Working Memory Recovery in Adolescents with Concussion: Longitudinal fMRI Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessments
2.3. Experimental Paradigm
2.4. Behavioral Data Analysis
2.5. fMRI Data Acquisition
2.6. fMRI Data Preprocessing
2.7. fMRI Data Subject-Level and Group-Level Analysis
2.7.1. Subject-Level Analysis
2.7.2. Group-Level Analysis
3. Results
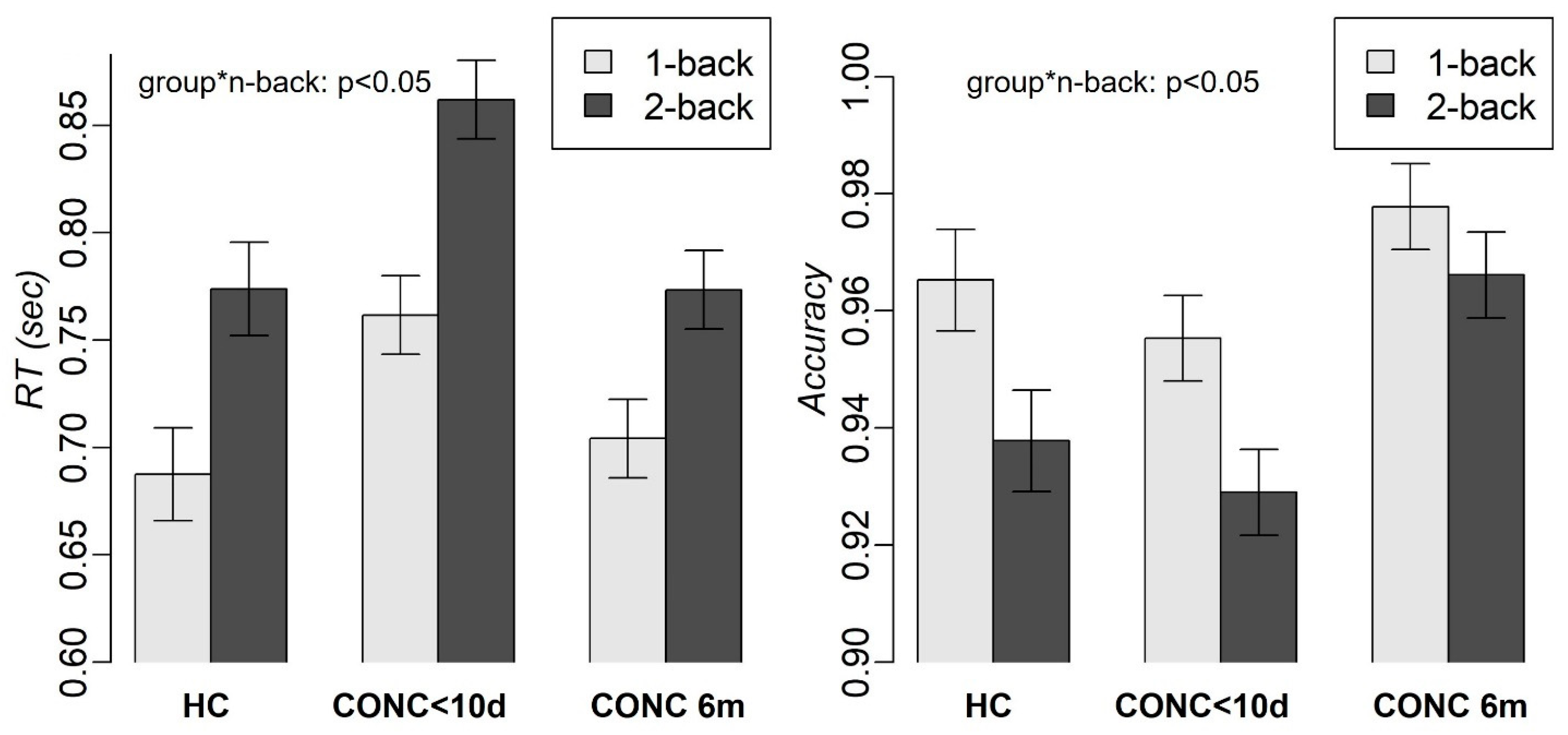
3.1. Demographics and Behavioral
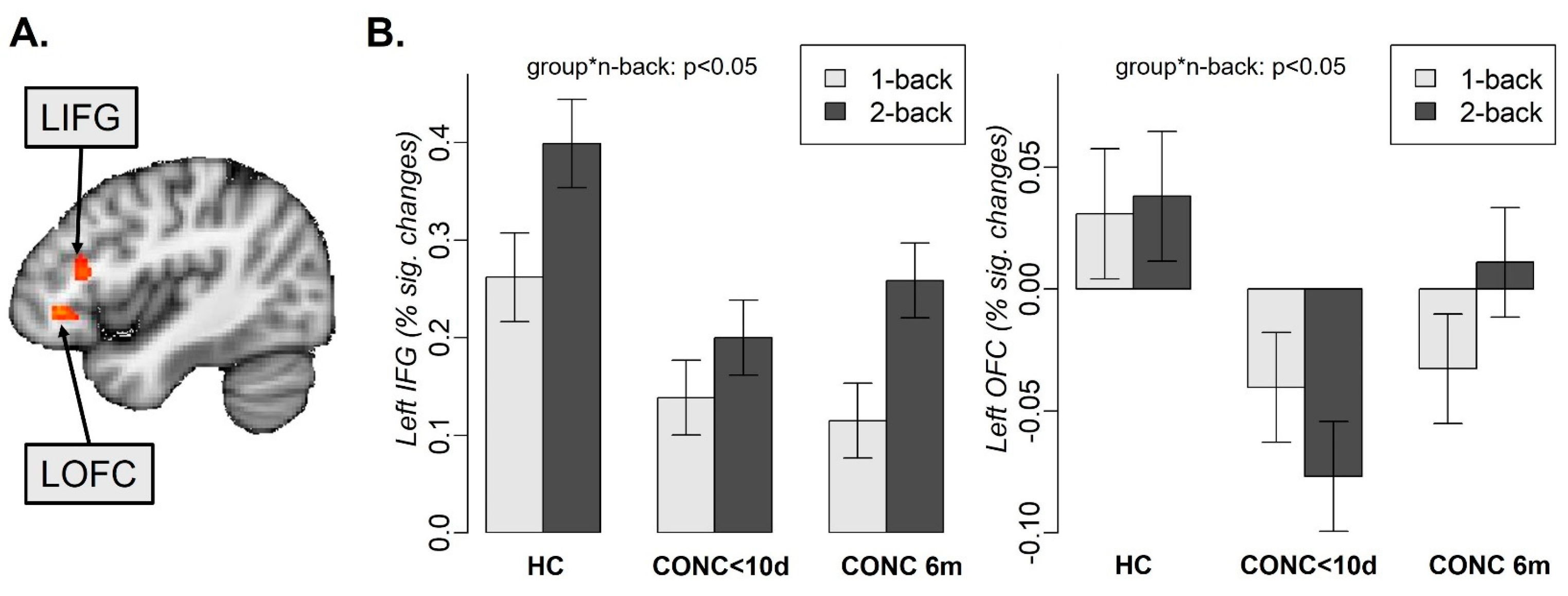
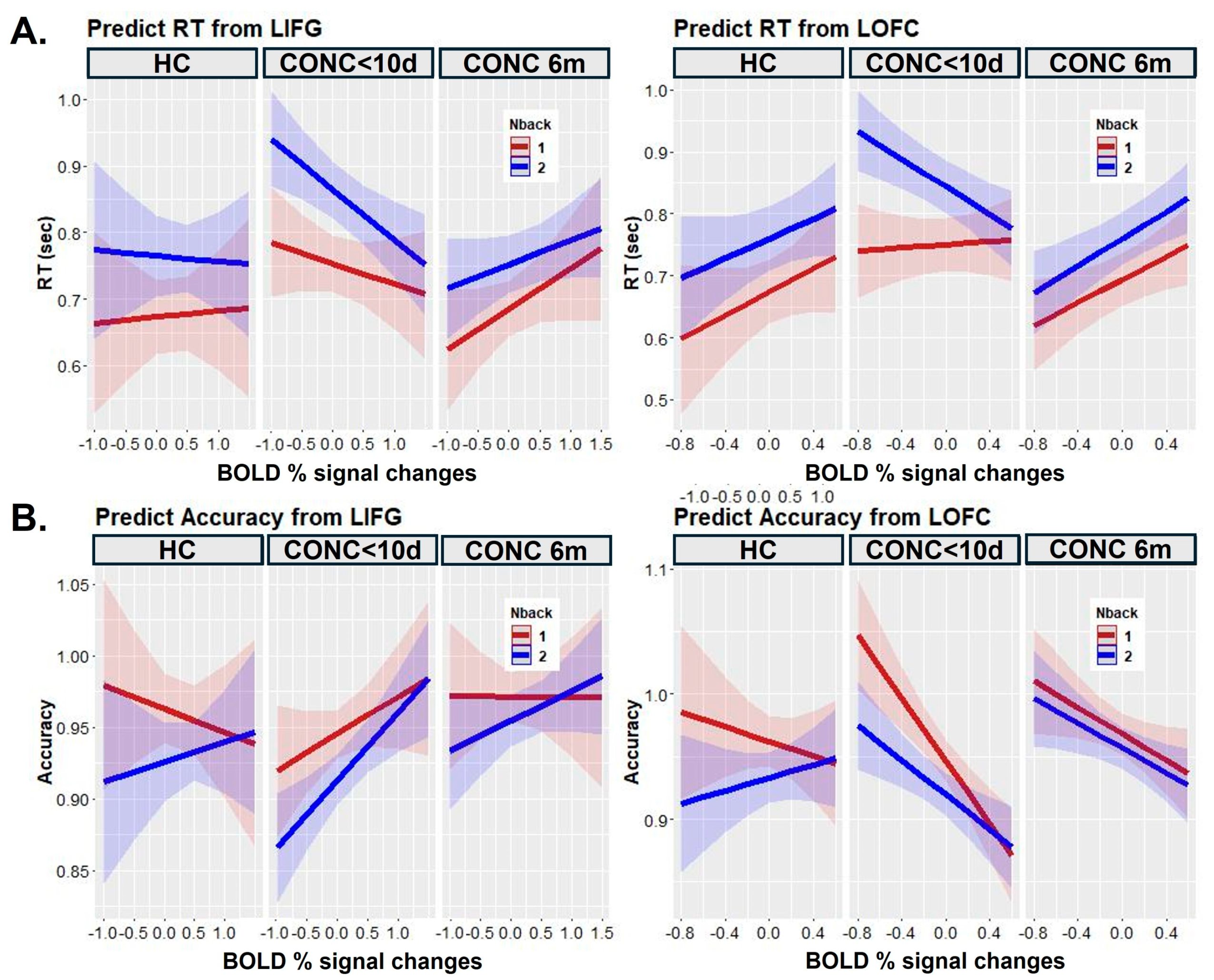
3.2. Neuroimaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veliz, P.; McCabe, S.E.; Eckner, J.T.; Schulenberg, J.E. Trends in the Prevalence of Concussion Reported by US Adolescents, 2016–2020. JAMA J. Am. Med. Assoc. 2021, 325, 1789–1791. [Google Scholar] [CrossRef]
- Yeates, K.O.; Kaizar, E.; Rusin, J.; Bangert, B.; Dietrich, A.; Nuss, K.; Wright, M.; Taylor, H.G. Reliable Change in Postconcussive Symptoms and Its Functional Consequences among Children with Mild Traumatic Brain Injury. Arch. Pediatr. Adolesc. Med. 2012, 166, 615–622. [Google Scholar] [CrossRef]
- Barlow, K.M.; Crawford, S.; Stevenson, A.; Sandhu, S.S.; Belanger, F.; Dewey, D. Epidemiology of Postconcussion Syndrome in Pediatric Mild Traumatic Brain Injury. Pediatrics 2010, 126, e374–e381. [Google Scholar] [CrossRef]
- Blume, H.; Hawash, K. Subacute Concussion-Related Symptoms and Postconcussion Syndrome in Pediatrics. Curr. Opin. Pediatr. 2012, 24, 724–730. [Google Scholar] [CrossRef]
- Kontos, A.P.; Deitrick, J.M.A.; Collins, M.W.; Mucha, A. Review of Vestibular and Oculomotor Screening and Concussion Rehabilitation. J. Athl. Train. 2017, 52, 256–261. [Google Scholar] [CrossRef]
- Mucha, A.; Collins, M.W.; Elbin, R.J.; Furman, J.M.; Troutman-Enseki, C.; Dewolf, R.M.; Marchetti, G.; Kontos, A.P. A Brief Vestibular/Ocular Motor Screening (VOMS) Assessment to Evaluate Concussions: Preliminary Findings. Am. J. Sports Med. 2014, 42, 2479–2486. [Google Scholar] [CrossRef]
- Wallace, B.; Lifshitz, J. Traumatic Brain Injury and Vestibulo-Ocular Function: Current Challenges and Future Prospects. Eye Brain 2016, 8, 153–164. [Google Scholar] [CrossRef]
- Baddeley, A. Working Memory. Curr. Biol. 2010, 20, R136–R140. [Google Scholar] [CrossRef]
- Conway, A.R.A.; Kane, M.J.; Engle, R.W. Working Memory Capacity and Its Relation to General Intelligence. Trends Cogn. Sci. 2003, 7, 547–552. [Google Scholar] [CrossRef]
- Quinn de Launay, K.; Martino, A.; Riggs, L.; Reed, N.; Beal, D.S. Pediatric Concussion Working Memory Outcomes: A Scoping Review. Brain Inj. 2021, 35, 1121–1133. [Google Scholar] [CrossRef]
- Stein, A.; Iyer, K.K.; Khetani, A.M.; Barlow, K.M. Changes in Working Memory-Related Cortical Responses Following Pediatric Mild Traumatic Brain Injury: A Longitudinal FMRI Study. J. Concussion 2021, 5, 205970022110065. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-Back Working Memory Paradigm: A Meta-Analysis of Normative Functional Neuroimaging Studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Manelis, A.; Reder, L.M. He Who Is Well Prepared Has Half Won the Battle: An FMRI Study of Task Preparation. Cereb. Cortex 2015, 25, 726–735. [Google Scholar] [CrossRef]
- Manelis, A.; Reder, L.M. Effective Connectivity among the Working Memory Regions during Preparation for and during Performance of the N-Back Task. Front. Hum. Neurosci. 2014, 8, 593. [Google Scholar] [CrossRef]
- Manelis, A.; Iyengar, S.; Swartz, H.A.; Phillips, M.L. Prefrontal Cortical Activation during Working Memory Task Anticipation Contributes to Discrimination between Bipolar and Unipolar Depression. Neuropsychopharmacology 2020, 45, 956–963. [Google Scholar] [CrossRef]
- Manelis, A.; Lima Santos, J.P.; Suss, S.J.; Holland, C.L.; Stiffler, R.S.; Bitzer, H.B.; Mailliard, S.; Shaffer, M.A.; Caviston, K.; Collins, M.W.; et al. Vestibular/Ocular Motor Symptoms in Concussed Adolescents Are Linked to Retrosplenial Activation. Brain Commun. 2022, 4, fcac123. [Google Scholar] [CrossRef]
- Schweinsburg, A.D.; Nagel, B.J.; Tapert, S.F. FMRI Reveals Alteration of Spatial Working Memory Networks across Adolescence. J. Int. Neuropsychol. Soc. 2005, 11, 631–644. [Google Scholar] [CrossRef]
- Schleepen, T.M.J.; Jonkman, L.M. The Development of Non-Spatial Working Memory Capacity during Childhood and Adolescence and the Role of Interference Control: An N-Back Task Study. Dev. Neuropsychol. 2010, 35, 37–56. [Google Scholar] [CrossRef]
- Geier, C.F.; Garver, K.; Terwilliger, R.; Luna, B. Development of Working Memory Maintenance. J. Neurophysiol. 2009, 101, 84–99. [Google Scholar] [CrossRef]
- Yaple, Z.; Arsalidou, M. N-Back Working Memory Task: Meta-Analysis of Normative FMRI Studies With Children. Child Dev. 2018, 89, 2010–2022. [Google Scholar] [CrossRef]
- Khetani, A.; Rohr, C.S.; Sojoudi, A.; Bray, S.; Barlow, K.M. Alteration in Cerebral Activation during a Working Memory Task after Pediatric Mild Traumatic Brain Injury: A Prospective Controlled Cohort Study. J. Neurotrauma 2019, 36, 3274–3283. [Google Scholar] [CrossRef]
- Westfall, D.R.; West, J.D.; Bailey, J.N.; Arnold, T.W.; Kersey, P.A.; Saykin, A.J.; McDonald, B.C. Increased Brain Activation during Working Memory Processing after Pediatric Mild Traumatic Brain Injury (MTBI). J. Pediatr. Rehabil. Med. 2015, 8, 297–308. [Google Scholar] [CrossRef]
- Brooks, B.L.; Virani, S.; Khetani, A.; Carlson, H.; Jadavji, Z.; Mauthner, M.; Low, T.A.; Plourde, V.; MacMaster, F.P.; Bray, S.; et al. Functional Magnetic Resonance Imaging Study of Working Memory Several Years after Pediatric Concussion. Brain Inj. 2020, 34, 895–904. [Google Scholar] [CrossRef]
- Hammeke, T.A.; McCrea, M.; Coats, S.M.; Verber, M.D.; Durgerian, S.; Flora, K.; Olsen, G.S.; Leo, P.D.; Gennarelli, T.A.; Rao, S.M. Acute and Subacute Changes in Neural Activation during the Recovery from Sport-Related Concussion. J. Int. Neuropsychol. Soc. 2013, 19, 863–872. [Google Scholar] [CrossRef]
- Keightley, M.L.; Singh Saluja, R.; Chen, J.K.; Gagnon, I.; Leonard, G.; Petrides, M.; Ptito, A. A Functional Magnetic Resonance Imaging Study of Working Memory in Youth after Sports-Related Concussion: Is It Still Working? J. Neurotrauma 2014, 31, 437–451. [Google Scholar] [CrossRef]
- Dettwiler, A.; Murugavel, M.; Putukian, M.; Cubon, V.; Furtado, J.; Osherson, D. Persistent Differences in Patterns of Brain Activation after Sports-Related Concussion: A Longitudinal Functional Magnetic Resonance Imaging Study. J. Neurotrauma 2014, 31, 180–188. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, C.H.; Liao, Y.P.; Hsu, H.L.; Tseng, Y.C.; Liu, H.L.; Chiu, W.T. Working Memory in Patients with Mild Traumatic Brain Injury: Functional MR Imaging Analysis. Radiology 2012, 264, 844–851. [Google Scholar] [CrossRef]
- McCrory, P.; Meeuwisse, W.; Dvorak, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J.; et al. Consensus Statement on Concussion in Sport—The 5th International Conference on Concussion in Sport Held in Berlin, October 2016. Br. J. Sports Med. 2017, 51, 838–847. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Sheehan, K.H.; Shytle, R.D.; Janavs, J.; Bannon, Y.; Rogers, J.E.; Milo, K.M.; Stock, S.L.; Wilkinson, B. Reliability and Validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J. Clin. Psychiatry 2010, 71, 313–326. [Google Scholar] [CrossRef]
- Lovell, M.R.; Iverson, G.L.; Collins, M.W.; Podell, K.; Johnston, K.M.; Pardini, D.; Pardini, J.; Norwig, J.; Maroon, J.C. Measurement of Symptoms Following Sports-Related Concussion: Reliability and Normative Data for the Post-Concussion Scale. Appl. Neuropsychol. 2006, 13, 166–174. [Google Scholar] [CrossRef]
- Tottenham, N.; Tanaka, J.W.; Leon, A.C.; McCarry, T.; Nurse, M.; Hare, T.A.; Marcus, D.J.; Westerlund, A.; Casey, B.J.; Nelson, C. The NimStim Set of Facial Expressions: Judgments from Untrained Research Participants. Psychiatry Res. 2009, 168, 242–249. [Google Scholar] [CrossRef]
- Ladouceur, C.D.; Silk, J.S.; Dahl, R.E.; Ostapenko, L.; Kronhaus, D.M.; Phillips, M.L. Fearful Faces Influence Attentional Control Processes in Anxious Youth and Adults. Emotion 2009, 9, 855–864. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Makowski, D.; Ben-Shachar, M.S.; Patil, I.; Lüdecke, D. Estimation of Model-Based Predictions, Contrasts and Means. Available online: https://easystats.github.io/modelbased/authors.html (accessed on 17 June 2024).
- Li, X.; Morgan, P.S.; Ashburner, J.; Smith, J.; Rorden, C. The First Step for Neuroimaging Data Analysis: DICOM to NIfTI Conversion. J. Neurosci. Methods 2016, 264, 47–56. [Google Scholar] [CrossRef]
- Lutkenhoff, E.S.; Rosenberg, M.; Chiang, J.; Zhang, K.; Pickard, J.D.; Owen, A.M.; Monti, M.M. Optimized Brain Extraction for Pathological Brains (OptiBET). PLoS ONE 2014, 9, e115551. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A Global Optimisation Method for Robust Affine Registration of Brain Images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Jenkinson, M.; Smith, S. Non-Linear Registration Aka Spatial Normalisation. In FMRIB Technial Report TR07JA2; FMRIB Centre: Oxford, UK, 2007; p. 22. [Google Scholar]
- Pruim, R.H.R.; Mennes, M.; van Rooij, D.; Llera, A.; Buitelaar, J.K.; Beckmann, C.F. ICA-AROMA: A Robust ICA-Based Strategy for Removing Motion Artifacts from FMRI Data. Neuroimage 2015, 112, 267–277. [Google Scholar] [CrossRef]
- Guillaume, B.; Hua, X.; Thompson, P.M.; Waldorp, L.; Nichols, T.E. Fast and Accurate Modelling of Longitudinal and Repeated Measures Neuroimaging Data. Neuroimage 2014, 94, 287–302. [Google Scholar] [CrossRef]
- Smith, S.M.; Nichols, T.E. Threshold-Free Cluster Enhancement: Addressing Problems of Smoothing, Threshold Dependence and Localisation in Cluster Inference. Neuroimage 2009, 44, 83–98. [Google Scholar] [CrossRef]
- Nystrom, L.E.; Braver, T.S.; Sabb, F.W.; Delgado, M.R.; Noll, D.C.; Cohen, J.D. Working Memory for Letters, Shapes, and Locations: FMRI Evidence against Stimulus-Based Regional Organization in Human Prefrontal Cortex. Neuroimage 2000, 11, 424–446. [Google Scholar] [CrossRef]
- Yarkoni, T.; Poldrack, R.A.; Nichols, T.E.; Van Essen, D.C.; Wager, T.D. Large-Scale Automated Synthesis of Human Functional Neuroimaging Data. Nat. Methods 2011, 8, 665–670. [Google Scholar] [CrossRef]
- Badre, D.; Wagner, A.D. Left Ventrolateral Prefrontal Cortex and the Cognitive Control of Memory. Neuropsychologia 2007, 45, 2883–2901. [Google Scholar] [CrossRef]
- Brass, M.; Von Cramon, D.Y. Selection for Cognitive Control: A Functional Magnetic Resonance Imaging Study on the Selection of Task-Relevant Information. J. Neurosci. 2004, 24, 8847–8852. [Google Scholar] [CrossRef]
- Suss, S.J.; Manelis, A.; Santos, J.P.L.; Holland, C.L.; Stiffler, R.S.; Bitzer, H.B.; Mailliard, S.; Shaffer, M.; Caviston, K.; Collins, M.W.; et al. Resting State Functional Connectivity between Dorsal Attentional Network and Right Inferior Frontal Gyrus in Concussed and Control Adolescents. J. Clin. Med. 2022, 11, 2293. [Google Scholar] [CrossRef]
- Coyle, H.L.; Bailey, N.W.; Ponsford, J.; Hoy, K.E. Recovery of Clinical, Cognitive and Cortical Activity Measures Following Mild Traumatic Brain Injury (MTBI): A Longitudinal Investigation. Cortex 2023, 165, 14–25. [Google Scholar] [CrossRef]
- Noonan, M.P.; Chau, B.K.H.; Rushworth, M.F.S.; Fellows, L.K. Contrasting Effects of Medial and Lateral Orbitofrontal Cortex Lesions on Credit Assignment and Decision-Making in Humans. J. Neurosci. 2017, 37, 7023–7035. [Google Scholar] [CrossRef]
- Labrenz, F.; Themann, M.; Wascher, E.; Beste, C.; Pfleiderer, B. Neural Correlates of Individual Performance Differences in Resolving Perceptual Conflict. PLoS ONE 2012, 7, 42849. [Google Scholar] [CrossRef]
- Lima Santos, J.P.; Jia-Richards, M.; Kontos, A.P.; Collins, M.W.; Versace, A. Emotional Regulation and Adolescent Concussion: Overview and Role of Neuroimaging. Int. J. Environ. Res. Public Health 2023, 20, 6274. [Google Scholar] [CrossRef]
- Manelis, A.; Stiffler, R.; Lockovich, J.C.; Almeida, J.R.C.; Aslam, H.A.; Phillips, M.L. Longitudinal Changes in Brain Activation during Anticipation of Monetary Loss in Bipolar Disorder. Psychol. Med. 2019, 49, 2781–2788. [Google Scholar] [CrossRef]

| Concussed | Controls | Statistics | |
|---|---|---|---|
| N | 45 | 32 | |
| Number of females (%) | 16 (35%) | 16 (50%) | ꭓ2 (1) = 0.4, p = 0.5 |
Race
|
|
| ꭓ2 (3) = 4.4, p = 0.2 |
| Mean age in years (SD) [min–max] | 15.9 (1.3) [13–17.9] | 15.5 (1.2) [13–17.4] | t(75) = 1.3, p = 0.2 |
| Mean IQ (SD) [min, max] | 104.3 (8.4) [83.0–122.0] | 107.9 (8.0) [92.0–124.0] | t(77) = −1.8, p = 0.07 |
| Mean VOMS scores total (SD) [min, max] | 43.9 (43.4) [0.0–193.0] | - | na |
| Mean PCSS score total (SD) [min, max] | 28.9 (21.3) [0.0–79.0] | - | na |
| Mean number of days between injury and scan (SD) [min, max] | 3.1 (2.3) [0–9] | - | na |
| Mean number of days between baseline scan and 6-month scan (SD) [min, max] | 190.3 (13) [168–209] | - | na |
| Mean number of days between injury and clearance for return to play (SD) [min, max] | 30.2 (33.9) [2–175] | - | na |
| Mean number of days between the clearance date and 6-month scan (SD) [min, max] | 159.9 (40) [25–200] | - | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manelis, A.; Lima Santos, J.P.; Suss, S.J.; Holland, C.L.; Perry, C.A.; Hickey, R.W.; Collins, M.W.; Kontos, A.P.; Versace, A. Working Memory Recovery in Adolescents with Concussion: Longitudinal fMRI Study. J. Clin. Med. 2024, 13, 3585. https://doi.org/10.3390/jcm13123585
Manelis A, Lima Santos JP, Suss SJ, Holland CL, Perry CA, Hickey RW, Collins MW, Kontos AP, Versace A. Working Memory Recovery in Adolescents with Concussion: Longitudinal fMRI Study. Journal of Clinical Medicine. 2024; 13(12):3585. https://doi.org/10.3390/jcm13123585
Chicago/Turabian StyleManelis, Anna, João P. Lima Santos, Stephen J. Suss, Cynthia L. Holland, Courtney A. Perry, Robert W. Hickey, Michael W. Collins, Anthony P. Kontos, and Amelia Versace. 2024. "Working Memory Recovery in Adolescents with Concussion: Longitudinal fMRI Study" Journal of Clinical Medicine 13, no. 12: 3585. https://doi.org/10.3390/jcm13123585





