Combined Central and Peripheral Demyelination (CCPD) Associated with MOG Antibodies: Report of Four New Cases and Narrative Review of the Literature
Abstract
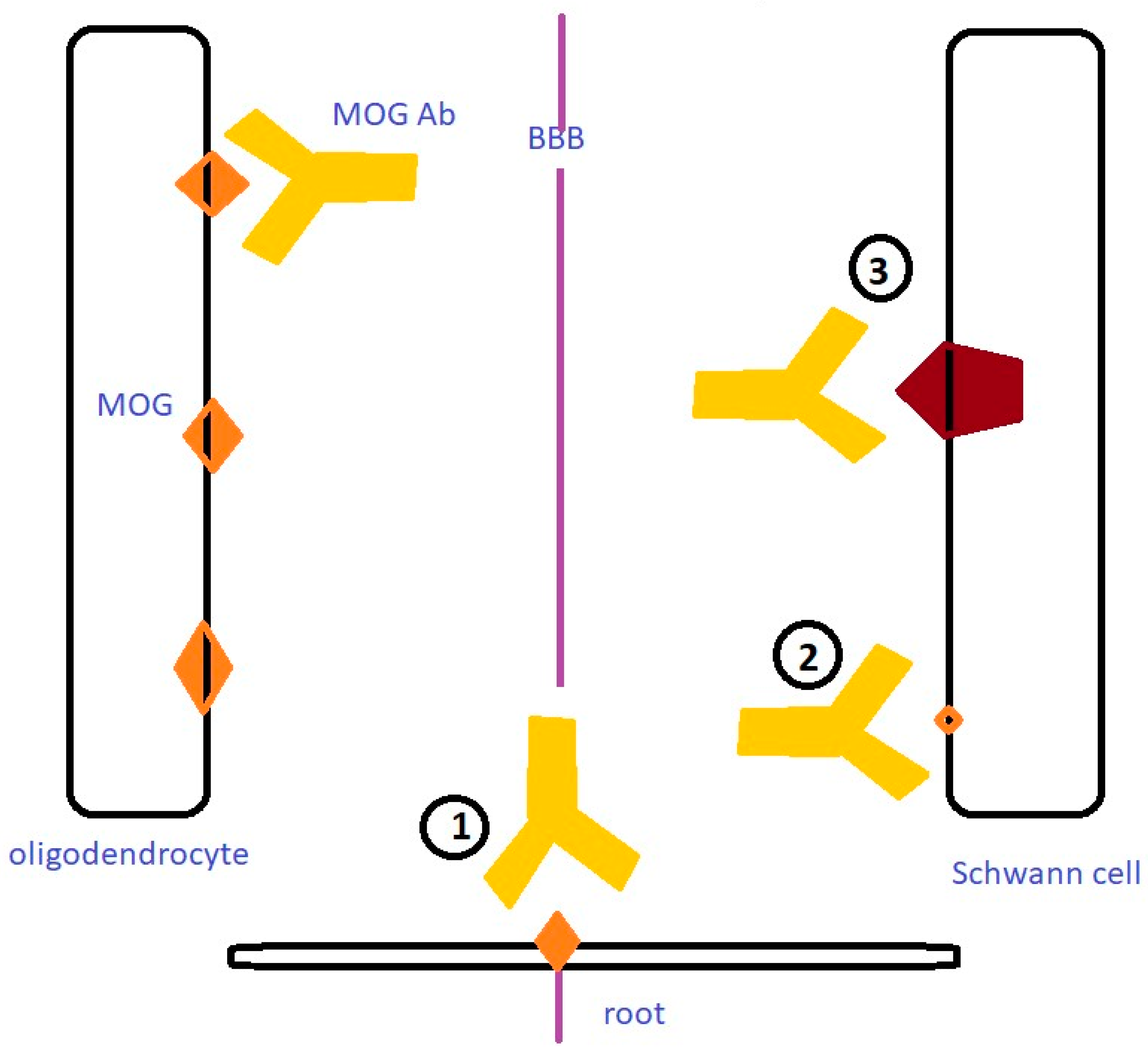
:1. Introduction
2. Materials and Methods
2.1. Case Presentation
2.2. Review of the Literature
2.3. Inclusion Criteria
- Case reports and case series were included.
- MOG antibody (IgG) seropositivity and demyelinating polyneuropathy, following diagnostic, clinical, and neurophysiological criteria, of the Joint Task Force of the EFNS and the PNS [21], without restrictions in terms of age, sex, stage, and duration of the disease.
2.4. Exclusion Criteria
- Reviews (systematic or other) and meta-analyses, clinical trials (randomized or not).
- Animal studies.
- Demyelination of the CNS not attributable to MOG antibodies, e.g., multiple sclerosis (MS) and seropositive aquaporin-4 neuromyelitis optica spectrum disorder (NMOSD).
- PNS involvement did not fulfill demyelinating electrodiagnostic criteria (e.g., axonal neuropathy, migrant sensory neuritis (Wartenberg neuritis), and pain/paresthesia without clear characterization.
- Secondary demyelinating diseases, such as infectious diseases (e.g., HIV), other inflammatory diseases (e.g., sarcoidosis), metabolic or toxic diseases (e.g., alcoholism), and inherited diseases (e.g., Charcot–Marie–Tooth)
2.5. Data Extraction
2.6. Risk of Bias
3. Results
3.1. Case Presentation
- Case A: A 20-year-old (September 1993) man, developed progressive weakness of the lower limbs and a T10 sensory level with hypoesthesia to heat, and numbness resulting, over 30 days, with difficulty standing and walking. Six months later, he was admitted to the hospital for further investigation; on examination, lower limb hypopallesthesia and spastic–ataxic gait, with downgoing plantar responses, were also noted. However, clinical findings in favor of PNS involvement were noted, i.e., diminished deep tendon reflexes in the upper limbs and almost absent in the lower limbs and mild distal lower limb weakness as well. CSF was unremarkable, with no cells, normal albumin levels, and presence of oligoclonal bands (OCBs). The MRI revealed brain lesions supratentorially, in the brainstem and cerebellum; multiple cervical and thoracic lesions, one longitudinal lesion (T6–T10 levels), with no gadolinium enhancement. Serum screening for systemic rheumatological disease, including anti-nuclear antibodies (ANAs) and antibodies to extractable nuclear antigens (anti-ENAs), was negative; complement C3 and C4 levels and vitamin B12 levels were within normal range. Although there was a strong suspicion of a diagnosis of MS, the signs of peripheral involvement led to an NCS examination that revealed demyelinating polyneuropathy (CIDP) according to EFNS/PNS electrodiagnostic criteria. Consequently, the diagnosis of CCPD was made, and genetic testing for Charcot–Marie–Tooth (CMT) disease, including CMT1-A and CMT-X subtypes, was negative. Due to a history of right optic neuritis (ON) 5 years before (1988), visual evoked potentials (VEPs) were performed showing a prolongation of P100 on right stimulation. He was treated with dexamethasone intramuscularly (8 mg × 5 days) for an MS attack and recovered fully after 40 days with mild residual numbness in his legs. After 6 months (November 1994), he developed dysesthesias and urinary incontinence and was subsequently treated with intravenous methylprednisolone (IVMP) (1 g × 5 days) and prednisolone p.o. (20 mg × 20 days) without substantial improvement; new lesions were found in a brain MRI. In the following years, the patient was treated by different physicians. During the following 8 years, he reported four clinical attacks that were treated with IVMP. He consecutively received several immunotherapies for MS, namely, intramuscular interferon beta-1a (30 mcg qWk) for 7 years that was stopped due to a clinical relapse with significant lower limb numbness; then, intravenous immune globulin pulse therapy (IVIG, 2 g/kg every 3 months) was administered for 5 years with no clinical response; then, azathioprine (50 mg TID) was tried for 1 year. Due to gastrointestinal side effects, azathioprine was switched to methotrexate (7.5 mg/week) for 1.5 years with additional clinical deterioration; gradually, he developed weakness and dysesthesia in his hands and legs, dysesthesia, and urinary incontinence. At that time, the causes of PNS involvement were further investigated; therefore, further serum screening was performed for neurofascin-155 and contactin-1 antibodies that tested negative; serum MOG antibodies were positive (1/80). A nerve biopsy was not performed, because the CCPD diagnosis was attributed consequently to MOG IgG positivity, and thus anti-B-cell therapy was initiated. Over the last 4 years, no relapses and clinical improvement have been reported under iv rituximab (600 mg every 6 months), and at the latest follow-up examination, the patient had normal sensory examination, mild distal limb weakness, and residual spastic–ataxic gait, requiring a walking aid. The timeline of the disease course is presented in Figure S1 (Supplementary Materials).
- Case B: A 67-year-old (2023) man, developed gradually, over 6 months, numbness in the upper and lower limbs, distal muscle weakness in the upper and lower limbs with a 4/5 score on the British Medical Research Council (BMRC) scale, lower limb hypopallesthesia (3/8 grade according to the graduated Rydel–Seiffer tuning fork), absent tendon reflexes in his lower limbs, ataxic gait with instability and falls, and hoarseness. The NCS findings were compatible with demyelinating neuropathy (CIDP) according to the EFNS/PNS electrodiagnostic criteria. A brain MRI showed supratentorial, non-enhancing, subcortical, and deep white matter brain lesions, whereas the spinal MRI was negative. CSF had increased albumin and negative OCBs. Accordingly, the diagnosis of CCPD was reached. A serum autoantibody screening for autoimmune CNS inflammatory disorders including ANAs, anti-ENAs, anti-double-stranded DNA (anti-dsDNA) antibodies, C3 and C4 levels, and anti-cardiolipin and anti-β-2 glycoprotein I (anti-β2GPI) antibodies was negative, as was the screening for infectious causes, including human immunodeficiency virus (HIV). Moreover, due to PNS involvement, further laboratory investigation was performed including serum antibodies against gangliosides and a paraneoplastic antibody panel, which were negative. Monoclonal gammopathy was also investigated and excluded. In addition, the laboratory investigation of CCPD also included MOG antibodies in the serum, which tested positive. A nerve biopsy was not required because CCPD was linked to MOGAD. He received IVMP (1 g/24 h) for 3 days with a good clinical response regarding muscle weakness (BMRC score 5/5); after a 13-month treatment period with p.o. methylprednisolone (16 mg/24 h slowly tapering to 4 mg/24 h), the neurological examination showed further improvement of the lower limb hypopallesthesia (4/8 Rydel–Seiffer score) and of the gait ataxia with only mild difficulty in tandem walking The timeline of the disease course is depicted in Figure S2 (Supplementary Materials).
- Case C: A 10-year-old (2018) boy, presented with over 10 days of progressive muscle weakness in the lower limbs, resulting in a 3/5 score on the BMRC scale, areflexia in the lower limbs, gait instability, and incontinence. A brain MRI showed multiple supratentorial, brainstem, and cerebellar non-enhancing lesions; non-active lesions were also found in the thoracic and lower cervical spine, with thickening and enhancement of the lumbosacral roots. NCS was consistent with demyelinating polyneuropathy (CIDP) according to EFNS/PNS electrodiagnostic criteria. The CSF revealed pleocytosis (lymphocytosis) and elevated albumin with negative OCBs. There were no findings of rheumatic disease autoantibodies in the serum (ANAs, anti-ENAs, anti-ds DNA, anti-cardiolipin, anti-β2GPI, and C3 and C4 levels); infectious causes of myelitis in the CSF including herpes simplex virus 1 (HSV-1) HSV-2), human herpes virus 6 (HHV-6), varicella zoster virus (VZV), cytomegalovirus, enterovirus, West Nile virus (WNV), Epstein–Barr virus (EBV), and HIV were also absent. Anti-ganglioside antibodies associated with autoimmune peripheral neuropathies were also investigated and were negative. NMOSD antibody serum screening for MOG IgGs was positive, and anti-AQP4 was negative. The patient was diagnosed with CCPD due to MOG antibodies, and therefore a nerve biopsy was not performed. He was treated with IVMP (1 g/24 h) for 5 days followed by a 5-day course of IVIG 2 g/kg with marked improvement during the first week, and he continued on oral corticosteroids. After 3 months, an MRI was unremarkable for new lesions, but after 9 months, new lesions were observed in the thoracic spine; after 13 months, visual acuity was diminished, compatible with optic neuritis. He received IVMP (1 g × 5 days) with marked improvement and was started on preventive therapy for MOGAD with rituximab (375 mg/m2 per week); due to an allergic reaction during the third infusion, rituximab was switched to p.o. mycophenolate mofetil (2 g/24 h), and since then (4.5 years), he has remained in a stable condition. The timeline of the disease course is presented in Figure S3 (Supplementary Materials).
- Case D: A 20-year-old (February 2022) woman, reported numbness in four limbs and muscle weakness in left limbs, beginning 2 weeks before admission. On examination, she had difficulty walking, left hemiplegia, “stocking-glove” distribution hypoesthesia with abolished ankle reflexes, and lower limb hypopallesthesia (4/8 grade according to the graduated Rydel–Seiffer tuning fork). An MRI of the brain and spine revealed multiple T2-weighted hyperintense lesions with no gadolinium enhancement; in detail, few for MS non-typical brain lesions and multiple spinal lesions at the C1–C2 levels with a central location on the axial plane, C3 level, and T5 and T8 levels. Due to the distribution of the aforementioned sensory symptoms, the patient was investigated for polyneuropathy NCS-confirmed demyelinating polyneuropathy (CIDP) according to the EFNS/PNS electrodiagnostic criteria. CSF showed elevated albumin and OCBs type II. Serum and CSF screening for infectious causes of myelitis (WNV, EBV, HIV, HSV-1, HSV-2, HHV-6, VZV, cytomegalovirus, and enterovirus) and serological testing for autoantibodies in systemic autoimmune diseases (ANAs, anti-ENAs, anti-ds DNA, anti-cardiolipin, anti-β2GPI, and C3 and C4 levels) were normal. In the context of atypical CNS demyelination, serum MOG antibodies tested positive, whereas anti-AQP4 tested negative, suggesting the diagnosis of MOGAD with CCPD; therefore, a nerve biopsy was not performed. She was treated with IVMP (1 g/24 h) for 5 days followed by p.o. corticosteroids (methylprednisolone), starting dose 64mg/day; the numbness subsided, and ankle reflexes and muscle strength and gait were restored over the next 2 weeks. Four months (June 2022) after the clinical attack, she had only residual left-hand numbness; on examination, there was lower-limb hypopallesthesia with normal muscle strength. The patient refused prophylactic treatment. However, 26 months after the first episode (April 2024), she relapsed with left hemiparesis and two new enhancing lesions in the brain and cervical MRI. The patient received IVMP (1 g × 5 days) with a favorable clinical response (resolution of hemiparesis) and was switched to long-term immunotherapy; she received the starting dose of iv rituximab (600 mg weekly for 4 weeks, May 2024), and subsequently, follow-up infusions (600 mg) have been planned for every 6 months. The timeline of the disease course is depicted in Figure S4 (Supplementary Materials).
3.2. Review of the Literature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barkhof, F.; Koeller, K.K. Demyelinating Diseases of the CNS (Brain and Spine). In Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; IDKD Springer Series; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-38489-0. [Google Scholar]
- Leray, E.; Moreau, T.; Fromont, A.; Edan, G. Epidemiology of Multiple Sclerosis. Rev. Neurol. 2016, 172, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. A Reassessment of the Distribution of Multiple Sclerosis. Acta Neurol. Scand. 1975, 51, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.H.; Samuels, M.A.; Klein, J.P.; Prasad, S. Adams and Victor’s Principles of Neurology, 12th ed.; McGraw Hill/Medical: New York, NY, USA; Chicago, IL, USA; San Francisco, CA, USA; Athens, Greece; London, UK, 2023; ISBN 978-1-264-26452-0. [Google Scholar]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2017, 7, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Balcerac, A.; Louapre, C. Genetics and Familial Distribution of Multiple Sclerosis: A Review. Rev. Neurol. 2022, 178, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Sahraian, M.A.; Naser Moghadasi, A. Multiple Sclerosis and Human Leukocyte Antigen Genotypes: Focus on the Middle East and North Africa Region. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217319881775. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, V.; Kursula, P. Multiple Sclerosis and Myelin Basic Protein: Insights into Protein Disorder and Disease. Amino Acids 2022, 54, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Höftberger, R.; Lassmann, H.; Berger, T.; Reindl, M. Pathogenic Autoantibodies in Multiple Sclerosis—From a Simple Idea to a Complex Concept. Nat. Rev. Neurol. 2022, 18, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, C.F.; Guo, Y.; Popescu, B.F.G.; Fujihara, K.; Itoyama, Y.; Misu, T. The Pathology of an Autoimmune Astrocytopathy: Lessons Learned from Neuromyelitis Optica. Brain Pathol. 2014, 24, 83–97. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; de Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International Consensus Diagnostic Criteria for Neuromyelitis Optica Spectrum Disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef]
- Mealy, M.A.; Mossburg, S.E.; Kim, S.-H.; Messina, S.; Borisow, N.; Lopez-Gonzalez, R.; Ospina, J.P.; Scheel, M.; Yeshokumar, A.K.; Awad, A.; et al. Long-Term Disability in Neuromyelitis Optica Spectrum Disorder with a History of Myelitis Is Associated with Age at Onset, Delay in Diagnosis/Preventive Treatment, MRI Lesion Length and Presence of Symptomatic Brain Lesions. Mult. Scler. Relat. Disord. 2019, 28, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Orphanet: Neuromyelitis Optica Spectrum Disorder. Available online: https://www.orpha.net/en/disease/detail/71211 (accessed on 17 May 2024).
- Banwell, B.; Bennett, J.L.; Marignier, R.; Kim, H.J.; Brilot, F.; Flanagan, E.P.; Ramanathan, S.; Waters, P.; Tenembaum, S.; Graves, J.S.; et al. Diagnosis of Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: International MOGAD Panel Proposed Criteria. Lancet Neurol. 2023, 22, 268–282. [Google Scholar] [CrossRef] [PubMed]
- De Mol, C.L.; Wong, Y.; van Pelt, E.D.; Wokke, B.; Siepman, T.; Neuteboom, R.F.; Hamann, D.; Hintzen, R.Q. The Clinical Spectrum and Incidence of Anti-MOG-Associated Acquired Demyelinating Syndromes in Children and Adults. Mult. Scler. 2020, 26, 806–814. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.; Hamilton-Shield, A.; Woodhall, M.; Messina, S.; Mariano, R.; Waters, P.; Ramdas, S.; Leite, M.I.; Palace, J. Prevalence and Incidence of Neuromyelitis Optica Spectrum Disorder, Aquaporin-4 Antibody-Positive NMOSD and MOG Antibody-Positive Disease in Oxfordshire, UK. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, S.; Suichi, T. Validation of the 2021 EAN/PNS Diagnostic Criteria for Chronic Inflammatory Demyelinating Polyneuropathy. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1237–1238. [Google Scholar] [CrossRef] [PubMed]
- Broers, M.C.; Bunschoten, C.; Nieboer, D.; Lingsma, H.F.; Jacobs, B.C. Incidence and Prevalence of Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Systematic Review and Meta-Analysis. Neuroepidemiology 2019, 52, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Mathey, E.K.; Park, S.B.; Hughes, R.A.C.; Pollard, J.D.; Armati, P.J.; Barnett, M.H.; Taylor, B.V.; Dyck, P.J.B.; Kiernan, M.C.; Lin, C.S.-Y. Chronic Inflammatory Demyelinating Polyradiculoneuropathy: From Pathology to Phenotype. J. Neurol. Neurosurg. Psychiatry 2015, 86, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on Management of Chronic Inflammatory Demyelinating Polyradiculoneuropathy: Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. J. Peripher. Nerv. Syst. 2010, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A.; Rees, J.H. Clinical and Epidemiologic Features of Guillain-Barré Syndrome. J. Infect. Dis. 1997, 176 (Suppl. S2), S92–S98. [Google Scholar] [CrossRef]
- Uncini, A.; Kuwabara, S. Electrodiagnostic Criteria for Guillain-Barrè Syndrome: A Critical Revision and the Need for an Update. Clin. Neurophysiol. 2012, 123, 1487–1495. [Google Scholar] [CrossRef]
- Van Doorn, P.A.; Van den Bergh, P.Y.K.; Hadden, R.D.M.; Avau, B.; Vankrunkelsven, P.; Attarian, S.; Blomkwist-Markens, P.H.; Cornblath, D.R.; Goedee, H.S.; Harbo, T.; et al. European Academy of Neurology/Peripheral Nerve Society Guideline on Diagnosis and Treatment of Guillain–Barré Syndrome. J. Peripher. Nerv. Syst. 2023, 28, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Cortese, A.; Franciotta, D.; Alfonsi, E.; Visigalli, N.; Zardini, E.; Diamanti, L.; Prunetti, P.; Osera, C.; Gastaldi, M.; Berzero, G.; et al. Combined Central and Peripheral Demyelination: Clinical Features, Diagnostic Findings, and Treatment. J. Neurol. Sci. 2016, 363, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kamm, C.; Zettl, U.K. Autoimmune Disorders Affecting Both the Central and Peripheral Nervous System. Autoimmun. Rev. 2012, 11, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liang, Y.; Cui, P.; Hao, J. The Clinical Features of Combined Central and Peripheral Demyelination and Antibodies against the Node of Ranvier. Mult. Scler. 2022, 28, 453–462. [Google Scholar] [CrossRef]
- Yuki, N. Fisher Syndrome and Bickerstaff Brainstem Encephalitis (Fisher-Bickerstaff Syndrome). J. Neuroimmunol. 2009, 215, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baumann, N.; Pham-Dinh, D. Biology of Oligodendrocyte and Myelin in the Mammalian Central Nervous System. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Lassmann, H.; Waehneldt, T.V.; Matthieu, J.M.; Linington, C. Differential Ultrastructural Localization of Myelin Basic Protein, Myelin/Oligodendroglial Glycoprotein, and 2’,3’-Cyclic Nucleotide 3’-Phosphodiesterase in the CNS of Adult Rats. J. Neurochem. 1989, 52, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Schluesener, H.J.; Sobel, R.A.; Linington, C.; Weiner, H.L. A Monoclonal Antibody against a Myelin Oligodendrocyte Glycoprotein Induces Relapses and Demyelination in Central Nervous System Autoimmune Disease. J. Immunol. 1987, 139, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Mohammad, S.; Tantsis, E.; Nguyen, T.K.; Merheb, V.; Fung, V.S.C.; White, O.B.; Broadley, S.; Lechner-Scott, J.; Vucic, S.; et al. Clinical Course, Therapeutic Responses and Outcomes in Relapsing MOG Antibody-Associated Demyelination. J. Neurol. Neurosurg. Psychiatry 2018, 89, 127–137. [Google Scholar] [CrossRef]
- Kawamura, N.; Yamasaki, R.; Yonekawa, T.; Matsushita, T.; Kusunoki, S.; Nagayama, S.; Fukuda, Y.; Ogata, H.; Matsuse, D.; Murai, H.; et al. Anti-Neurofascin Antibody in Patients with Combined Central and Peripheral Demyelination. Neurology 2013, 81, 714–722. [Google Scholar] [CrossRef]
- Vazquez Do Campo, R.; Stephens, A.; Marin Collazo, I.V.; Rubin, D.I. MOG Antibodies in Combined Central and Peripheral Demyelination Syndromes. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e503. [Google Scholar] [CrossRef]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing Narrative Literature Reviews for Peer-Reviewed Journals: Secrets of the Trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.scribd.com/document/434616519/ANDJ-Narrative-Review-Checklist (accessed on 16 June 2024).
- Dinoto, A.; Licciardi, N.M.; Reindl, M.; Chiodega, V.; Schanda, K.; Carta, S.; Höftberger, R.; Ferrari, S.; Mariotto, S. Peripheral Neuropathy and MOG-IgG: A Clinical and Neuropathological Retrospective Study. Mult. Scler. Relat. Disord. 2022, 68, 104214. [Google Scholar] [CrossRef]
- Rinaldi, S.; Davies, A.; Fehmi, J.; Beadnall, H.N.; Wang, J.; Hardy, T.A.; Barnett, M.H.; Broadley, S.A.; Waters, P.; Reddel, S.W.; et al. Overlapping Central and Peripheral Nervous System Syndromes in MOG Antibody-Associated Disorders. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e924. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Nair, S.S.; Jaganmohan, D.; Unnikrishnan, G.; Nair, M. Relapsing Lumbosacral Myeloradiculitis: An Unusual Presentation of MOG Antibody Disease. Mult. Scler. 2020, 26, 509–511. [Google Scholar] [CrossRef]
- Shima, T.; Tsujino, A. MOG Antibody-Related Disease with Recurrent Optic Neuritis and Sensory Polyradiculoneuropathy: A Case Report. Mult. Scler. Relat. Disord. 2020, 46, 102597. [Google Scholar] [CrossRef]
- Nakamura, M.; Fujimori, J.; Kobayashi, M.; Ishigaki, A.; Kikuchi, H.; Miyazawa, K.; Sato, K.; Kawasaski, E.; Suzuki, Y.; Nakashima, I. Refractory Case of Myelin Oligodendrocyte Glycoprotein Antibody-Associated Encephalomyelitis with Lumbosacral Radiculitis. Clin. Exp. Neuroimmunol. 2020, 11, 126–130. [Google Scholar] [CrossRef]
- Nakamura, T.; Kaneko, K.; Watanabe, G.; Harashima, S.; Kawasaki, E.; Tsukita, K.; Takahashi, T.; Nakashima, I.; Misu, T.; Suzuki, Y. Myelin Oligodendrocyte Glycoprotein-IgG-Positive, Steroid-Responsive Combined Central and Peripheral Demyelination with Recurrent Peripheral Neuropathy. Neurol. Sci. 2021, 42, 1135–1138. [Google Scholar] [CrossRef]
- Kang, M.S.; Kim, M.K.; Kim, Y.E.; Kim, J.H.; Kim, B.J.; Lee, H.L. Two Cases of Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease Presenting with Cauda Equina Syndrome without Conus Myelitis. Mult. Scler. Relat. Disord. 2021, 52, 103017. [Google Scholar] [CrossRef]
- Akbar, A.; Blume, G.M.; Creeden, S.; Ahmad, S. COVID-19 and MOG-IgG-Associated Acquired Demyelinating Polyneuropathy Compatible with Chronic Inflammatory Demyelinating Polyneuropathy in a Previously Healthy Girl. Bayl. Univ. Med. Cent. Proc. 2022, 35, 719–721. [Google Scholar] [CrossRef]
- Elterefi, A.E.; Elbashari, M.Y.; Alzaabi, A.; Abouelnaga, M.E.; Eissa, H. Combined Central and Peripheral Demyelination in a Patient of Multifocal Motor Neuropathy and Positive Anti-Myelin Oligodendrocyte Glycoprotein (MOG) Antibodies. Cureus 2022, 14, e32143. [Google Scholar] [CrossRef] [PubMed]
- Spiezia, A.L.; Carotenuto, A.; Iovino, A.; Moccia, M.; Gastaldi, M.; Iodice, R.; Tedeschi, E.; Petracca, M.; Lavorgna, L.; d’Ambrosio, A.; et al. AQP4-MOG Double-Positive Neuromyelitis Optica Spectrum Disorder: Case Report with Central and Peripheral Nervous System Involvement and Review of Literature. Int. J. Mol. Sci. 2022, 23, 14559. [Google Scholar] [CrossRef] [PubMed]
- Bosisio, L.; Gastaldi, M.; Inglese, M.; Rossi, A.; Franciotta, D.; Cataldi, M.; Leone, C.; Giacomini, T.; Benedetti, L.; Nobili, L.; et al. Asynchronous Combined Central and Peripheral Demyelination (CCPD) in a Girl with Anti-MOG Positivity: A Case Report and Review of the Literature. J. Neuroimmunol. 2023, 384, 578213. [Google Scholar] [CrossRef] [PubMed]
- Fuse, K.; Araki, A.; Morozumi, S.; Yasui, K. A patient with anti-myelin oligodendrocyte glycoprotein antibody-associated combined central and peripheral demyelination with anti-galactocerebroside and anti-GM1 antibodies. Rinsho Shinkeigaku 2023, 63, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A.; Kikuchi, K.; Horita, H.; Ogata, H.; Hamano, S.-I. Pediatric Anti-Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease With Combined Central and Peripheral Demyelination. Pediatr. Neurol. 2024, 152, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetc, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; The Joanna Briggs Institute: Adelaide, Australia, 2020; ISBN 978-0-648-84880-6. [Google Scholar]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological Quality of Case Series Studies: An Introduction to the JBI Critical Appraisal Tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Matsuse, D.; Yamasaki, R.; Kawamura, N.; Matsushita, T.; Yonekawa, T.; Hirotani, M.; Murai, H.; Kira, J. A Nationwide Survey of Combined Central and Peripheral Demyelination in Japan. J. Neurol. Neurosurg. Psychiatry 2016, 87, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Shirani, A.; Arcot Jayagopal, L.; Piccione, E.; Hartman, E.; Zabad, R.K. Anti-Neurofascin Antibodies Associated with White Matter Diseases of the Central Nervous System: A Red Flag or a Red Herring? Brain Sci. 2022, 12, 1124. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.L.; Tait, S.; Melrose, S.; Johnson, R.; Zonta, B.; Court, F.A.; Macklin, W.B.; Meek, S.; Smith, A.J.H.; Cottrell, D.F.; et al. Neurofascins Are Required to Establish Axonal Domains for Saltatory Conduction. Neuron 2005, 48, 737–742. [Google Scholar] [CrossRef]
- Chang, S.-H.; Wang, J.; Zhang, X.; Zhao, N.; Jia, K.; Yi, M.; Zhang, Q.-X.; Zhai, H.; Li, X.-W.; Yang, C.-S.; et al. The Prevalence of Anti-Neurofascin-155 Antibodies in Patients with Neuromyelitis Optica Spectrum Disorders. Clin. Exp. Immunol. 2021, 206, 1–11. [Google Scholar] [CrossRef]
- Devaux, J.J.; Miura, Y.; Fukami, Y.; Inoue, T.; Manso, C.; Belghazi, M.; Sekiguchi, K.; Kokubun, N.; Ichikawa, H.; Wong, A.H.Y.; et al. Neurofascin-155 IgG4 in Chronic Inflammatory Demyelinating Polyneuropathy. Neurology 2016, 86, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.M.; Malotka, J.; Kawakami, N.; Derfuss, T.; Khademi, M.; Olsson, T.; Linington, C.; Odaka, M.; Tackenberg, B.; Prüss, H.; et al. Neurofascin as a Target for Autoantibodies in Peripheral Neuropathies. Neurology 2012, 79, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Querol, L.; Nogales-Gadea, G.; Rojas-Garcia, R.; Diaz-Manera, J.; Pardo, J.; Ortega-Moreno, A.; Sedano, M.J.; Gallardo, E.; Berciano, J.; Blesa, R.; et al. Neurofascin IgG4 Antibodies in CIDP Associate with Disabling Tremor and Poor Response to IVIg. Neurology 2014, 82, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Sotgiu, S.; Giua, A.; Murrighile, M.R.; Ortu, R. A Case of Anti-Myelin-Associated Glycoprotein Polyneuropathy and Multiple Sclerosis: One Disease Instead of Two? BMJ Case Rep. 2009, 2009, bcr06.2008.0212. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Suzuki, H.; Ichihashi, J.; Inada, R.; Miyamoto, K.; Takahashi, T.; Mitsui, Y.; Fujihara, K.; Kusunoki, S. Acute Combined Central and Peripheral Demyelination Showing Anti-Aquaporin 4 Antibody Positivity. Intern. Med. 2012, 51, 2443–2447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Chen, H.; Zhuang, W.-P.; Li, H.-L. The Clinical Features of Combined Central and Peripheral Demyelination in Chinese Patients. J. Neuroimmunol. 2018, 317, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Miron, S. Myelin Associated Antibodies: Myelin-Associated Glycoprotein Autoantibodies, Myelin Basic Protein Autoantibodies and Myelin Proteolipid Autoantibodies in Neurologic Diseases. In Autoantibodies, 2nd ed.; Shoenfeld, Y., Gershwin, M.E., Meroni, P.L., Eds.; Elsevier: Burlington, NJ, USA, 2007; pp. 619–626. ISBN 978-0-444-52763-9. [Google Scholar]
- Ambrosius, W.; Michalak, S.; Kozubski, W.; Kalinowska, A. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Current Insights into the Disease Pathophysiology, Diagnosis and Management. Int. J. Mol. Sci. 2020, 22, 100. [Google Scholar] [CrossRef]
- Pagany, M.; Jagodic, M.; Schubart, A.; Pham-Dinh, D.; Bachelin, C.; Baron van Evercooren, A.; Lachapelle, F.; Olsson, T.; Linington, C. Myelin Oligodendrocyte Glycoprotein Is Expressed in the Peripheral Nervous System of Rodents and Primates. Neurosci. Lett. 2003, 350, 165–168. [Google Scholar] [CrossRef]
| History | Sex | Age | PNS | ON | Myelitis | Sequence of Nervous System Involvement | Antibodies | CSF | MRI Features | Treatment | Comments | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-A | History of ON | M | 48 | CIDP | − | + | Concurrent | Neurofascin-155 (−) Contactin-1 (−) | Normal OCB (+) | Brain Spine (C–T) | IVMP Interferon beta-1a AZA, MTX, RTX | Relapses | |
| Patient-B | M | 67 | CIDP | − | − | Concurrent | Gangliosides (−), Paraneoplasmatic (−) | Pr+ OCB (−) | Brain | IVMP improvement | Monophasic | ||
| Patient-C | M | 10 | CIDP | UON | + | Concurrent | AQP4 (−) Gangliosides (−) | Pr+ Cells + OCB (−) | Brain Spine (C–T) Cauda equina | IVMP, IVIG RTX, MM | Relapses | ||
| Patient-D | F | 20 | CIDP | − | + | Concurrent | PR+ OCBs+, (type II) | Brain Spine Gd+ | IVMP Complete resolution | Relapses | |||
| 1 | Dinoto et al., 2022 [37] | M | 55 | CIDP | − | + | Myelitis first | Contactin (−) Neurofascin 155 (−) | Pr+ OCB NI | Spine (C) | No treatment | No MOG protein expression on peripheral nerve assessed by Western blot was observed Multifocal swellings in nerve US | |
| 2 | Dinoto et al., 2022 [37] | F | 74 | CIDP | UON | − | ON preceded 10 MO | MOG (+) If 1>1/160 CBA Contactin (−) Neurofascin 155 (−) | Normal OCB (−) | NI | IVMP complete resolution | No MOG protein expression on peripheral nerve assessed by Western blot was observed | |
| 3 | Rinaldi et al., 2021 [38] | F | 9 | AIDP | − | + | Concurrent | AQP4, NF186, CNTN1, CASPR1, LGI1, GQ1b, sulfatide (−) | OCB (−) | Spine (T3–T10) Conus Caude Equina Gd+ | IVMP-IVIG incomplete resolution | Ongoing paraplegia thought to be secondary to spinal cord ischaemia /necrosis | |
| 4 | Rinaldi et al., 2021 [38] | F | 26 | MMN | UON | − | PNS preceded 30 MO | GM1, GQ1b, NF186, CNTN1, CASPR1, LGI1, sulfatide (−) | not performed | Normal spinal | IVIG complete resolution | Relapsing Monthly IVIG | |
| 5 | Rinaldi et al., 2021 [38] | 3Ms Post-partum | F | 31 | Radiculitis | − | + | Concurrent | AQP4, NF186, CNTN1, CASPR1, LGI1, GQ1b, sulfatide (−) | Pr+ OCB (−) | Spinal T8 Conus Cauda equina Gd+ | IVMP Complete resolution | Monophasic |
| 6 | Rinaldi et al., 2021 [38] | F | 34 | Radiculitis | BON | − | Concurrent | AQP4, NF186, CNTN1, CASPR1, LGI1, GQ1b, sulfatide (−) | Pr + OCB (−) | Spinal (C2, S1) cauda equina Gd+ | IVMP, IVIG Complete resolution | Monophasic | |
| 7 | Rinaldi et al., 2021 [38] | F | 54 | L Brachial neuritis | BON | − | ON first 23 MO | AQP4, NF186, CNTN1, CASPR1, LGI1, GQ1b, sulfatide (−) | OCBs (+) (serum and CSF) | NORMAL Spinal | Spontaneous recovery | Relapsing | |
| 8 | Rinaldi et al., 2021 [38] | H1N1 vaccine 2Ws before | M | 58 | L Brachial neuritis | − | + | Brachial first 72 MO | AQP4 (−) GM1 (+) | OCBs (−) | Spinal T6–T10 Gd+ | Steroids Partial resolution | Relapsing |
| 9 | Vasquez Do Campo et al., 2018 [34] | no preceding infection or vaccination | M | 18 | MADSAM | − | + | Concurrent over 3 W | AQP4, Contactin-1 Gngliosides, Sufatides, MAG, Paraneo (−) | Brain Spinal (C–T) Conus Cauda Equina Gd+ | IVMP Clinical improvement-mild residuals | Sural biopsy: demyelinating MOG-IgG1 not detected after 9 M | |
| 10 | Sundaram et al., 2019 [39] | DM | M | 51 | Radiculitis | − | + | Myelitis First 7 MO | AQP4 (−) | Pr+ OCBs (−) | Brain Spinal T12 Conus Cauda Equina Gd+ | IVMP, AZA METH, RTX | relapsing |
| 11 | Shima and Tsujino, 2020 [40] | ON 20 Ys before | M | 46 | CIDP | UON | − | Concurrent | AQP4, Gangliosides, Paraneo Plasmatic Neurofascin 155 Contactin (−) | Pr+ OCB (−) | Cauda Equina | IVMP, PE steroids po Only ON improvement | No other CNS involvement |
| 12 | Nakamura et al., 2020 [41] | INFL A infection | M | 40 | Radiculitis | − | + | Concurrent | Pr+ OCBs (−) | Brain Spine C3-Conus Cauda Equina Gd+ | IVMP, PE, IVIG, CP No clinical improvement | ||
| 13 | Nakamura et al., 2021 [42] | 3 MOs after normal delivery | F | 32 | CIDP | − | + | Concurrent | AQP4, Neurofascin 155, Ganglioside (−) | PR+ cells+ OCBs (−) | Brain Spinal C4, C7, T9) Cauda Equina Gd+ | IVIG IVMP resolution | relapses PNS steroid response |
| 14 | Kang et al., 2021 [43] | No history infection or vaccination | M | 72 | Radiculitis | − | − | No CNS | Ganglioside, AQP4, MAG | CSF Normal | Cauda Equina Gd+ | IVMP Improvement | |
| 15 | Kang et al., 2021 [43] | No infection or vaccination DM, HTN | M | 46 | CIDP | − | − | No CNS | AQP4 (−) | NI | Cauda Equina Gd+ | IVMP | |
| 16 | Akbar et al., 2022 [44] | POST-COVID | F | 9 | CIDP | − | − | No CNS | Gangliosides (−) | Pr + OCBs (−) | Cauda Equina Gd+ | IVIG (every 6–8 Ws) improvement | No CNS involvement |
| 17 | Elterefi et al., 2022 [45] | M | 24 | MMN | − | + | Concurrent | AQP4, Gangliosides (−) | Pr + OCBs (+) | Spinal (C–T) Gd + | IVIG, STEROIDS significant clinical improvement residual deficits | ||
| 18 | Spiezia et al., 2022 [46] | NI | F | 62 | CIDP | BON | + | ON first PNS myelitis 4 MO later | AQP4 (+) | OCBs (+) | Brain Spinal C2–T1 Conus Cauda Equina Gd+ | AZA, RTX Clinical improvement | Reduction in AQP4, MOG titres Improvement NCSs |
| 19 | Bosello et al., 2023 [47] | SARS-CoV-2 1M before DM, HTN | F | 74 | CIDP | UON | − | ON first CIDP 1 MO later | AQP4, Gangliosides (−) | normalL OCBs (−) | Normal MR brain/spine roots | STEROIDS IVIG improvement | Monophasic |
| 20 | Fuse et al., 2023 [48] | NI | M | 48 | CIDP | − | + | Concurrent | GM1, galactocerebroside (−) | NI | SpinailNI | STEROIDS PE Improvement | relapses |
| 21 | Bosisio et al., 2023 [47] | NI | F | 7 | CIDP | UON | NI | ON first 8 Ys | NI | Asynchronous | |||
| 22 | Horiguchi et al., 2024 [49] | six Ds after upper respiratory tract infection | F | 10 | CIDP | BON | + | Concurrent | AQP4, neurofascin 155, Contactin-1 Gangliosides (−) | Pr+ Cells + OCBs (+) | Brain Optic nerves Cauda Equina Gd + | IVMP improvement | Monophasic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, M.; Tzanetakos, D.; Moschovos, C.; Korona, A.; Vartzelis, G.; Voudris, K.; Fanouraki, S.; Dimitriadou, E.-M.; Papadimas, G.; Tzartos, J.S.; et al. Combined Central and Peripheral Demyelination (CCPD) Associated with MOG Antibodies: Report of Four New Cases and Narrative Review of the Literature. J. Clin. Med. 2024, 13, 3604. https://doi.org/10.3390/jcm13123604
Papadopoulou M, Tzanetakos D, Moschovos C, Korona A, Vartzelis G, Voudris K, Fanouraki S, Dimitriadou E-M, Papadimas G, Tzartos JS, et al. Combined Central and Peripheral Demyelination (CCPD) Associated with MOG Antibodies: Report of Four New Cases and Narrative Review of the Literature. Journal of Clinical Medicine. 2024; 13(12):3604. https://doi.org/10.3390/jcm13123604
Chicago/Turabian StylePapadopoulou, Marianna, Dimitrios Tzanetakos, Christos Moschovos, Anastasia Korona, George Vartzelis, Konstantinos Voudris, Stella Fanouraki, Evangelia-Makrina Dimitriadou, Georgios Papadimas, John S. Tzartos, and et al. 2024. "Combined Central and Peripheral Demyelination (CCPD) Associated with MOG Antibodies: Report of Four New Cases and Narrative Review of the Literature" Journal of Clinical Medicine 13, no. 12: 3604. https://doi.org/10.3390/jcm13123604
APA StylePapadopoulou, M., Tzanetakos, D., Moschovos, C., Korona, A., Vartzelis, G., Voudris, K., Fanouraki, S., Dimitriadou, E.-M., Papadimas, G., Tzartos, J. S., Giannopoulos, S., & Tsivgoulis, G. (2024). Combined Central and Peripheral Demyelination (CCPD) Associated with MOG Antibodies: Report of Four New Cases and Narrative Review of the Literature. Journal of Clinical Medicine, 13(12), 3604. https://doi.org/10.3390/jcm13123604








