Outcomes and Impact of Pre-ECMO Clinical Course in Severe COVID-19-Related ARDS Treated with VV-ECMO: Data from an Italian Referral ECMO Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients and ECMO Management
2.3. Outcomes and Data Collection
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Supportive Care and Medications Delivered before ECMO Initiation
3.3. Pre-ECMO Ventilator Setting and Arterial Blood Gases
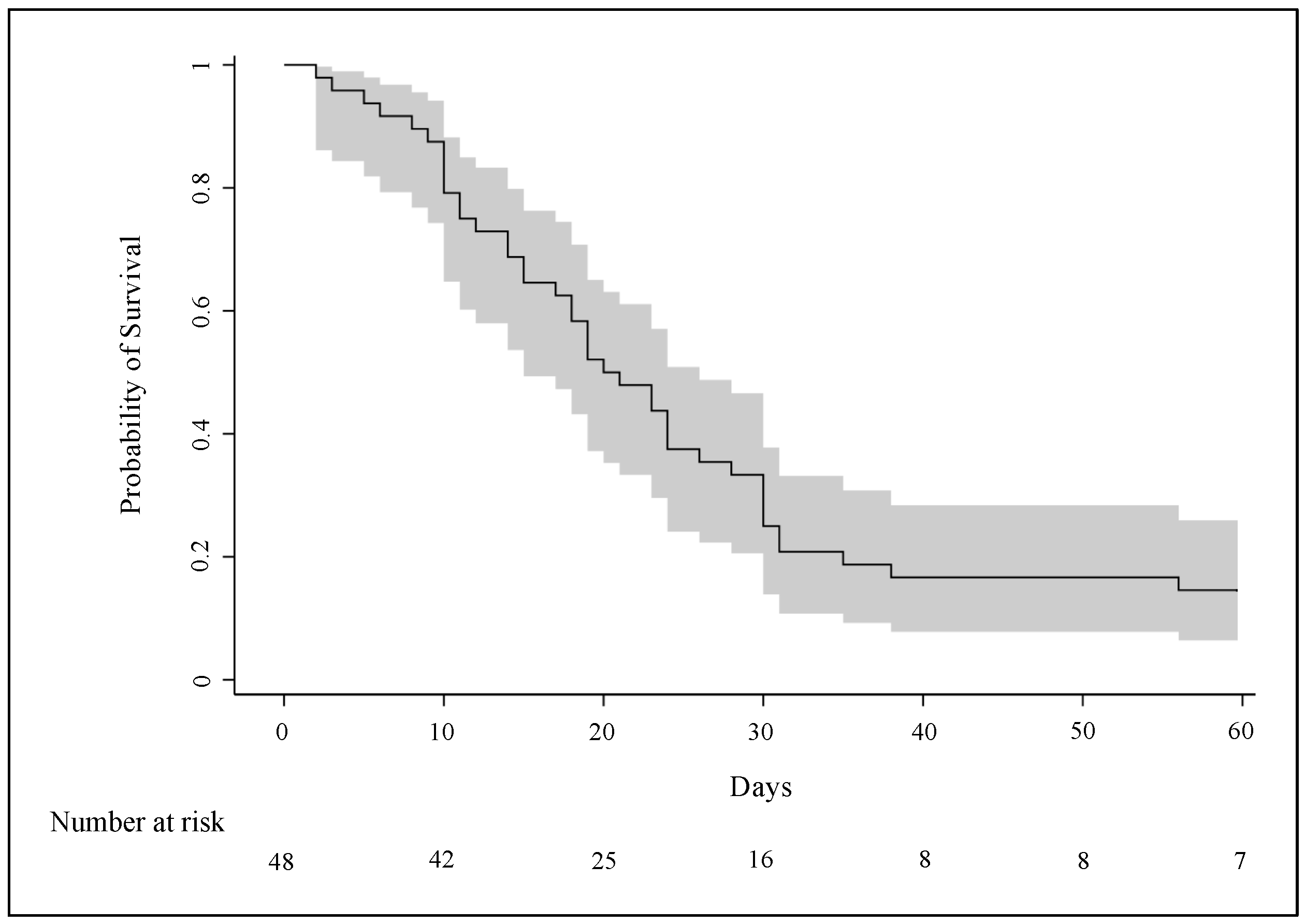
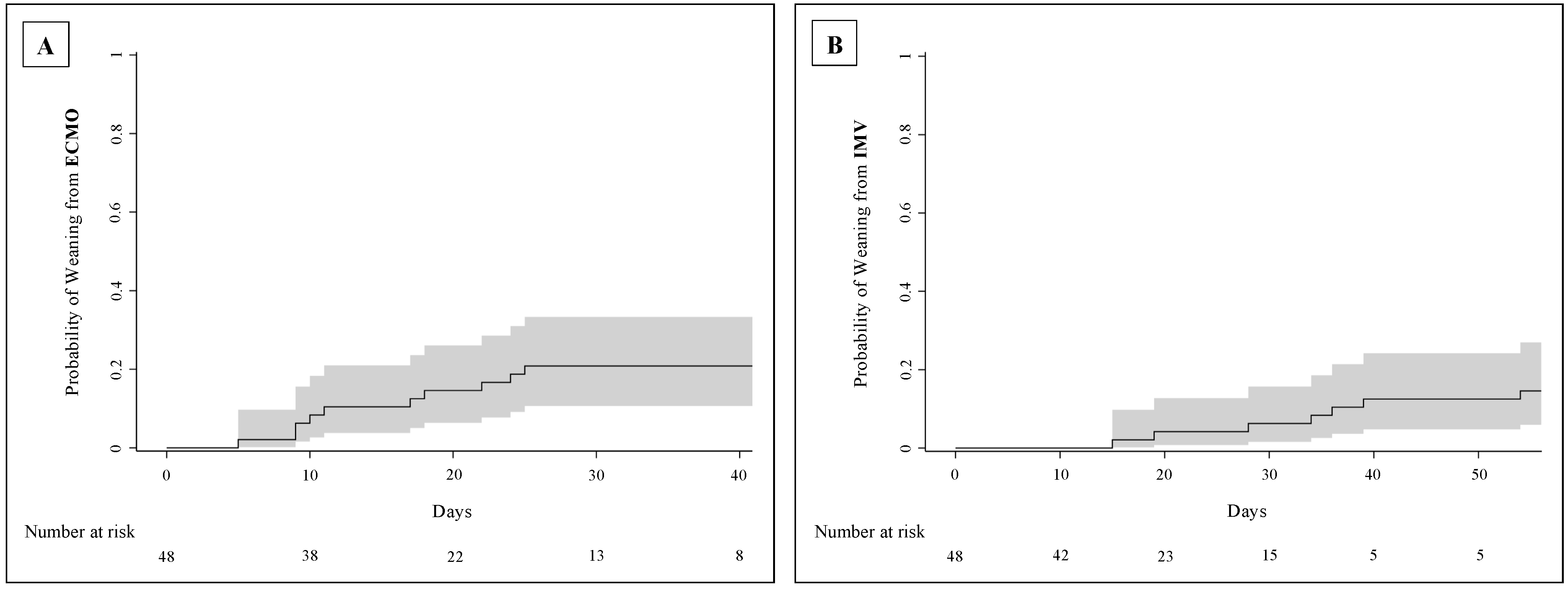
3.4. Clinical Outcomes
3.5. Complications after ECMO Initiation
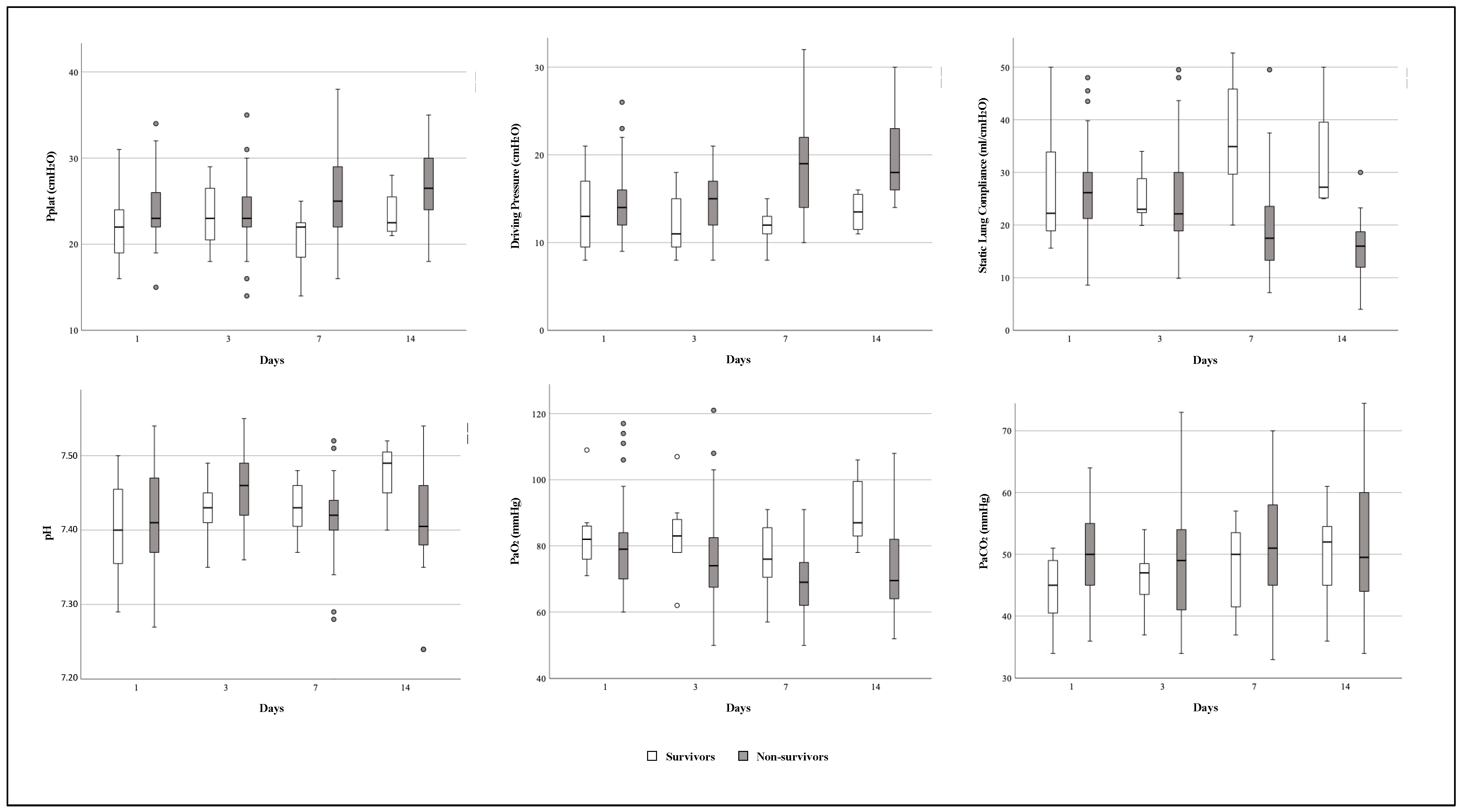
3.6. Time Course of Respiratory Mechanics and Arterial Blood Gases Variables during ECMO Support
3.7. Differences between Patients Treated in the First and Subsequent Waves of COVID-19 Outbreak
4. Discussion
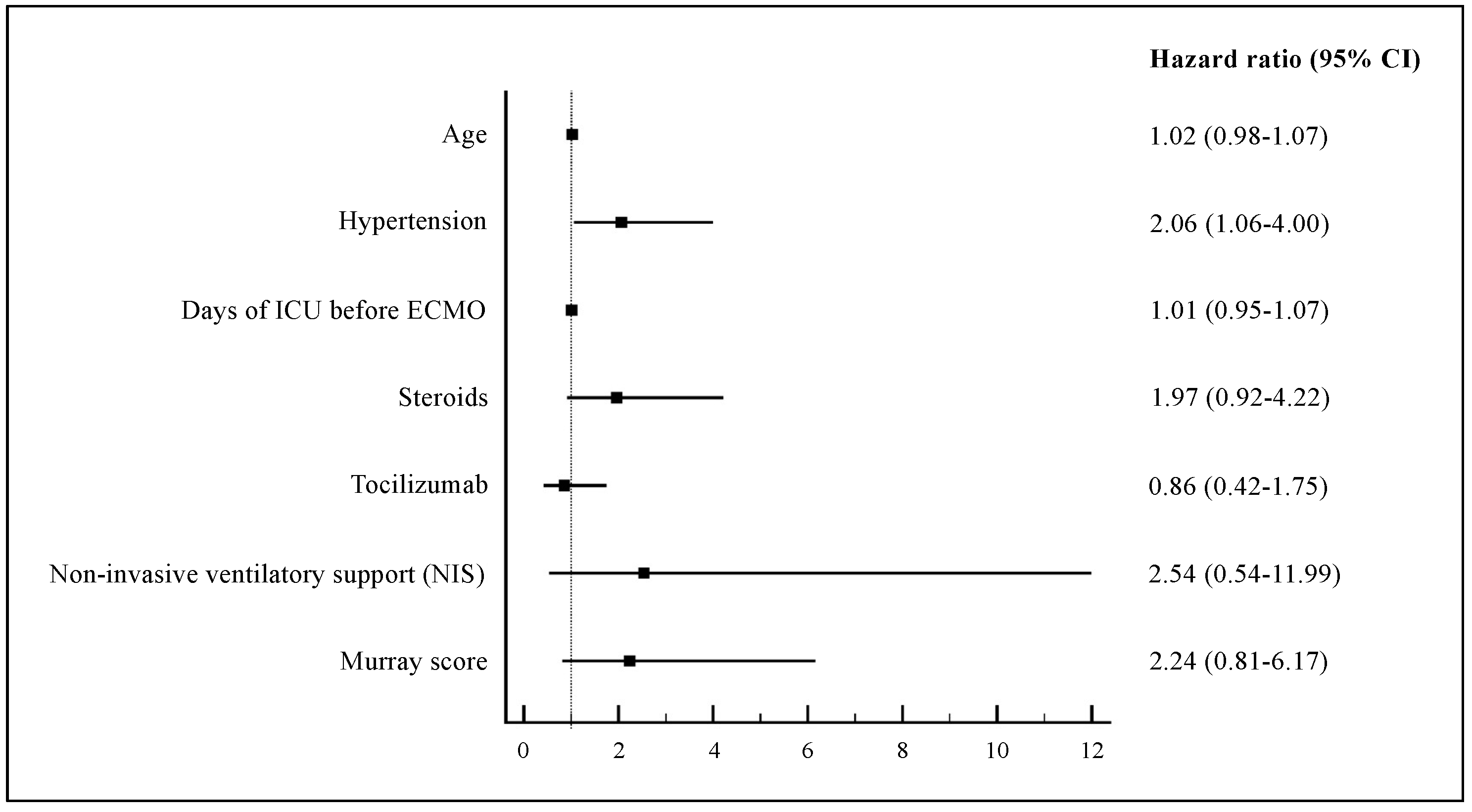
4.1. In-Hospital Mortality and Pre-ECMO Clinical Characteristics
4.2. Supportive Cares and Medications before ECMO Implantation
4.3. Complications after ECMO Initiation
4.4. Length of ECMO Support and Other Clinical Outcomes
4.5. Evolution of Respiratory Mechanics during ECMO Support
4.6. Patient Management in First Versus Subsequent Waves of COVID-19 Outbreak
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combes, A.; Schmidt, M.; Hodgson, C.L.; Fan, E.; Ferguson, N.D.; Fraser, J.F.; Jaber, S.; Pesenti, A.; Ranieri, M.; Rowan, K.; et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensiv. Care Med. 2020, 46, 2464–2476. [Google Scholar] [CrossRef]
- Bertini, P.; Guarracino, F.; Falcone, M.; Nardelli, P.; Landoni, G.; Nocci, M.; Paternoster, G. ECMO in COVID-19 Patients: A Systematic Review and Meta-analysis. Cardiothorac. Vasc. Anesth. 2022, 36, 2700–2706. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): Pooled analysis of early reports. J. Crit. Care 2020, 58, 27–28. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, G.; Fisher, D.; Brodie, D. Treating the Most Critically Ill Patients With COVID-19: The Evolving Role of Extracorporeal Membrane Oxygenation. JAMA 2022, 327, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.; Murdoch, D.R. Clinical course and mortality risk of severe COVID-19. Lancet 2020, 395, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Badulak, J.; Antonini, M.V.; Stead, C.M.; Shekerdemian, L.; Raman, L.; Paden, M.L.; Agerstrand, C.; Bartlett, R.H.; Barrett, N.; Combes, A.; et al. Extracorporeal Membrane Oxygenation for COVID-19: Updated 2021 Guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021, 67, 485–495. [Google Scholar] [CrossRef]
- World Health Organization. Living Guidance for Clinical Management of COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 14 April 2022).
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensiv. Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef]
- Patroniti, N.; Zangrillo, A.; Pappalardo, F.; Peris, A.; Cianchi, G.; Braschi, A.; Iotti, G.A.; Arcadipane, A.; Panarello, G.; Ranieri, V.M.; et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: Preparation for severe respiratory emergency outbreaks. Intensiv. Care Med. 2011, 37, 1447–1457. [Google Scholar] [CrossRef]
- Montrucchio, G.; Sales, G.; Urbino, R.; Simonetti, U.; Bonetto, C.; Cura Stura, E.; Simonato, E.; Fuoco, G.; Fanelli, V.; Brazzi, L. ECMO Support and Operator Safety in the Context of COVID-19 Outbreak: A Regional Center Experience. Membranes 2021, 11, 334. [Google Scholar] [CrossRef]
- Extracorporeal Life Support Organization (ELSO). ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support. Available online: https://www.elso.org (accessed on 31 January 2023).
- Extracorporeal Life Support Organization (ELSO). ELSO Anticoagulation Guidelines. Available online: https://www.elso.org/Resources/Guidelines.aspx (accessed on 31 January 2023).
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Rozencwajg, S.; Guihot, A.; Franchineau, G.; Lescroat, M.; Bréchot, N.; Hékimian, G.; Lebreton, G.; Autran, B.; Luyt, C.E.; Combes, A.; et al. Ultra-Protective Ventilation Reduces Biotrauma in Patients on Venovenous Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. Crit. Care Med. 2019, 47, 1505–1512. [Google Scholar] [CrossRef]
- Abrams, D.; Schmidt, M.; Pham, T.; Beitler, J.R.; Fan, E.; Goligher, E.C.; McNamee, J.J.; Patroniti, N.; Wilcox, M.E.; Combes, A.; et al. Mechanical Ventilation for Acute Respiratory Distress Syndrome during Extracorporeal Life Support. Research and Practice. Am. J. Respir. Crit. Care Med. 2020, 201, 514–525. [Google Scholar] [CrossRef]
- Goligher, E.C.; Tomlinson, G.; Hajage, D.; Wijeysundera, D.N.; Fan, E.; Jüni, P.; Brodie, D.; Slutsky, A.S.; Combes, A. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome and Posterior Probability of Mortality Benefit in a Post Hoc Bayesian Analysis of a Randomized Clinical Trial. JAMA 2018, 320, 2251–2259. [Google Scholar] [CrossRef]
- Ramanathan, K.; Shekar, K.; Ling, R.R.; Barbaro, R.P.; Wong, S.N.; Tan, C.S.; Rochwerg, B.; Fernando, S.M.; Takeda, S.; MacLaren, G.; et al. Extracorporeal membrane oxygenation for COVID-19: A systematic review and meta-analysis. Crit. Care 2021, 25, 211. [Google Scholar] [CrossRef] [PubMed]
- ISARIC Clinical Characterisation Group. COVID-19 symptoms at hospital admission vary with age and sex: Results from the ISARIC prospective multinational observational study. Infection 2021, 49, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Iwashyna, T.J.; Slutsky, A.S.; Fan, E.; Bartlett, R.H.; Tonna, J.E.; Hyslop, R.; Fanning, J.J.; et al. Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020, 396, 1071–1078. [Google Scholar] [CrossRef]
- Lorusso, R.; Combes, A.; Lo Coco, V.; De Piero, M.E.; Belohlavek, J.; EuroECMO COVID-19 WorkingGroup; Euro-ELSO Steering Committee. ECMO for COVID-19 patients in Europe and Israel. Intensiv. Care Med. 2021, 47, 344–348. [Google Scholar] [CrossRef]
- Lebreton, G.; Schmidt, M.; Ponnaiah, M.; Folliguet, T.; Para, M.; Guihaire, J.; Lansac, E.; Sage, E.; Cholley, B.; Mégarbane, B.; et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: A multicentre cohort study. Lancet Respir. Med. 2021, 9, 851–862. [Google Scholar] [CrossRef]
- Kunavarapu, C.; Yeramaneni, S.; Melo, J.; Sterling, R.K.; Huskey, L.C.; Sears, L.; Burch, C.; Rodriguez, S.M.; Habib, P.J.; Triana, F.; et al. Clinical outcomes of severe COVID-19 patients receiving early VV-ECMO and the impact of pre-ECMO ventilator use. Int. J. Artif. Organs 2021, 44, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Rabie, A.A.; Azzam, M.H.; Al-Fares, A.A.; Abdelbary, A.; Mufti, H.N.; Hassan, I.F.; Chakraborty, A.; Oza, P.; Elhazmi, A.; Alfoudri, H.; et al. Implementation of new ECMO centers during the COVID-19 pandemic: Experience and results from the Middle East and India. Intensiv. Care Med. 2021, 47, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hajage, D.; Landoll, M.; Pequignot, B.; Langouet, E.; Amalric, M.; Mekontso-Dessap, A.; Chiscano-Camon, L.; Surman, K.; Finnerty, D.; et al. Comparative outcomes of extracorporeal membrane oxygenation for COVID-19 delivered in experienced European centres during successive SARS-CoV-2 variant outbreaks (ECMO-SURGES): An international, multicentre, retrospective cohort study. Lancet Respir. Med. 2023, 11, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.H.; Saha, B.K.; Medarov, B.I. Clinical Characteristics Between Survivors and Nonsurvivors of COVID-19 Patients Requiring Extracorporeal Membrane Oxygenation (ECMO) Support: A Systematic Review and Meta-Analysis. J. Intensiv. Care Med. 2022, 37, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Calvez, V.; Savoia, C. Hypertension and COVID-19: Current Evidence and Perspectives. High Blood Press. Cardiovasc. Prev. 2022, 29, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, V.; Giani, M.; Grasselli, G.; Mojoli, F.; Martucci, G.; Grazioli, L.; Alessandri, F.; Mongodi, S.; Sales, G.; Montrucchio, G.; et al. Extracorporeal membrane oxygenation for COVID-19 and influenza H1N1 associated acute respiratory distress syndrome: A multicenter retrospective cohort study. Crit. Care 2022, 26, 34. [Google Scholar] [CrossRef]
- Fan, E.; Beitler, J.R.; Brochard, L.; Calfee, C.S.; Ferguson, N.D.; Slutsky, A.S.; Brodie, D. COVID-19-associated acute respiratory distress syndrome: Is a different approach to management warranted? Lancet Respir. Med. 2020, 8, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Botta, M.; Tsonas, A.M.; Pillay, J.; Boers, L.S.; Algera, A.G.; Bos, L.D.J.; Dongelmans, D.A.; Hollmann, M.W.; Horn, J.; Vlaar, A.P.J.; et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): A national, multicentre, observational cohort study. Lancet Respir. Med. 2021, 9, 139–148. [Google Scholar] [CrossRef] [PubMed]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensiv. Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329–2330. [Google Scholar] [CrossRef]
- Boscolo, A.; Pasin, L.; Sella, N.; Pretto, C.; Tocco, M.; Tamburini, E.; Rosi, P.; Polati, E.; Donadello, K.; Gottin, L.; et al. Outcomes of COVID-19 patients intubated after failure of non-invasive ventilation: A multicenter observational study. Sci. Rep. 2021, 11, 17730. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Q.; Green, A.; Chandel, A.; Lantry, J.; Desai, M.; Simou, J.; Osborn, E.; Singh, R.; Puri, N.; Moran, P.; et al. Impact of Noninvasive Respiratory Support in Patients With COVID-19 Requiring V-V ECMO. ASAIO J. 2022, 68, 171–177. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [PubMed]
- Fanelli, V.; Montrucchio, G.; Sales, G.; Simonetti, U.; Bonetto, C.; Rumbolo, F.; Mengozzi, G.; Urbino, R.; Pizzi, C.; Richiardi, L.; et al. Effects of Steroids and Tocilizumab on the Immune Response Profile of Patients with COVID-19-Associated ARDS Requiring or Not Veno-Venous Extracorporeal Membrane Oxygenation. Membranes 2021, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Yusuff, H.; Zochios, V.; Brodie, D. Thrombosis and coagulopathy in COVID-19 patients rceiving ECMO: A narrative review of current literature. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3312–3317. [Google Scholar] [CrossRef] [PubMed]
- Shih, E.; DiMaio, J.M.; Squiers, J.J.; Banwait, J.K.; Kussman, H.M.; Meyers, D.P.; Meidan, T.G.; Sheasby, J.; George, T.J. Bloodstream and respiratory coinfections in patients with COVID-19 on ECMO. J Card Surg. 2022, 37, 3609–3618. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Heinsar, S.; Jeong, I.S.; Shekar, K.; Bassi, G.L.; Jung, J.S.; Suen, J.Y.; Fraser, J.F. ECMO use in COVID-19: Lessons from past respiratory virus outbreaks-a narrative review. Crit. Care 2020, 24, 301. [Google Scholar] [CrossRef] [PubMed]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Combes, A.; Agerstrand, C.; Annich, G.; Diaz, R.; Fan, E.; Hryniewicz, K.; Lorusso, R.; et al. Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet 2021, 398, 1230–1238. [Google Scholar] [CrossRef]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensiv. Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef]
- Smith, D.E.; Chang, S.H.; Geraci, T.C.; James, L.; Kon, Z.N.; Carillo, J.A.; Alimi, M.; Williams, D.; Scheinerman, J.A.; Cerfolio, R.J.; et al. One-Year Outcomes with Venovenous Extracorporeal Membrane Oxygenation Support for Severe COVID-19. Ann. Thorac. Surg. 2022, 114, 70–75. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 48) | Survivors (n = 7) | Non-Survivors (n = 41) | p-Value |
|---|---|---|---|---|
| Age, years, median (IQR) | 54 (49–61) | 50 (48–53) | 55 (50–61) | 0.457 |
| Sex, male, n (%) | 37 (77.1) | 6 (85.7) | 31 (75.6) | 0.557 |
| BMI, kg/m2, median (IQR) | 29.6 (27.5–33.9) | 31.8 (28.4–34.7) | 29.4 (27.1–33.3) | 0.430 |
| Pre-existing comorbidities: | ||||
| No comorbidity, n (%) | 7 (14.6) | 2 (28.6) | 5 (12.2) | 0.267 |
| Obesity, n (%) | 23 (47.9) | 4 (57.1) | 19 (46.3) | 0.696 |
| Active smoking, n (%) | 8 (16.7) | 1 (14.3) | 7 (17.1) | 0.855 |
| Hypertension, n (%) | 21 (43.8) | 1 (14.3) | 20 (48.8) | 0.089 |
| CAD, n (%) | 1 (2.1) | 0 | 1 (2.4) | 1.000 |
| Lung disease (asthma/COPD), n (%) | 6 (12.5) | 0 | 6 (14.6) | 0.279 |
| Diabetes mellitus, n (%) | 7 (14.6) | 0 | 7 (17.1) | 0.237 |
| Hypothyroidism, n (%) | 4 (8.3) | 0 | 4 (9.8) | 1.000 |
| Chronic immunosuppression, n (%) | 1 (2.1) | 0 | 1 (2.4) | 1.000 |
| Severity score at ECMO implantation: | ||||
| SOFA | 10 (8–12) | 12 (7–12) | 10 (8–12) | 0.309 |
| APACHE II | 24 (19–26) | 25 (20–26) | 24 (19–25) | 0.499 |
| Pre-existing bacterial co-infection, n (%) | 19 (39.6) | 2 (28.6) | 17 (41.5) | 0.687 |
| Days from COVID-19 onset to ECMO, median (IQR) | 17 (12–22) | 15 (13–19) | 17 (11–22) | 0.835 |
| Days from hospitalization to ECMO, median (IQR) | 12 (7–17) | 11 (6–12) | 12 (8–17) | 0.412 |
| Days of ICU before ECMO, median (IQR) | 7 (3–11) | 4 (2–8) | 7 (4–11) | 0.151 |
| Mobile ECMO, n (%) | 41 (85.4) | 7 (100) | 34 (82.9) | 0.237 |
| Non-invasive ventilatory support (NIS) before intubation, n (%): | 43 (89.6) | 4 (57.1) | 39 (95.1) | 0.018 |
| HFNC, n (%) | 6 (12.5) | 0 | 6 (14.6) | |
| CPAP, n (%) | 34 (70.8) | 3 (42.9) | 31 (75.6) | |
| NIV, n (%) | 15 (31.3) | 2 (28.6) | 13(31.7) | |
| Days of NIS, median (IQR) | 5 (3–10) | 6 (0–10) | 5 (3–9) | 0.519 |
| Days of invasive mechanical ventilation (IMV), median (IQR) | 5 (3–8) | 4 (2–7) | 5 (3–8) | 0.527 |
| Total days of ventilation (NIS + IMV), median (IQR) | 11 (8–15) | 8 (7–13) | 11 (8–15) | 0.355 |
| Rescue therapies: | ||||
| LRM, n (%) | 23 (47.9) | 3 (42.9) | 20 (48.8) | 1.000 |
| Pronation, n (%) | 43 (89.6) | 6 (85.7) | 37 (90.2) | 0.562 |
| iNO, n (%) | 17 (35.4) | 3 (42.9) | 14 (34.1) | 0.686 |
| COVID-19-targeted therapies: | ||||
| Steroids, n (%) | 32 (66.7) | 2 (28.6) | 30 (73.2) | 0.033 |
| Tocilizumab, n (%) | 13 (27.1) | 0 | 13 (31.7) | 0.081 |
| Hyperimmune plasma, n (%) | 6 (12.5) | 1 (14.3) | 5 (12.2) | 1.000 |
| Remdesevir, n (%) | 14 (29.2) | 2 (28.6) | 12 (29.3) | 1.000 |
| Pre-ECMO ventilator setting and arterial blood gases: | ||||
| TV/PBW, mL/kg, median (IQR) | 6.7 (6.1–7.4) | 6.3 (6.0–7.0) | 6.7 (6.1–7.5) | 0.143 |
| RR, breaths/min, median (IQR) | 28 (24–30) | 30 (28–35) | 26 (24–30) | 0.286 |
| PEEP, cmH2O, median (IQR) | 12 (10–14) | 10 (8–12) | 12 (10–14) | 0.311 |
| FiO2, %, median (IQR) | 100 (100–100) | 100 (85–100) | 100 (100–100) | 0.506 |
| Pplat, cmH2O, median (IQR) | 28 (23–30) | 29 (25–31) | 28 (23–30) | 0.700 |
| DP, cmH2O, median (IQR) | 16 (12–19) | 17 (12–22) | 16 (12–19) | 0.820 |
| Static lung compliance, mL/cmH2O, median (IQR) | 29.2 (23.2–37.8) | 28.5 (19–40.3) | 29.2 (23.2–37.8) | 0.703 |
| pH, median (IQR) | 7.34 (7.30–7.40) | 7.33 (7.26–7.37) | 7.34 (7.31–7.41) | 0.433 |
| PaO2/FiO2, mmHg, median (IQR) | 64 (55–72) | 64 (57–71) | 64 (53–72) | 0.941 |
| PaCO2, mmHg, median (IQR) | 59 (54–68) | 55.4 (54–61) | 59 (54–68) | 0.353 |
| HCO−3, mmol/L, median (IQR) | 33 (29–36) | 31 (26–36) | 33 (30–36) | 0.517 |
| Murray score, median (IQR) | 3.3 (3.0–3.6) | 3.3 (3.0–3.5) | 3.3 (3.3–3.7) | 0.198 |
| Complications | Total (n = 48) | Survivors (n = 7) | Non-Survivors (n = 41) | p-Value |
|---|---|---|---|---|
| ECMO-related: | ||||
| Cannula thrombosis, n (%) | 2 (4.2) | 1 (14.3) | 1 (2.4) | 0.273 |
| Membrane clotting, n (%) | 14 (29.2) | 1 (14.3) | 13 (31.7) | 0.349 |
| Circuit change, n (%) | 13 (27.1) | 1 (14.3) | 12 (29.3) | 0.410 |
| Bleeding, n (%) | 41 (85.4) | 4 (57.1) | 37 (90.2) | 0.053 |
| Hemorrhagic stroke, n (%) | 3 (6.3) | 0 | 3 (7.3) | 1.000 |
| Hemorrhagic shock, n (%) | 2 (4.2) | 0 | 2 (4.9) | 1.000 |
| Superinfection, n (%) | 44 (91.7) | 6 (85.7) | 38 (92.7) | 0.480 |
| VAP, n (%) | 39 (81.3) | 5 (71.4) | 34 (82.9) | 0.601 |
| BSI, n (%) | 18 (37.5) | 4 (57.1) | 14 (34.1) | 0.400 |
| Septic shock, n (%) | 27 (56.3) | 3 (42.9) | 24 (58.5) | 0.683 |
| Deep venous thrombosis, n (%) | 5 (10.4) | 3 (42.9) | 2 (4.9) | 0.018 |
| Pulmonary thromboembolism, n (%) | 3 (6.3) | 0 | 3 (7.3) | 1.000 |
| Acute kidney disease, n (%) | 21 (43.8) | 3 (42.9) | 18 (43.9) | 1.000 |
| Renal replacement therapy, n (%) | 11 (22.9) | 2 (28.6) | 9 (22.0) | 0.653 |
| Tracheostomy, n (%) | 16 (33.3) | 6 (85.7) | 10 (24.4) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, G.; Montrucchio, G.; Sanna, V.; Collino, F.; Fanelli, V.; Filippini, C.; Simonetti, U.; Bonetto, C.; Morscio, M.; Verderosa, I.; et al. Outcomes and Impact of Pre-ECMO Clinical Course in Severe COVID-19-Related ARDS Treated with VV-ECMO: Data from an Italian Referral ECMO Center. J. Clin. Med. 2024, 13, 3545. https://doi.org/10.3390/jcm13123545
Sales G, Montrucchio G, Sanna V, Collino F, Fanelli V, Filippini C, Simonetti U, Bonetto C, Morscio M, Verderosa I, et al. Outcomes and Impact of Pre-ECMO Clinical Course in Severe COVID-19-Related ARDS Treated with VV-ECMO: Data from an Italian Referral ECMO Center. Journal of Clinical Medicine. 2024; 13(12):3545. https://doi.org/10.3390/jcm13123545
Chicago/Turabian StyleSales, Gabriele, Giorgia Montrucchio, Valentina Sanna, Francesca Collino, Vito Fanelli, Claudia Filippini, Umberto Simonetti, Chiara Bonetto, Monica Morscio, Ivo Verderosa, and et al. 2024. "Outcomes and Impact of Pre-ECMO Clinical Course in Severe COVID-19-Related ARDS Treated with VV-ECMO: Data from an Italian Referral ECMO Center" Journal of Clinical Medicine 13, no. 12: 3545. https://doi.org/10.3390/jcm13123545
APA StyleSales, G., Montrucchio, G., Sanna, V., Collino, F., Fanelli, V., Filippini, C., Simonetti, U., Bonetto, C., Morscio, M., Verderosa, I., Urbino, R., & Brazzi, L. (2024). Outcomes and Impact of Pre-ECMO Clinical Course in Severe COVID-19-Related ARDS Treated with VV-ECMO: Data from an Italian Referral ECMO Center. Journal of Clinical Medicine, 13(12), 3545. https://doi.org/10.3390/jcm13123545






