The Effect of Obesity on Repolarization and Other ECG Parameters
Abstract
:1. Introduction
2. Materials and Methods
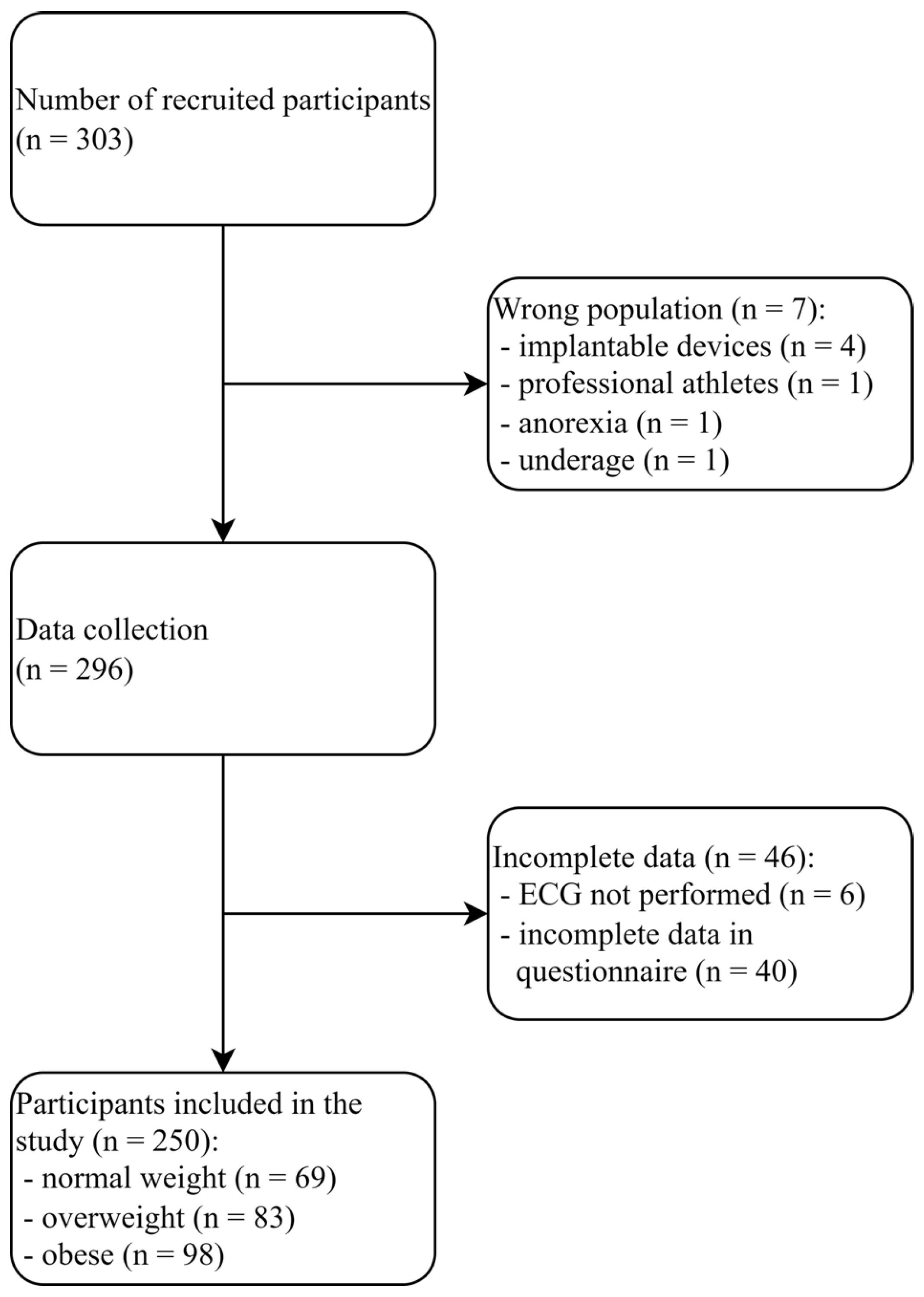
2.1. Study Population and Trial Design
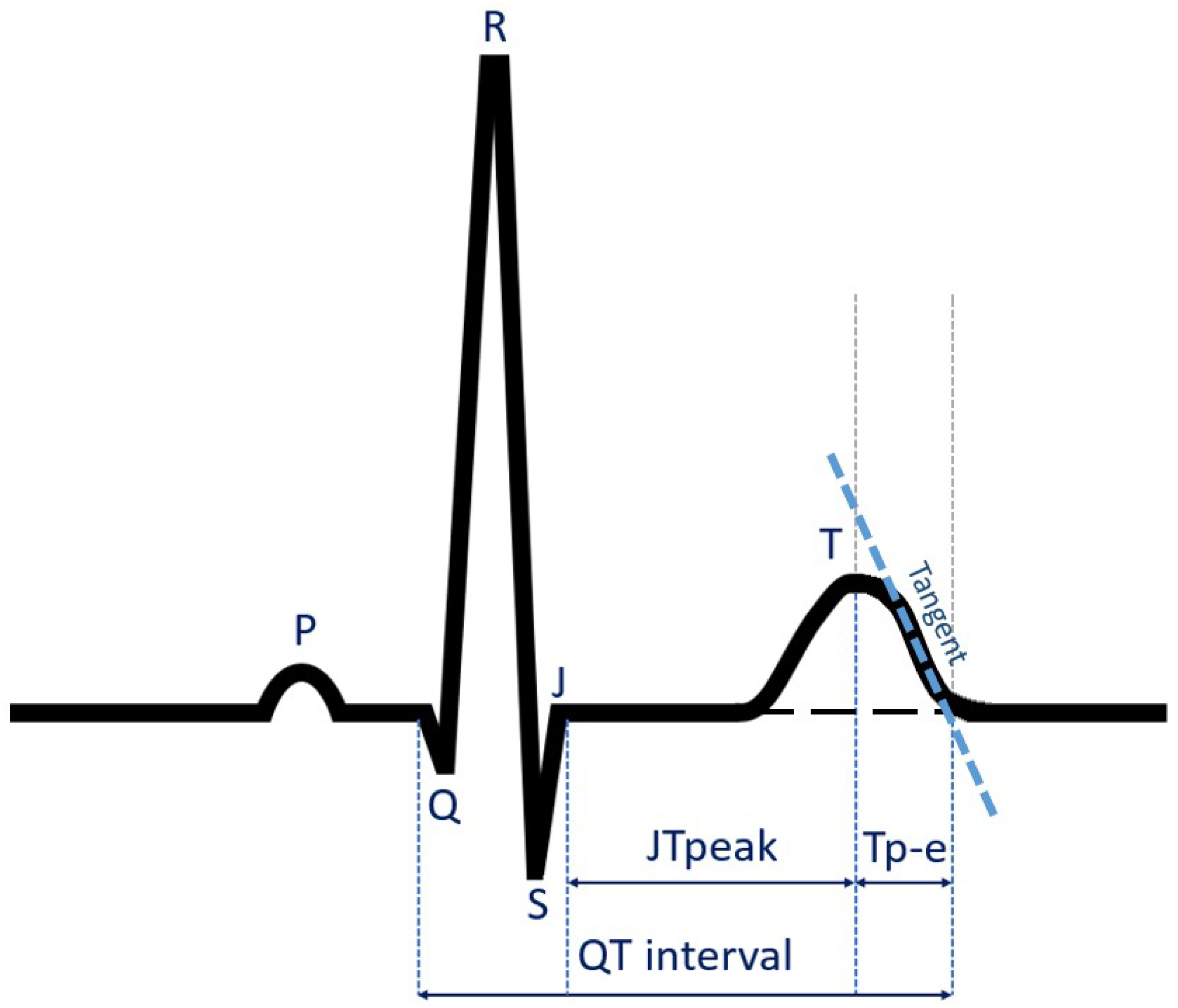
2.2. Electrocardiographic Analysis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristic
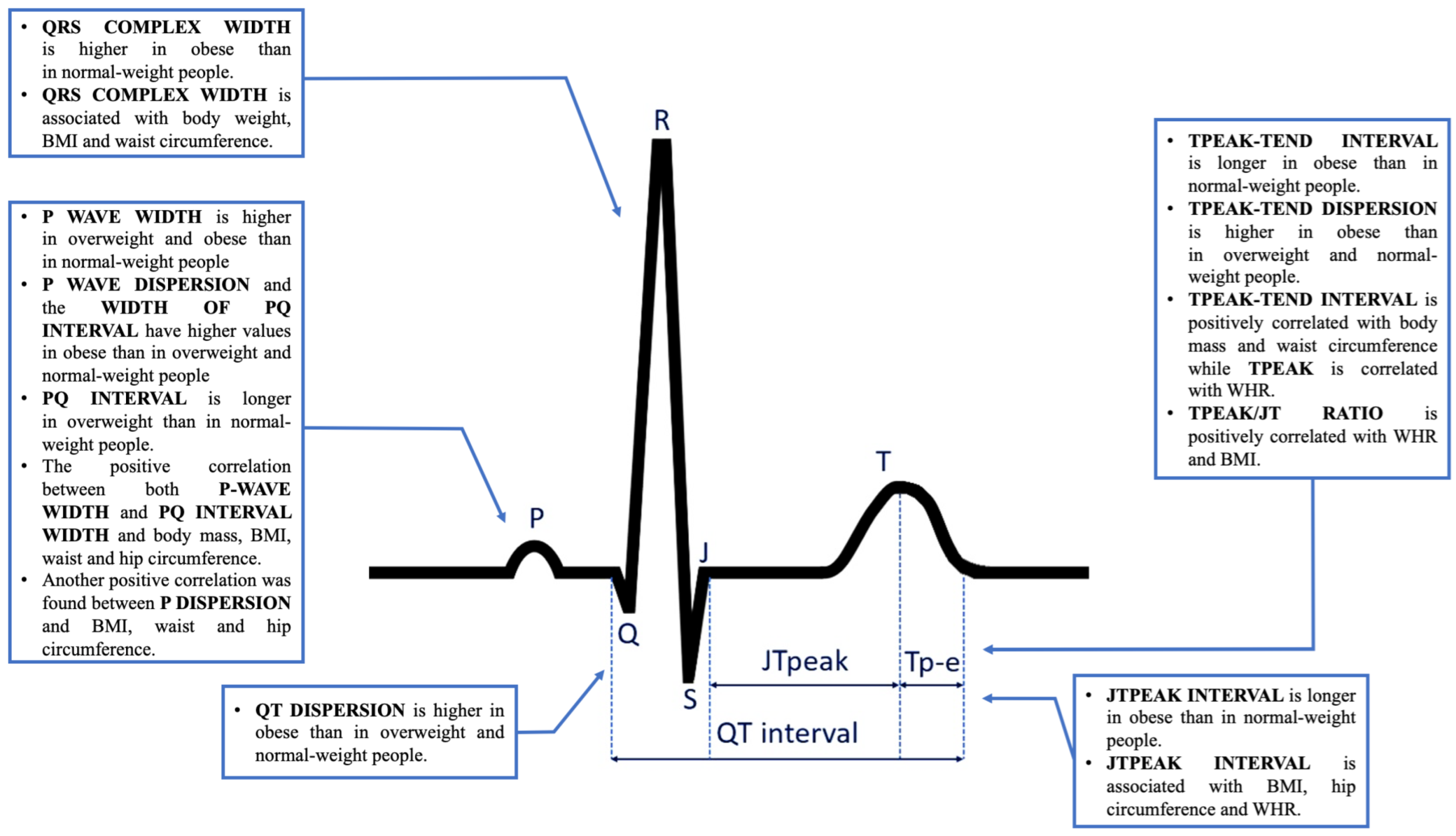
3.2. Analysis of 12-Lead ECG Parameters in Studied Subgroups
3.3. Linear Relationship between Body Mass Parameters and 12-Lead ECG Parameters in the Entire Study Group
3.4. Backward Stepwise Multiple Regression Model
4. Discussion
4.1. Limitations
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 12 August 2023).
- Global Health Estimates: Leading Causes of Death. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 12 August 2023).
- Dixon, A.E.; Peters, U. The effect of obesity on lung function. Expert. Rev. Respir. Med. 2018, 12, 755–767. [Google Scholar] [CrossRef]
- Yu, W.; Rohli, K.E.; Yang, S.; Jia, P. Impact of obesity on COVID-19 patients. J. Diabetes Complicat. 2021, 35, 107817. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Obesity is Common, Serious, and Costly. Available online: https://www.cdc.gov/obesity/php/about/index.html (accessed on 12 August 2023).
- The Lancet Gastroenterology Hepatology. Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021, 6, 411. [Google Scholar] [CrossRef]
- Global Health Observatory Data Repository. Overweight/Obesity. Available online: https://apps.who.int/gho/data/node.main.A896?lang=en (accessed on 15 August 2023).
- Binu, A.J.; Srinath, S.C.; Cherian, K.E.; Jacob, J.R.; Paul, T.V.; Kapoor, N. A Pilot Study of Electrocardiographic Features in Patients with Obesity from a Tertiary Care Centre in Southern India (Electron). Med. Sci. 2022, 10, 56. [Google Scholar] [CrossRef]
- Kumar, T.; Jha, K.; Sharan, A.; Sakshi, P.; Kumar, S.; Kumari, A. Study of the effect of obesity on QT-interval among adults. J. Family Med. Prim. Care. 2019, 8, 1626–1629. [Google Scholar]
- Omran, J.; Firwana, B.; Koerber, S.; Bostick, B.; Alpert, M.A. Effect of obesity and weight loss on ventricular repolarization: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 520–530. [Google Scholar] [CrossRef]
- Inanir, M.; Sincer, I.; Erdal, E.; Gunes, Y.; Cosgun, M.; Mansiroglu, A.K. Evaluation of electrocardiographic ventricular repolarization parameters in extreme obesity. J. Electrocardiol. 2019, 53, 36–39. [Google Scholar] [CrossRef]
- Bağcı, A.; Aksoy, F.; Baş, H.A.; Işık, İ.B.; Orhan, H. The effect of Systolic and diastolic blood pressure on Tp-e interval in patients divided according to World Health Organization classification for body mass index. Clin. Exp. Hypertens. 2021, 43, 642–646. [Google Scholar] [CrossRef]
- Braschi, A.; Abrignani, M.G.; Francavilla, V.C.; Francavilla, G. Novel electrocardiographic parameters of altered repolarization in uncomplicated overweight and obesity. Obesity 2011, 19, 875–881. [Google Scholar] [CrossRef]
- Al-Mosawi, A.A.; Nafakhi, H.; Hassan, M.B.; Alareedh, M.; Al-Nafakh, H.A. ECG markers of arrythmogenic risk relationships with pericardial fat volume and BMI in patients with coronary atherosclerosis. J. Electrocardiol. 2018, 51, 569–572. [Google Scholar] [CrossRef]
- Tse, G.; Yan, B.P. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace 2017, 19, 712–721. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Corrao, A.; Carnovale, M.; Di Diego, I.; Lospinuso, I.; Caltabiano, C.; Mezzadri, M.; Rossi, P.; Magrì, D. Noninvasive Hemodynamic Monitoring in Advanced Heart Failure Patients: New Approach for Target Treatments. Biomedicines 2022, 10, 2407. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Carnovale, M.; Corrao, A.; Di Diego, I.; Lospinuso, I.; Caltabiano, C.; Mezzadri, M.; Rossi, P.; Magrì, D. Short-Period Temporal Dispersion Repolarization Markers in Elderly Patients with Decompensated Heart Failure. Clin. Ter. 2022, 173, 356–361. [Google Scholar]
- Tse, G.; Gong, M.; Meng, L.; Wong, C.W.; Georgopoulos, S.; Bazoukis, G.; Wong, M.C.; Letsas, K.P.; Vassiliou, V.S.; Xia, Y.; et al. Meta-analysis of Tpeak-Tend and Tpeak-Tend/QT ratio for risk stratification in congenital long QT syndrome. J. Electrocardiol. 2018, 51, 396–401. [Google Scholar] [CrossRef]
- Tse, G.; Gong, M.; Meng, L.; Wong, C.W.; Bazoukis, G.; Chan, M.T.; Wong, M.C.; Letsas, K.P.; Baranchuk, A.; Yan, G.X.; et al. Predictive Value of T peak—T end Indices for Adverse Outcomes in Acquired QT Prolongation: A Meta-Analysis. Front. Physiol. 2018, 9, 1226. [Google Scholar] [CrossRef]
- Markiewicz-Łoskot, G.; Moric-Janiszewska, E.; Mazurek, B.; Łoskot, M.; Bartusek, M.; Skierska, A.; Szydłowski, L. Electrocardiographic T-wave parameters in families with long QT syndrome. Adv. Clin. Exp. Med. 2018, 27, 501–507. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Gao, C.; Zhu, J.; Yang, X. Tpeak-Tend/QT interval predicts ST-segment resolution and major adverse cardiac events in acute ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention. Medicine 2018, 97, e12943. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, Z.; Wu, Y.; Chen, R.; Li, M.; Chen, X.; Qin, S.; Liang, Y.; Su, Y.; Ge, J. Electrocardiographic parameters effectively predict ventricular tachycardia/fibrillation in acute phase and abnormal cardiac function in chronic phase of ST-segment elevation myocardial infarction. J. Cardiovasc. Electrophysiol. 2018, 29, 756–766. [Google Scholar] [CrossRef]
- Andršová, I.; Hnatkova, K.; Šišáková, M.; Toman, O.; Smetana, P.; Huster, K.M.; Barthel, P.; Novotný, T.; Schmidt, G.; Malik, M. Heart Rate Dependency and Inter-Lead Variability of the T Peak—T End Intervals. Front. Physiol. 2020, 11, 595815. [Google Scholar] [CrossRef]
- Gupta, P.; Patel, C.; Patel, H.; Narayanaswamy, S.; Malhotra, B.; Green, J.T.; Yan, G.X. T(p-e)/QT ratio as an index of arrhythmogenesis. J. Electrocardiol. 2008, 41, 567–574. [Google Scholar] [CrossRef]
- Rosenthal, T.M.; Masvidal, D.; Abi Samra, F.M.; Bernard, M.L.; Khatib, S.; Polin, G.M.; Rogers, P.A.; Xue, J.Q.; Morin, D.P. Optimal method of measuring the T-peak to T-end interval for risk stratification in primary prevention. Europace 2018, 20, 698–705. [Google Scholar] [CrossRef]
- Seyfeli, E.; Duru, M.; Kuvandik, G.; Kaya, H.; Yalcin, F. Effect of obesity on P-wave dispersion and QT dispersion in women. Int. J. Obes. 2006, 30, 957–961. [Google Scholar] [CrossRef]
- Waheed, S.; Dawn, B.; Gupta, K. Association of corrected QT interval with body mass index, and the impact of this association on mortality: Results from the Third National Health and Nutrition Examination Survey. Obes. Res. Clin. Pract. 2017, 11, 426–434. [Google Scholar] [CrossRef]
- Guo, X.; Li, Z.; Guo, L.; Yu, S.; Yang, H.; Zheng, L.; Pan, G.; Zhang, Y.; Sun, Y.; Pletcher, M.J. Effects of Metabolically Healthy and Unhealthy Obesity on Prolongation of Corrected QT Interval. Am. J. Cardiol. 2017, 119, 1199–1204. [Google Scholar] [CrossRef]
- Kosar, F.; Aksoy, Y.; Ari, F.; Keskin, L.; Sahin, I. P-wave duration and dispersion in obese subjects. Ann. Noninvasive Electrocardiol. 2008, 13, 3–7. [Google Scholar] [CrossRef]
- Cosgun, M.; Sincer, I.; Inanir, M.; Erdal, E.; Mansiroglu, A.K.; Gunes, Y. P-wave Duration and Dispersion in Lone Obesity. J. Coll. Physicians Surg. Pak. 2021, 30, 567–570. [Google Scholar]
- Bocchi, F.; Marques-Vidal, P.; Pruvot, E.; Waeber, G.; Vollenweider, P.; Gachoud, D. Clinical and biological determinants of P-wave duration: Cross-sectional data from the population-based CoLaus|PsyCoLaus study. BMJ Open. 2020, 10, e038828. [Google Scholar] [CrossRef]
- Russo, V.; Ammendola, E.; De Crescenzo, I.; Docimo, L.; Santangelo, L.; Calabrò, R. Severe obesity and P-wave dispersion: The effect of surgically induced weight loss. Obes. Surg. 2008, 18, 90–96. [Google Scholar] [CrossRef]
- Duru, M.; Seyfeli, E.; Kuvandik, G.; Kaya, H.; Yalcin, F. Effect of weight loss on P wave dispersion in obese subjects. Obesity 2006, 14, 1378–1382. [Google Scholar] [CrossRef]
- Falchi, A.G.; Grecchi, I.; Muggia, C.; Tinelli, C. Weight loss and P wave dispersion: A preliminary study. Obes. Res. Clin. Pract. 2014, 8, e614–e617. [Google Scholar] [CrossRef]
- Chousou, P.A.; Chattopadhyay, R.; Tsampasian, V.; Vassiliou, V.S.; Pugh, P.J. Electrocardiographic Predictors of Atrial Fibrillation. Med. Sci. 2023, 11, 30. [Google Scholar] [CrossRef]
- Wang, Y.S.; Chen, G.Y.; Li, X.H.; Zhou, X.; Li, Y.G. Prolonged P-wave duration is associated with atrial fibrillation recurrence after radiofrequency catheter ablation: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 227, 355–359. [Google Scholar] [CrossRef]
- Kawczynski, M.J.; Van De Walle, S.; Maesen, B.; Isaacs, A.; Zeemering, S.; Hermans, B.; Vernooy, K.; Maessen, J.G.; Schotten, U.; Bidar, E. Preoperative P-wave parameters and risk of atrial fibrillation after cardiac surgery: A meta-analysis of 20,201 patients. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac220. [Google Scholar] [CrossRef]
- Pranata, R.; Yonas, E.; Vania, R. Prolonged P-wave duration in sinus rhythm pre-ablation is associated with atrial fibrillation recurrence after pulmonary vein isolation-A systematic review and meta-analysis. Ann. Noninvasive Electrocardiol. 2019, 24, e12653. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Kühl, J.T.; Pietersen, A.; Graff, C.; Lind, B.; Struijk, J.J.; Olesen, M.S.; Sinner, M.F.; Bachmann, T.N.; Haunsø, S.; et al. P-wave duration and the risk of atrial fibrillation: Results from the Copenhagen ECG Study. Heart Rhythm. 2015, 12, 1887–1895. [Google Scholar] [CrossRef]
- Pérez-Riera, A.R.; de Abreu, L.C.; Barbosa-Barros, R.; Grindler, J.; Fernandes-Cardoso, A.; Baranchuk, A. P-wave dispersion: An update. Indian. Pacing Electrophysiol. J. 2016, 16, 126–133. [Google Scholar] [CrossRef]
- Intzes, S.; Zagoridis, K.; Symeonidou, M.; Spanoudakis, E.; Arya, A.; Dinov, B.; Dagres, N.; Hindricks, G.; Bollmann, A.; Kanoupakis, E.; et al. P-wave duration and atrial fibrillation recurrence after catheter ablation: A systematic review and meta-analysis. Europace 2023, 25, 450–459. [Google Scholar] [CrossRef]
- Weng, L.C.; Hall, A.W.; Choi, S.H.; Jurgens, S.J.; Haessler, J.; Bihlmeyer, N.A.; Grarup, N.; Lin, H.; Teumer, A.; Li-Gao, R.; et al. Genetic Determinants of Electrocardiographic P-Wave Duration and Relation to Atrial Fibrillation. Circ. Genom. Precis. Med. 2020, 13, 387–395. [Google Scholar] [CrossRef]
- Hari, K.J.; Nguyen, T.P.; Soliman, E.Z. Relationship between P-wave duration and the risk of atrial fibrillation. Expert. Rev. Cardiovasc. Ther. 2018, 16, 837–843. [Google Scholar] [CrossRef]
- Dzikowicz, D.J.; Carey, M.G. Obesity and hypertension contribute to prolong QRS complex duration among middle-aged adults. Ann. Noninvasive Electrocardiol. 2019, 24, e12665. [Google Scholar] [CrossRef]
- Rao, A.C.; Ng, A.C.; Sy, R.W.; Chia, K.K.; Hansen, P.S.; Chiha, J.; Kilian, J.; Kanagaratnam, L.B. Electrocardiographic QRS duration is influenced by body mass index and sex. Int. J. Cardiol. Heart Vasc. 2021, 37, 100884. [Google Scholar] [CrossRef]
- Sobhani, S.; Sara, R.; Aghaee, A.; Pirzadeh, P.; Miandehi, E.E.; Shafiei, S.; Akbari, M.; Eslami, S. Body mass index, lipid profile, and hypertension contribute to prolonged QRS complex. Clin. Nutr. ESPEN. 2022, 50, 231–237. [Google Scholar] [CrossRef]
- Sun, G.Z.; Li, Y.; Zhou, X.H.; Guo, X.F.; Zhang, X.G.; Zheng, L.Q.; Li, Y.; Jiao, Y.D.; Sun, Y.X. Association between obesity and ECG variables in children and adolescents: A cross-sectional study. Exp. Ther. Med. 2013, 6, 1455–1462. [Google Scholar] [CrossRef]
- Figliozzi, S.; Stazi, A.; Pinnacchio, G.; Laurito, M.; Parrinello, R.; Villano, A.; Russo, G.; Milo, M.; Mollo, R.; Lanza, G.A.; et al. Use of T-wave alternans in identifying patients with coronary artery disease. J. Cardiovasc Med. 2016, 17, 20–25. [Google Scholar] [CrossRef]
- Hekkanen, J.J.; Kenttä, T.V.; Holmström, L.; Tulppo, M.P.; Ukkola, O.H.; Pakanen, L.; Junttila, M.J.; Huikuri, H.V.; Perkiömäki, J.S. Association of electrocardiographic spatial heterogeneity of repolarization and spatial heterogeneity of atrial depolarization with left ventricular fibrosis. Europace 2023, 25, 820–827. [Google Scholar] [CrossRef]
- Aromolaran, A.S.; Boutjdir, M. Cardiac Ion Channel Regulation in Obesity and the Metabolic Syndrome: Relevance to Long QT Syndrome and Atrial Fibrillation. Front. Physiol. 2017, 8, 431. [Google Scholar] [CrossRef]
- McCauley, M.D.; Hong, L.; Sridhar, A.; Menon, A.; Perike, S.; Zhang, M.; da Silva, I.B.; Yan, J.; Bonini, M.G.; Ai, X.; et al. Ion Channel and Structural Remodeling in Obesity-Mediated Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2020, 13, e008296. [Google Scholar] [CrossRef]
- Sharma, S.; Adrogue, J.V.; Golfman, L.; Uray, I.; Lemm, J.; Youker, K.; Noon, G.P.; Frazier, O.H.; Taegtmeyer, H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004, 18, 1692–1700. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Morrow, J.P.; Katchman, A.; Son, N.H.; Trent, C.M.; Khan, R.; Shiomi, T.; Huang, H.; Amin, V.; Lader, J.M.; Vasquez, C.; et al. Mice with cardiac overexpression of peroxi-some proliferator-activated receptor γ have impaired repolarization and spontaneous fatal ventricular ar-rhythmias. Circulation 2011, 124, 2812–2821. [Google Scholar] [CrossRef]
- Purohit, A.; Rokita, A.G.; Guan, X.; Chen, B.; Koval, O.M.; Voigt, N.; Neef, S.; Sowa, T.; Gao, Z.; Luczak, E.D.; et al. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 2013, 128, 1748–1757. [Google Scholar] [CrossRef]
- Joseph, L.C.; Barca, E.; Subramanyam, P.; Komrowski, M.; Pajvani, U.; Colecraft, H.M.; Hirano, M.; Morrow, J.P. Inhibition of NAPDH Oxidase 2 (NOX2) Prevents Oxidative Stress and Mitochondrial Abnormalities Caused by Saturated Fat in Cardiomyocytes. PLoS ONE 2016, 11, e0145750. [Google Scholar] [CrossRef]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef]
- Prospective Studies Collaboration; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900,000 adults: Collabo-rative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef]
- Şaylık, F.; Çınar, T.; Hayıroğlu, M.İ. Effect of the Obesity Paradox on Mortality in Patients with Acute Coronary Syndrome: A Comprehensive Meta-analysis of the Literature. Balkan Med. J. 2023, 40, 93–103. [Google Scholar] [CrossRef]
- Chang, Y.; Kim, B.K.; Yun, K.E.; Cho, J.; Zhang, Y.; Rampal, S.; Zhao, D.; Jung, H.S.; Choi, Y.; Ahn, J.; et al. Metabolically-healthy obesity and coronary artery calcification. J. Am. Coll. Cardiol. 2014, 63, 2679–2686. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, W.Q.; Qiu, B.; Zhang, L.J.; Qin, J.; Tang, F.J.; Wang, H.T.; Li, H.J.; Hao, Y.R. Assessing causal estimates of the association of obesity-related traits with coronary artery disease using a Mendelian randomization approach. Sci. Rep. 2018, 8, 7146. [Google Scholar] [CrossRef]
- Law, W.Y.; Huang, G.L.; Yang, C.C. Effect of Body Mass Index in Coronary CT Angiography Performed on a 256-Slice Multi-Detector CT Scanner. Diagnostics 2022, 12, 319. [Google Scholar] [CrossRef]
- Bagi, Z.; Broskova, Z.; Feher, A. Obesity and coronary microvascular disease—Implications for adipose tissue-mediated remote inflammatory response. Curr. Vasc. Pharmacol. 2014, 12, 453–461. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Zellweger, M.J.; Burger, P.C.; Mueller-Brand, J.; Pfisterer, M.E. Is obesity per se as weighty as other risk factors of coronary artery disease? J. Nucl. Cardiol. 2004, 11, S16. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association scientific statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar]
- Salem, J.E.; Alexandre, J.; Bachelot, A.; Funck-Brentano, C. Influence of steroid hormones on ventricular repolarization. Pharmacol. Ther. 2016, 167, 38–47. [Google Scholar] [CrossRef]
- Zukowski, M.; Biernawska, J.; Kotfis, K.; Kaczmarczyk, M.; Bohatyrewicz, R.; Blaszczyk, W.; Zegan-Baranska, M.; Ostrowski, M.; Brykczynski, M.; Ciechanowicz, A. Factors influencing QTc interval prolongation during kidney transplantation. Ann. Transplant. 2011, 16, 43–49. [Google Scholar] [CrossRef]
- Alexopoulos, D.; Christodoulou, J.; Toulgaridis, T.; Sitafidis, G.; Manias, O.; Hahalis, G.; Vagenakis, A.G. Repolarization abnormalities with prolonged hyperventilation in apparently healthy subjects: Incidence, mechanisms and affecting factors. Eur. Heart J. 1996, 17, 1432–1437. [Google Scholar] [CrossRef]
- Mirowsky, J.E.; Carraway, M.S.; Dhingra, R.; Tong, H.; Neas, L.; Diaz-Sanchez, D.; Cascio, W.E.; Case, M.; Crooks, J.L.; Hauser, E.R.; et al. Exposures to low-levels of fine particulate matter are associated with acute changes in heart rate variability, cardiac repolarization, and circulating blood lipids in coronary artery disease patients. Environ. Res. 2022, 214 Pt 1, 113768. [Google Scholar] [CrossRef]
- Kazanski, V.; Mitrokhin, V.M.; Mladenov, M.I.; Kamkin, A.G. Cytokine Effects on Mechano-Induced Electrical Activity in Atrial Myocardium. Immunol. Invest. 2017, 46, 22–37. [Google Scholar] [CrossRef]
- Piccirillo, G.; Magrì, D.; Matera, S.; Marigliano, V. Emotions that afflict the heart: Influence of the autonomic nervous system on temporal dispersion of myocardial repolarization. J. Cardiovasc. Electrophysiol. 2008, 19, 185–187. [Google Scholar] [CrossRef]
- Wang, L. ACE inhibitors suppress ischemia-induced arrhythmias by reducing the spatial dispersion of ven-tricular repolarization. Cardiology 1999, 92, 106–109. [Google Scholar] [CrossRef]
- Viitasalo, M.; Oikarinen, L.; Swan, H.; Väänänen, H.; Järvenpää, J.; Hietanen, H.; Karjalainen, J.; Toivonen, L. Effects of beta-blocker therapy on ventricular repolarization documented by 24-h electrocardiography in patients with type 1 long-QT syndrome. J. Am. Coll. Cardiol. 2006, 48, 747–753. [Google Scholar] [CrossRef]
- Yıldırım, D.İ.; Hayıroğlu, M.İ.; Ünal, N.; Eryılmaz, M.A. Evaluation of varenicline usage on ventricular repolar-ization after smoking cessation. Ann. Noninvasive Electrocardiol. 2019, 24, e12609. [Google Scholar] [CrossRef]
| Parameter | %/n or Mean ± SD |
|---|---|
| age (years) | 59.94 ± 13.22 |
| sex (%/n) | |
| Male | 41.6/104 |
| Female | 58.4/146 |
| height (cm) | 167.37 ± 9.76 |
| weight (kg) | 80.42 ± 17.50 |
| BMI (kg/m2) | 28.64 ± 4.99 |
| waist circumference (cm) | 95.99 ± 14.26 |
| hip circumference (cm) | 106.60 ± 12.16 |
| WHR | 0.95 ± 0.74 |
| hypertension (%/n) | 52.8/132 |
| myocardial infarction (%/n) | 6.4/16 |
| stroke (%/n) | 2.8/7 |
| atrial fibrillation (%/n) | 8.8/22 |
| deep vein thrombosis (%/n) | 3.6/9 |
| type 2 diabetes (%/n) | 13.2/33 |
| thyroid disease (%/n) | 16.4/41 |
| smoking (%/n) | 13.2/33 |
| Parameter | Obesity (A, n = 98) | Overweight (B, n = 83) | Control Group (C, n = 69) | p < 0.05 |
|---|---|---|---|---|
| age (years) | 61.18 ± 11.07 | 53.40 ± 13.70 | 58.83 ± 15.33 | ns |
| sex (%/n) | ||||
| Male | 50.0/49 | 50.6/42 | 18.8/13 | A, B vs. C |
| Female | 50.0/49 | 49.4/41 | 81.2/56 | A, B vs. C |
| height (cm) | 168.14 ± 9.65 | 168.64 ± 10.36 | 164.76 ± 8.78 | ns |
| weight (kg) | 95.09 ± 13.24 | 78.39 ± 11.10 | 62.02 ± 7.99 | A vs. B, C B vs. C |
| BMI (kg/m2) | 33.62 ± 3.26 | 27.56 ± 1.34 | 22.86 ± 1.71 | A vs. B, C B vs. C |
| waist circumference (cm) | 107.29 ± 10.16 | 95.29 ± 8.55 | 79.72 ± 7.55 | A vs. B, C B vs. C |
| hip circumference (cm) | 115.23 ± 7.32 | 104.26 ± 5.23 | 93.83 ± 14.14 | A vs. B, C B vs. C |
| WHR | 0.93 ± 0.08 | 0.91 ± 0.09 | 1.05 ± 1.56 | ns |
| hypertension (%/n) | 64.3/63 | 50.6/42 | 39.1/27 | A, B vs. C |
| myocardial infarction (%/n) | 7.1/7 | 7.2/6 | 4.3/3 | ns |
| stroke (%/n) | 2.0/2 | 3.6/3 | 2.9/2 | ns |
| atrial fibrillation (%/n) | 8.2/8 | 12.0/10 | 5.8/4 | ns |
| deep vein thrombosis (%/n) | 7.1/7 | 1.2/1 | 1.4/1 | ns |
| type 2 diabetes (%/n) | 21.4/21 | 10.8/9 | 4.3/3 | A vs. C |
| thyroid disease (%/n) | 16.3/16 | 13.2/11 | 20.3/14 | ns |
| smoking (%/n) | 9.2/9 | 15.8/13 | 15.9/11 | ns |
| Parameter | Mean | Confidence Interval −95.000% | Confidence Interval +95.000% | SD | Coefficients of Variability |
|---|---|---|---|---|---|
| HR (bpm) | 66.73 | 66.25 | 68.21 | 11.87 | 17.79 |
| P-wave width (ms) | 109.63 | 107.18 | 112.08 | 19.65 | 17.92 |
| P disp (ms) | 34.45 | 31.91 | 36.99 | 20.38 | 59.16 |
| PQ interval (ms) | 168.19 | 164.37 | 172.01 | 30.68 | 18.24 |
| QRS complex width (ms) | 103.70 | 101.17 | 106.23 | 20.34 | 19.62 |
| QT interval (ms) | 389.79 | 385.72 | 393.86 | 32.69 | 8.39 |
| QTc interval (ms) | 408.54 | 405.37 | 411.71 | 25.46 | 6.23 |
| QTd (ms) | 35.02 | 31.97 | 38.06 | 24.45 | 69.82 |
| QRS axis (°) | 26.24 | 21.09 | 31.38 | 41.31 | 157.46 |
| Sokolow–Lyon index LV (mm) | 18.40 | 17.67 | 19.13 | 5.87 | 31.93 |
| Sokolow–Lyon index RV (mm) | 3.63 | 3.27 | 3.99 | 2.26 | 62.20 |
| Tpeak-Tend (ms) | 94.66 | 92.01 | 97.31 | 21.28 | 22.48 |
| (Tpeak-Tend) disp (ms) | 39.17 | 36.76 | 41.59 | 19.38 | 49.46 |
| (Tpeak-Tend)/QT | 0.24 | 0.23 | 0.24 | 0.05 | 23.28 |
| (Tpeak-Tend)/QTc | 0.22 | 0.22 | 0.23 | 0.04 | 19.35 |
| JTpeak (ms) | 199.89 | 195.92 | 203.85 | 31.83 | 15.92 |
| JT interval (ms) | 293.54 | 289.39 | 297.70 | 33.34 | 11.36 |
| JTpeak/JT | 0.69 | 0.68 | 0.69 | 0.07 | 10.03 |
| (Tpeak-Tend)/JTpeak | 0.42 | 0.40 | 0.44 | 0.17 | 40.93 |
| Tpeak (mV) | 0.40 | 0.37 | 0.43 | 0.24 | 61.05 |
| Tpeak/JT (mV/ms) | 0.00 | 0.00 | 0.00 | 0.00 | 62.92 |
| JTpeak-JTend (ms) | 95.93 | 92.37 | 99.49 | 28.57 | 29.79 |
| (JTpeak-JTend) disp (ms) | 44.90 | 41.23 | 48.56 | 29.34 | 65.36 |
| Parameter | Obesity (A, n = 98) | Overweight (B, n = 83) | Control Group (C, n = 69) | p < 0.05 |
|---|---|---|---|---|
| HR (bpm) | 66.50 ± 11.45 | 66.04 ± 12.10 | 67.90 ± 12.26 | ns |
| P-wave width (ms) | 113.12 ± 19.98 | 111.66 ± 17.92 | 102.22 ± 19.45 | A, B vs. C |
| P disp (ms) | 40.08 ± 19.39 | 31.01 ± 21.58 | 30.59 ± 18.66 | A vs. B, C |
| PQ interval (ms) | 177.45 ± 29.74 | 167.73 ± 28.92 | 155.58 ± 29.86 | A vs. B, C B vs. C |
| QRS complex width (ms) | 107.24 ± 21.34 | 102.47 ± 23.26 | 100.14 ± 13.42 | A vs. C |
| QT interval (ms) | 392.66 ± 25.77 | 390.19 ± 40.85 | 385.23 ± 30.32 | ns |
| QTc interval (ms) | 411.50 ± 23.43 | 406.95 ± 30.23 | 406.25 ± 21.61 | ns |
| QTd (ms) | 39.63 ± 23.14 | 32.02 ± 27.95 | 32.06 ± 20.77 | A vs. B, C |
| QRS axis (°) | 17.32 ± 38.36 | 27.05 ± 41.37 | 37.93 ± 42.80 | ns |
| Sokolow-index LV (mm) | 17.11 ± 5.15 | 19.77 ± 6.16 | 18.58 ± 6.15 | ns |
| Sokolow-index RV (mm) | 3.73 ± 2.23 | 3.32 ± 2.40 | 3.71 ± 2.22 | ns |
| Tpeak-Tend (ms) | 97.08 ± 23.38 | 95.88 ± 23.71 | 89.74 ± 12.88 | A vs. C |
| (Tpeak-Tend) disp (ms) | 43.29 ± 24.14 | 37.34 ±17.75 | 35.52 ± 11.03 | A vs. B, C |
| (Tpeak-Tend)/QT | 0.23 ± 0.05 | 0.25 ± 0.07 | 0.23 ± 0.03 | ns |
| (Tpeak-Tend)/QTc | 0.22 ± 0.04 | 0.23 ± 0.05 | 0.22 ± 0.03 | ns |
| JTpeak (ms) | 205.92 ± 28.04 | 198.77 ± 32.39 | 192.67 ± 34.88 | A vs. C |
| JT interval (ms) | 292.82 ± 28.67 | 295.52 ± 36.14 | 292.20 ± 36.25 | ns |
| JTpeak/JT | 0.69 ± 0.07 | 0.67 ± 0.07 | 0.69 ± 0.05 | ns |
| (Tpeak-Tend)/JTpeak | 0.46 ± 0.16 | 0.35 ± 0.21 | 0.45 ± 0.11 | ns |
| Tpeak (mV) | 0.39 ± 0.26 | 0.40 ± 0.25 | 0.41 ± 0.21 | ns |
| Tpeak/JT (mV/ms) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ns |
| JTpeak-JTend (ms) | 99.55 ± 34.53 | 95.98 ± 29.64 | 90.72 ± 13.54 | A vs. C |
| (JTpeak-JTend) disp (ms) | 48.22 ± 37.60 | 44.04 ± 26.32 | 41.19 ± 16.33 | ns |
| Parameter | Body Weight (kg) | BMI (kg/m2) | Waist Circumference (cm) | Hip Circumference (cm) | WHR |
|---|---|---|---|---|---|
| HR (bpm) | ns | ns | ns | ns | ns |
| P-wave width (ms) | 0.31 | 0.25 | 0.30 | 0.20 | ns |
| P disp (ms) | ns | 0.15 | 0.16 | 0.17 | ns |
| PQ interval (ms) | 0.38 | 0.33 | 0.40 | 0.32 | ns |
| QRS complex width (ms) | 0.16 | 0.16 | 0.14 | ns | ns |
| QT interval (ms) | ns | ns | ns | ns | ns |
| QTTc interval (ms) | ns | ns | ns | ns | ns |
| QTd (ms) | ns | ns | ns | ns | ns |
| QRS axis (°) | ns | ns | ns | ns | ns |
| Sokolow-index LV (mm) | ns | ns | ns | ns | ns |
| Sokolow-index RV (mm) | ns | ns | ns | ns | ns |
| Tpeak-Tend (ms) | 0.16 | ns | 0.16 | ns | ns |
| (Tpeak-Tend) disp (ms) | ns | ns | ns | ns | ns |
| (Tpeak-Tend)/QT | ns | ns | ns | ns | ns |
| (Tpeak-Tend)/QTc | ns | ns | ns | ns | ns |
| JTpeak (ms) | ns | 0.15 | ns | 0.19 | 0.18 |
| JT interval (ms) | ns | ns | ns | ns | ns |
| JTpeak/JT | ns | ns | ns | ns | ns |
| (Tpeak-Tend)/JTpeak | ns | ns | ns | ns | ns |
| Tpeak (mV) | ns | ns | ns | ns | 0.16 |
| Tpeak/JT (mV/ms) | ns | 0.15 | ns | ns | 0.16 |
| JTpeak-JTend (ms) | ns | ns | ns | ns | ns |
| (JTpeak-JTend) disp (ms) | ns | ns | ns | ns | ns |
| Age | WHR | Type 2 Diabetes | Smoking | |
|---|---|---|---|---|
| Regression coefficient (RC) | 0.439 | 17.563 | 13.064 | 6.259 |
| SEM of Rc | 0.163 | 3.032 | 6.081 | 2.803 |
| p | <0.01 | <0.001 | <0.05 | <0.05 |
| p for the model | p < 0.001 | |||
| Male | BMI (kg/m2) | β-Blockers | |
|---|---|---|---|
| Regression coefficient (RC) | 0.001 | 0.001 | −0.001 |
| SEM of Rc | 0.000 | 0.000 | 0.000 |
| p | <0.001 | <0.001 | <0.05 |
| p for the model | p < 0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dykiert, I.A.; Kraik, K.; Jurczenko, L.; Gać, P.; Poręba, R.; Poręba, M. The Effect of Obesity on Repolarization and Other ECG Parameters. J. Clin. Med. 2024, 13, 3587. https://doi.org/10.3390/jcm13123587
Dykiert IA, Kraik K, Jurczenko L, Gać P, Poręba R, Poręba M. The Effect of Obesity on Repolarization and Other ECG Parameters. Journal of Clinical Medicine. 2024; 13(12):3587. https://doi.org/10.3390/jcm13123587
Chicago/Turabian StyleDykiert, Irena A., Krzysztof Kraik, Lidia Jurczenko, Paweł Gać, Rafał Poręba, and Małgorzata Poręba. 2024. "The Effect of Obesity on Repolarization and Other ECG Parameters" Journal of Clinical Medicine 13, no. 12: 3587. https://doi.org/10.3390/jcm13123587





