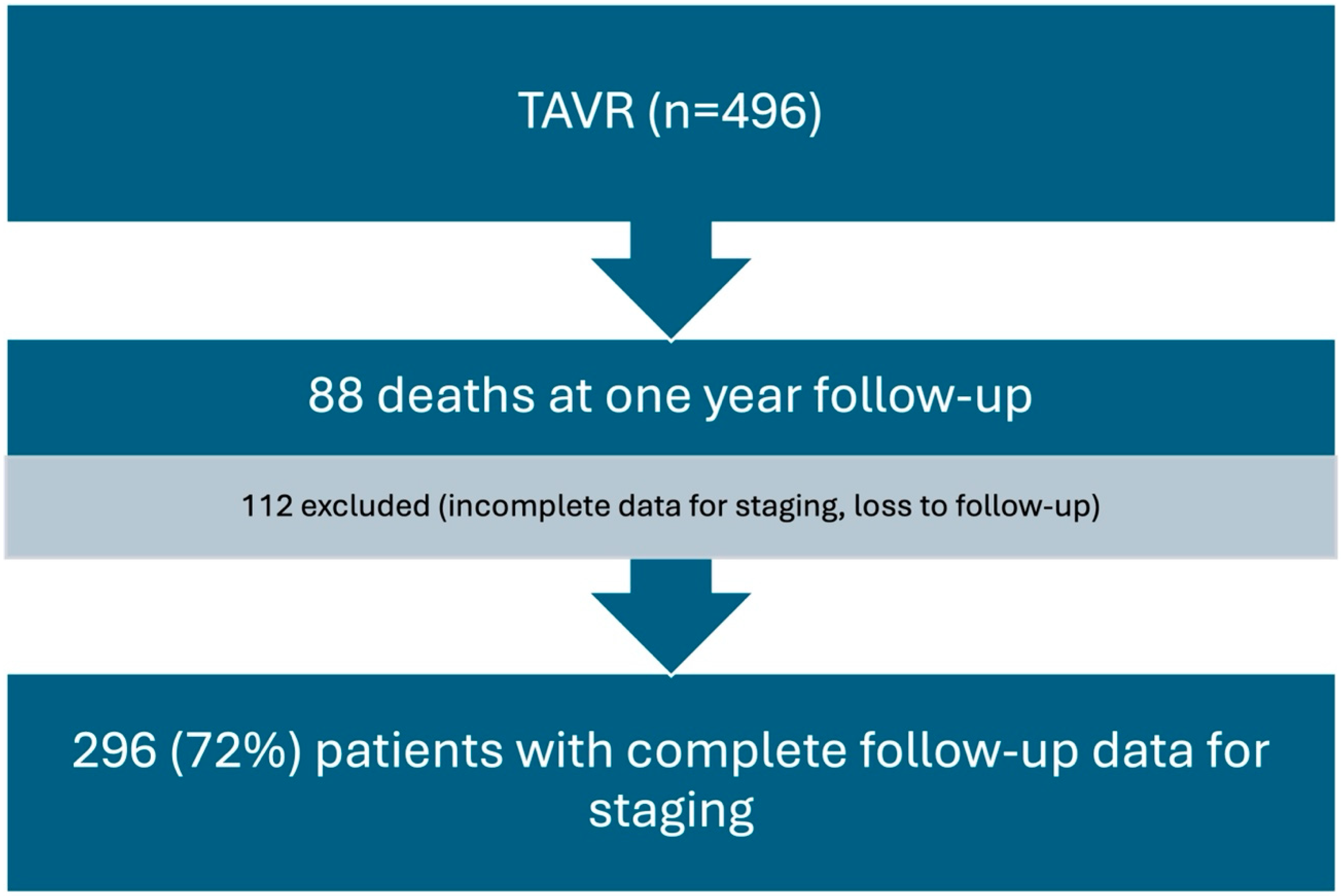
2. Methods
2.1. Design and Setting
2.2. Definitions, Outcome, and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Echocardiographic Characteristics
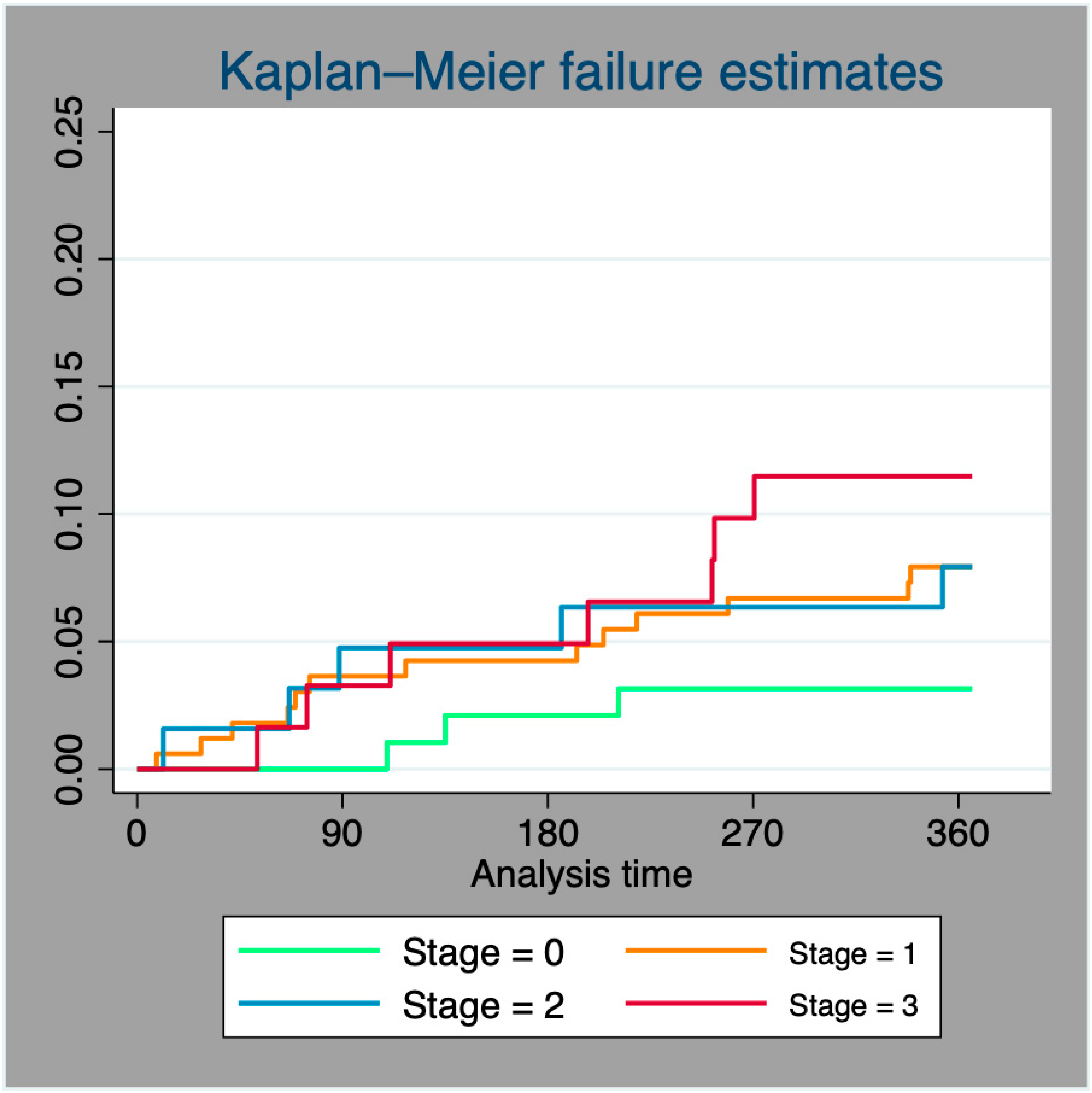
3.3. One-Year Mortality
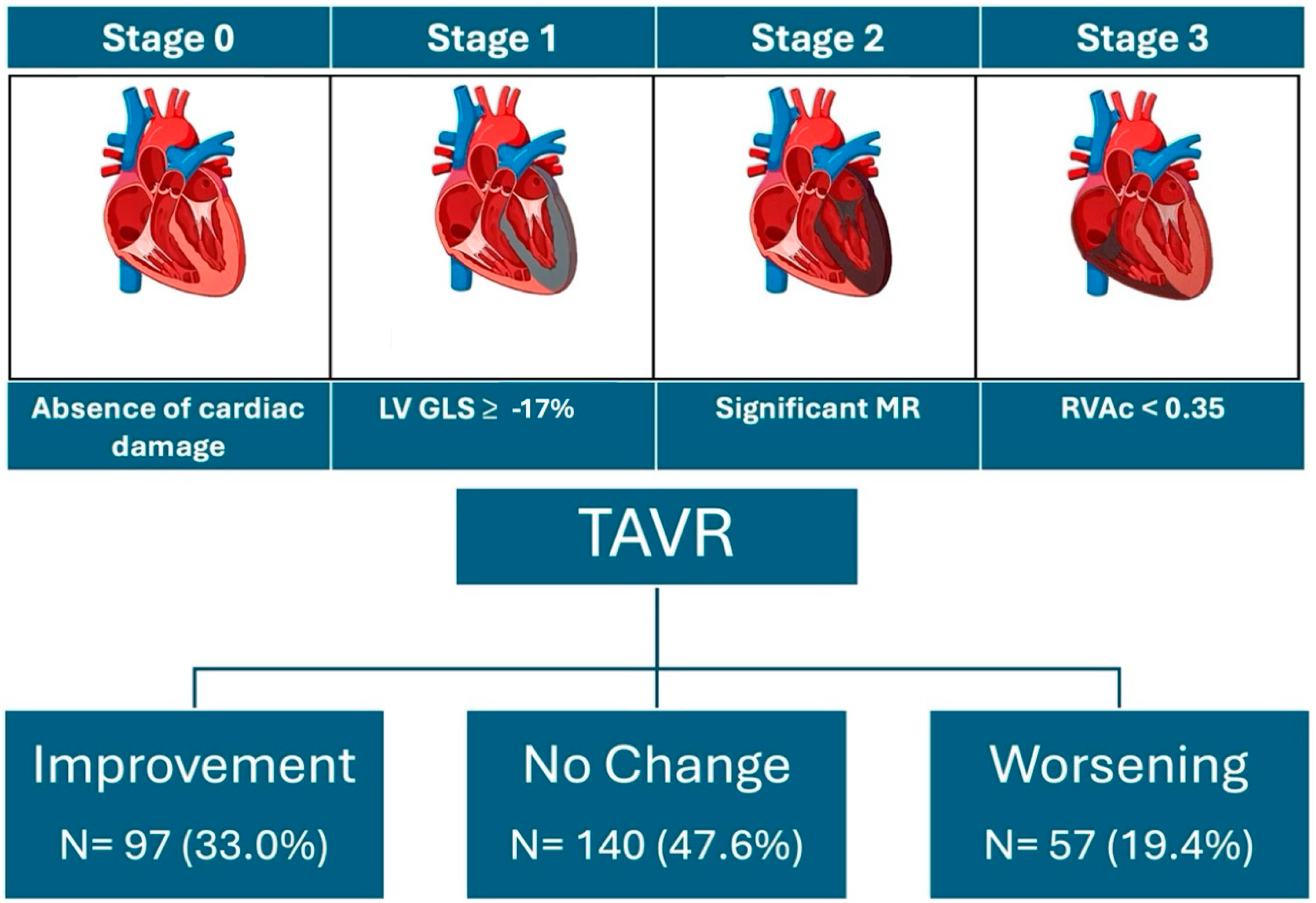
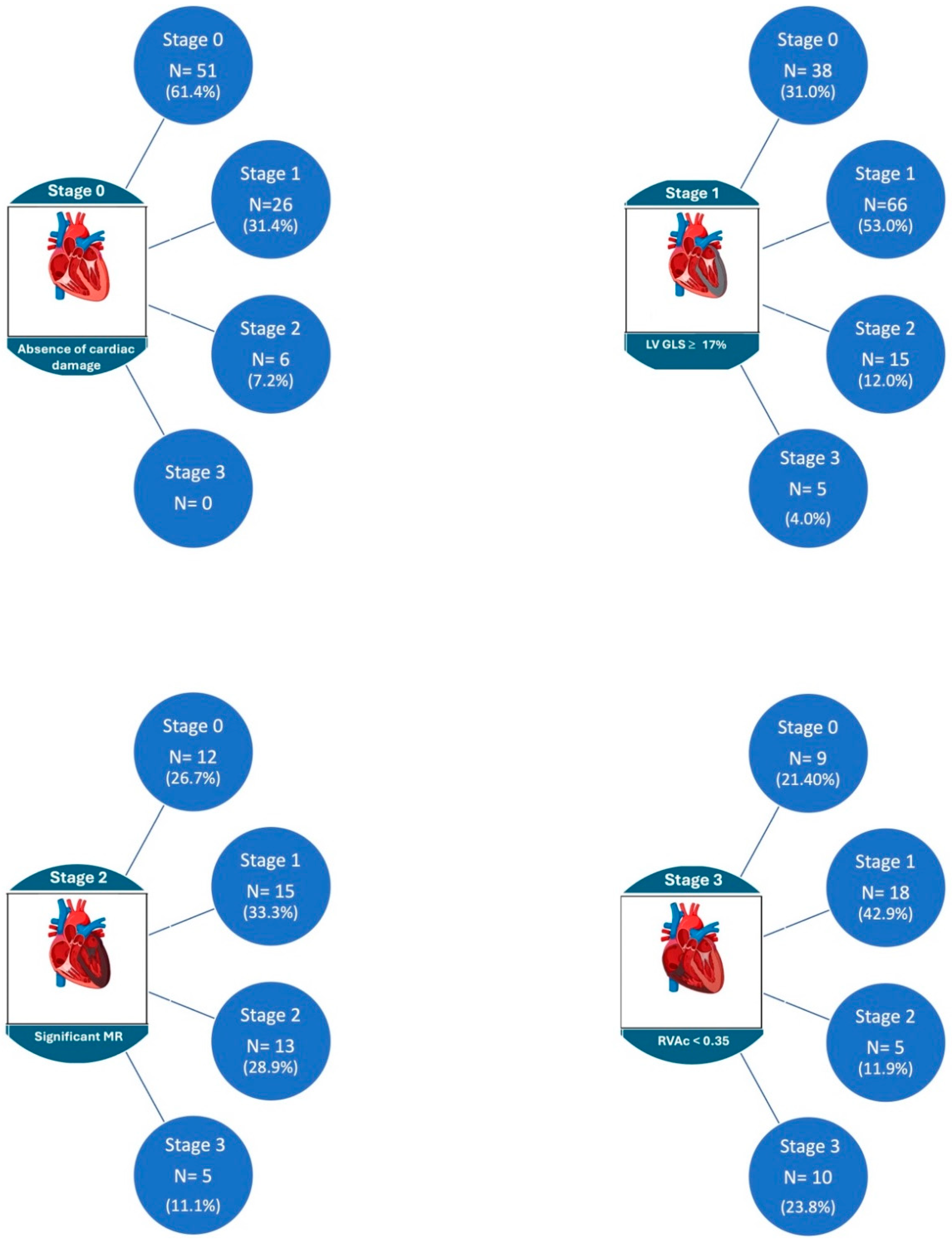
3.4. Evolution of Cardiac Damage at One-Year Follow-Up
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coffey, S.; Cairns, B.J.; Iung, B. The modern epidemiology of heart valve disease. Heart 2016, 102, 75–85. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; A Jaber, W.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Heg, D.; Lanz, J.; Praz, F.; Brugger, N.; Stortecky, S.; Windecker, S.; Pilgrim, T. Refined staging classification of cardiac damage associated with aortic stenosis and outcomes after transcatheter aortic valve implantation. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Ortiz, E.; Olmos, C.; Carrión-Sanchez, I.; Jiménez-Quevedo, P.; Nombela-Franco, L.; Párraga, R.; Gil-Abizanda, S.; Mahía, P.; Luaces, M.; de Agustín, J.A.; et al. Redefining cardiac damage staging in aortic stenosis: The value of GLS and RVAc. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Bax, J.J.; Zhao, Y.; Makkar, R.R.; Kapadia, S.; Thourani, V.H.; Mack, M.J.; Nazif, T.M.; et al. Evolution and Prognostic Impact of Cardiac Damage After Aortic Valve Replacement. J. Am. Coll. Cardiol. 2022, 80, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Han, X.; Chen, Y.; Chen, H.; Dong, S.; Song, B. Impact of Chronic Kidney Disease on the Prognosis of Transcatheter Aortic Valve Replacement in Patients with Aortic Stenosis: A Meta-Analysis of 133624 Patients. Ann. Thorac. Cardiovasc. Surg. 2022, 28, 83–95. [Google Scholar] [CrossRef]
- Gupta, T.; Goel, K.; Kolte, D.; Khera, S.; Villablanca, P.A.; Aronow, W.S.; Bortnick, A.E.; Slovut, D.P.; Taub, C.C.; Kizer, J.R.; et al. Association of Chronic Kidney Disease With In-Hospital Outcomes of Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 2050–2060. [Google Scholar] [CrossRef]
- Allende, R.; Webb, J.G.; Munoz-Garcia, A.J.; de Jaegere, P.; Tamburino, C.; Dager, A.E.; Cheema, A.; Serra, V.; Amat-Santos, I.; Velianou, J.L.; et al. Advanced chronic kidney disease in patients undergoing transcatheter aortic valve implantation: Insights on clinical outcomes and prognostic markers from a large cohort of patients. Eur. Heart J. 2014, 35, 2685–2696. [Google Scholar] [CrossRef]
- Gracia, E.; Wang, T.Y.; Callahan, S.; Bilfinger, T.; Tannous, H.; Pyo, R.; Kort, S.; Skopicki, H.; Weinstein, J.; Patel, N.; et al. Impact of Severity of Chronic Kidney Disease on Management and Outcomes Following Transcatheter Aortic Valve Replacement With Newer-Generation Transcatheter Valves. J. Invasive Cardiol. 2020, 32, 25–29. [Google Scholar]
- Matsumoto, S.; Ohno, Y.; Miyamoto, J.; Ikari, Y.; Tada, N.; Naganuma, T.; Yamawaki, M.; Yamanaka, F.; Shirai, S.; Mizutani, K.; et al. Impact of diabetes mellitus on outcome after transcatheter aortic valve replacement: Identifying high-risk diabetic population from the OCEAN-TAVI registry. Catheter. Cardiovasc. Interv. 2021, 98, E1058–E1065. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, Y.; Vemulapalli, S.; Chakravarty, T.; Li, Z.; Kapadia, S.; Holmes, D.; Matsouaka, R.A.; Wang, A.; Cheng, W.; Forrester, J.S.; et al. Clinical Impact of Diabetes Mellitus on Outcomes After Transcatheter Aortic Valve Replacement: Insights From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circ. Cardiovasc. Interv. 2017, 10, e005417. [Google Scholar] [CrossRef]
- Chorin, E.; Finkelstein, A.; Banai, S.; Aviram, G.; Barkagan, M.; Barak, L.; Keren, G.; Steinvil, A. Impact of Diabetes Mellitus and Hemoglobin A1C on Outcome After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2015, 116, 1898–1903. [Google Scholar] [CrossRef]
- Muratori, M.; Fusini, L.; Tamborini, G.; Ali, S.G.; Gripari, P.; Fabbiocchi, F.; Salvi, L.; Trabattoni, P.; Roberto, M.; Agrifoglio, M.; et al. Mitral valve regurgitation in patients undergoing TAVI: Impact of severity and etiology on clinical outcome. Int. J. Cardiol. 2020, 299, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Kiramijyan, S.; Magalhaes, M.A.; Koifman, E.; Didier, R.; Escarcega, R.O.; Minha, S.; Baker, N.C.; Negi, S.I.; Torguson, R.; Gai, J.; et al. Impact of baseline mitral regurgitation on short- and long-term outcomes following transcatheter aortic valve replacement. Am. Heart J. 2016, 178, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.; Aneni, E.; Escolar, E.; Mihos, C.G.; Xydas, S.; LaPietra, A.; Beohar, N.; Arenas, I.A. Tricuspid regurgitation and in-hospital outcomes after transcatheter aortic valve replacement in high-risk patients. J. Thorac. Dis. 2020, 12, 2963–2970. [Google Scholar] [CrossRef]
- Amat-Santos, I.J.; Castrodeza, J.; Nombela-Franco, L.; Muñoz-García, A.J.; Gutiérrez-Ibanes, E.; Hernández, J.M.d.l.T.; Córdoba-Soriano, J.G.; Jiménez-Quevedo, P.; Hernández-García, J.M.; González-Mansilla, A.; et al. Tricuspid but not Mitral Regurgitation Determines Mortality After TAVI in Patients With Nonsevere Mitral Regurgitation. Rev. Esp. Cardiol. 2018, 71, 357–364. [Google Scholar] [CrossRef]
- Alushi, B.; Beckhoff, F.; Leistner, D.; Franz, M.; Reinthaler, M.; Stähli, B.E.; Morguet, A.; Figulla, H.R.; Doenst, T.; Maisano, F.; et al. Pulmonary Hypertension in Patients With Severe Aortic Stenosis: Prognostic Impact After Transcatheter Aortic Valve Replacement: Pulmonary Hypertension in Patients Undergoing TAVR. JACC Cardiovasc. Imaging 2019, 12, 591–601. [Google Scholar] [CrossRef]
- Puehler, T.; Pommert, N.S.; Freitag-Wolf, S.; Seoudy, H.; Ernst, M.; Haneya, A.; Sathananthan, J.; Sellers, S.L.; Meier, D.; Schöttler, J.; et al. Tricuspid Regurgitation and TAVR: Outcomes, Risk Factors and Biomarkers. J. Clin. Med. 2024, 13, 1474. [Google Scholar] [CrossRef]
- Ito, S.; Pislaru, S.V.; Soo, W.M.; Huang, R.; Greason, K.L.; Mathew, V.; Sandhu, G.S.; Eleid, M.F.; Suri, R.M.; Oh, J.K.; et al. Impact of right ventricular size and function on survival following transcatheter aortic valve replacement. Int. J. Cardiol. 2016, 221, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Grevious, S.N.; Fernandes, M.F.; Annor, A.K.; Ibrahim, M.; Croix, G.R.S.; de Marchena, E.; Cohen, M.G.; Alfonso, C.E. Prognostic Assessment of Right Ventricular Systolic Dysfunction on Post-Transcatheter Aortic Valve Replacement Short-Term Outcomes: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e014463. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, X.; Yu, L.; Sun, Y.; Jaiswal, S.; Zhu, Q.; Chen, H.; He, Y.; Wang, L.; Ren, K.; et al. Impact of tricuspid regurgitation and right ventricular dysfunction on outcomes after transcatheter aortic valve replacement: A systematic review and meta-analysis. Clin. Cardiol. 2019, 42, 206–212. [Google Scholar] [CrossRef] [PubMed]
| Baseline Stage | Stage 0 | Stage 1 | Stage 2 | Stage 3 | p Value |
|---|---|---|---|---|---|
| Age, years | 81.8 ± 6.4 | 81.7 ± 5.8 | 82.2 ± 5.5 | 83.3 ± 5.6 | 0.301 |
| BMI (kg/m2) | 27.9 ± 4.7 | 28.0 ± 5.9 | 28.7 ± 7.1 | 28.0 ± 5.2 | 0.780 |
| HTA (%) | 24.0 | 42.6 | 17.3 | 16.0 | 0.836 |
| DM (%) | 27.3 | 41.7 | 16.6 | 14.4 | 0.738 |
| DLP (%) | 25.4 | 39.6 | 17.1 | 17.9 | 0.364 |
| CKD (%) | 17.6 | 37.2 | 21.6 | 23.5 | 0.017 |
| CAD (%) | 22.6 | 45.3 | 16.8 | 15.3 | 0.900 |
| Logistic EuroSCORE | 11.9 ± 7.2 | 17.5 ± 12.7 | 19.0 ± 14.9 | 26.4 ± 15.0 | <0.001 |
| LVEF % | 64.1 ± 5.1 | 55.4 ± 9.4 | 54.5 ± 13.2 | 53.1 ± 12.5 | <0.001 |
| LVEDV, mL/m2 | 51.3 ± 13.3 | 53.3 ± 22.9 | 58.0 ± 18.9 | 62.1 ± 24.3 | 0.003 |
| LVGLS, % | −17.8 ± 2.8 | −15.4 ± 4.2 | −15.3 ± 4.6 | −15.1 ± 42 | <0.001 |
| LV mass, g/m2 | 116.2 ± 25.0 | 127.2 ± 28.9 | 132.6 ± 34.5 | 140.7 ± 38.9 | <0.001 |
| LAVI, mL/m2 | 42.7 ± 16.3 | 46.1 ± 16.7 | 57.5 ± 20.4 | 66.5 ± 76.6 | <0.001 |
| E/e’ ratio | 13.8 ± 4.7 | 14.7 ± 6.3 | 17.3 ± 6.1 | 19.5 ± 6.5 | <0.001 |
| PASP, mmHg | 31.0 ± 10.3 | 31.9 ± 10.7 | 37.5 ± 12.5 | 57.2 ± 14.3 | <0.001 |
| TAPSE, mm | 22.5 ± 4.2 | 20.9 ± 4.0 | 20.6 ± 4.1 | 15.8 ± 3.8 | <0.001 |
| RVAc | 0.81 ± 0.3 | 0.74 ± 0.3 | 0.69 ± 0.3 | 0.28 ± 0.5 | <0.001 |
| Stage 1-Year FU | Stage 0 | Stage 1 | Stage 2 | Stage 3 | p Value |
|---|---|---|---|---|---|
| LVEF % | 61.4 ± 5.5 | 58.2 ± 9.3 | 56.6 ± 9.0 | 57.7 ± 8.9 | <0.001 |
| LVEDV, mL/m2 | 49.6 ± 14.9 | 50.8 ± 15.5 | 52.1 ± 21.1 | 56.0 ± 21.7 | 0.223 |
| LV mass, gr/m2 | 110.7 ± 31.8 | 111.4 ± 31.0 | 113.8 ± 26.6 | 120.6 ± 26.1 | 0.307 |
| LVGLS, % | −17.8 ± 2.8 | −15.4 ± 4.2 | −15.3 ± 4.6 | −15.1 ± 42 | <0.001 |
| LAVI, mL/m2 | 41.4 ± 16.1 | 43.9 ± 17.8 | 55.3 ± 21.7 | 71.0 ± 74.4 | <0.001 |
| E/e’ ratio | 13.8 ± 5.1 | 14.6 ± 5.5 | 15.8 ± 5.4 | 15.9 ± 4.7 | 0.284 |
| PASP, mmHg | 26.9 ± 7.4 | 28.5 ± 11.3 | 31.5 ± 13.3 | 37.3 ± 13.6 | <0.001 |
| TAPSE, mm | 21.8 ± 4.1 | 20.4 ± 3.9 | 20.7 ± 4.6 | 18.4 ± 4.5 | <0.001 |
| RVAc | 0.87 ± 0.3 | 0.81 ± 0.3 | 0.77 ± 0.3 | 0.58 ± 0.3 | <0.001 |
| Univariable Logistic Regression Analysis | Multivariable Logistic Regression Analysis | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | |
| HTA | 1.49 (0.76–2.93) | 0.236 | ||
| DM | 1.55 (1.14–2.17) | 0.014 | 1.38 (1.09–2.48) | 0.047 |
| DLP | 1.27 (0.74–2.17) | 0.374 | ||
| CKD | 2.14 (1.20–3.79) | 0.010 | 2.19 (1.15–4.20) | 0.024 |
| CAD | 1.58 (0.93–2.67) | 0.092 | ||
| EuroSCORE | 1.00 (0.99–1.02) | 0.599 | ||
| LVEF | 1.02 (1.00–1.06) | 0.029 | ||
| GLS | 0.92 (0.86–0.98) | 0.015 | ||
| MR ≥ 2 | 1.83 (1.01–3.32) | 0.047 | 3.24 (1.07–9.87) | <0.001 |
| TR ≥ 2 | 1.73 (1.36–3.18) | 0.038 | 2.33 (1.01–3.99) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islas, F.; O’Neill-González, P.; Jiménez-Quevedo, P.; Nombela-Franco, L.; Gil-Abizanda, S.; Mahía-Casado, P.; Rivadeneira-Ruiz, M.; Pozo-Osinalde, E.; Carbone, A.; Olmos, C. Aortic Stenosis and the Evolution of Cardiac Damage after Transcatheter Aortic Valve Replacement. J. Clin. Med. 2024, 13, 3539. https://doi.org/10.3390/jcm13123539
Islas F, O’Neill-González P, Jiménez-Quevedo P, Nombela-Franco L, Gil-Abizanda S, Mahía-Casado P, Rivadeneira-Ruiz M, Pozo-Osinalde E, Carbone A, Olmos C. Aortic Stenosis and the Evolution of Cardiac Damage after Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine. 2024; 13(12):3539. https://doi.org/10.3390/jcm13123539
Chicago/Turabian StyleIslas, Fabián, Patrick O’Neill-González, Pilar Jiménez-Quevedo, Luis Nombela-Franco, Sandra Gil-Abizanda, Patricia Mahía-Casado, María Rivadeneira-Ruiz, Eduardo Pozo-Osinalde, Andreina Carbone, and Carmen Olmos. 2024. "Aortic Stenosis and the Evolution of Cardiac Damage after Transcatheter Aortic Valve Replacement" Journal of Clinical Medicine 13, no. 12: 3539. https://doi.org/10.3390/jcm13123539
APA StyleIslas, F., O’Neill-González, P., Jiménez-Quevedo, P., Nombela-Franco, L., Gil-Abizanda, S., Mahía-Casado, P., Rivadeneira-Ruiz, M., Pozo-Osinalde, E., Carbone, A., & Olmos, C. (2024). Aortic Stenosis and the Evolution of Cardiac Damage after Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine, 13(12), 3539. https://doi.org/10.3390/jcm13123539







