A Comprehensive Review of Biologics in Phase III and IV Clinical Trials for Atopic Dermatitis
Abstract
1. Introduction
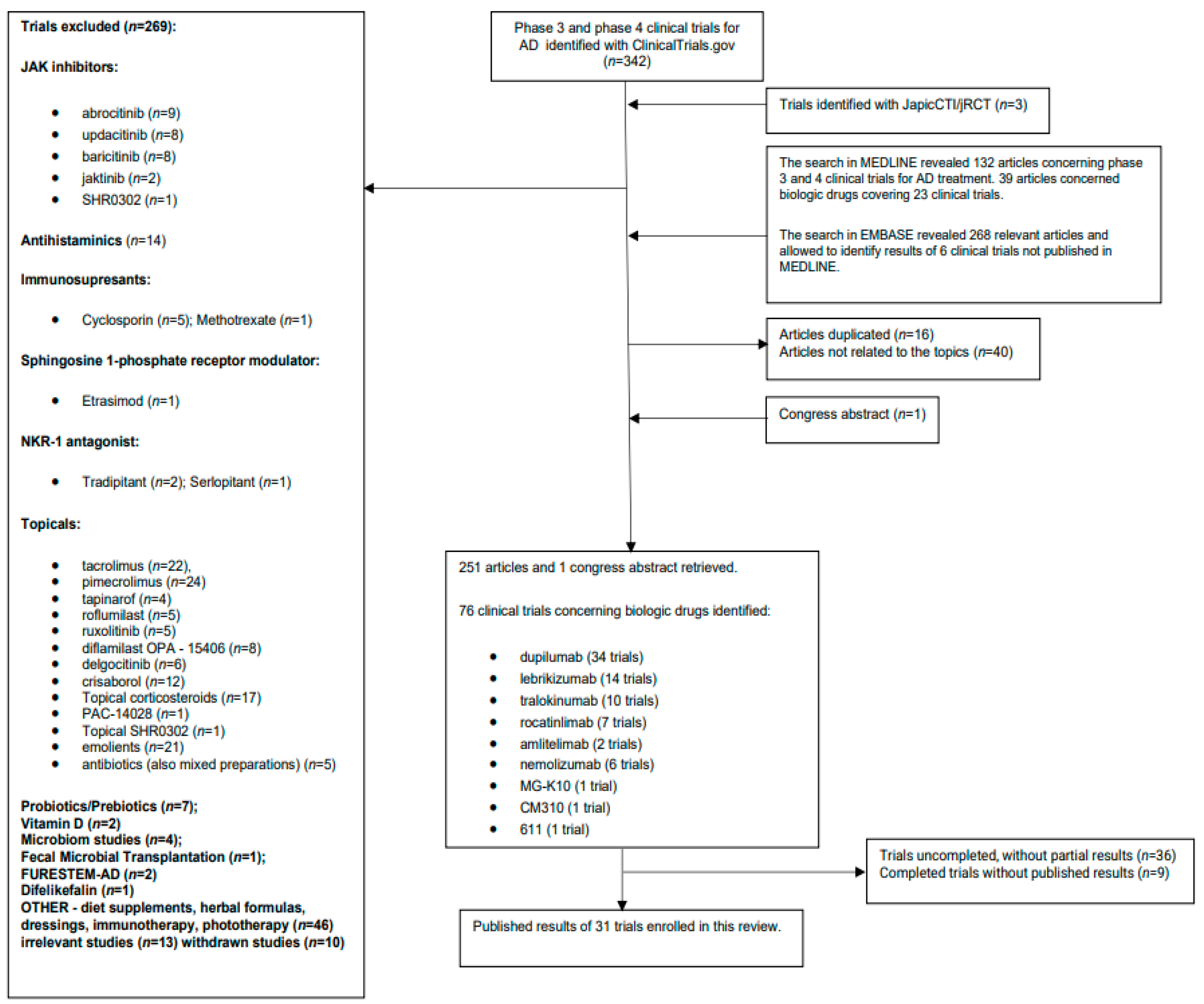
2. Methods
3. Results
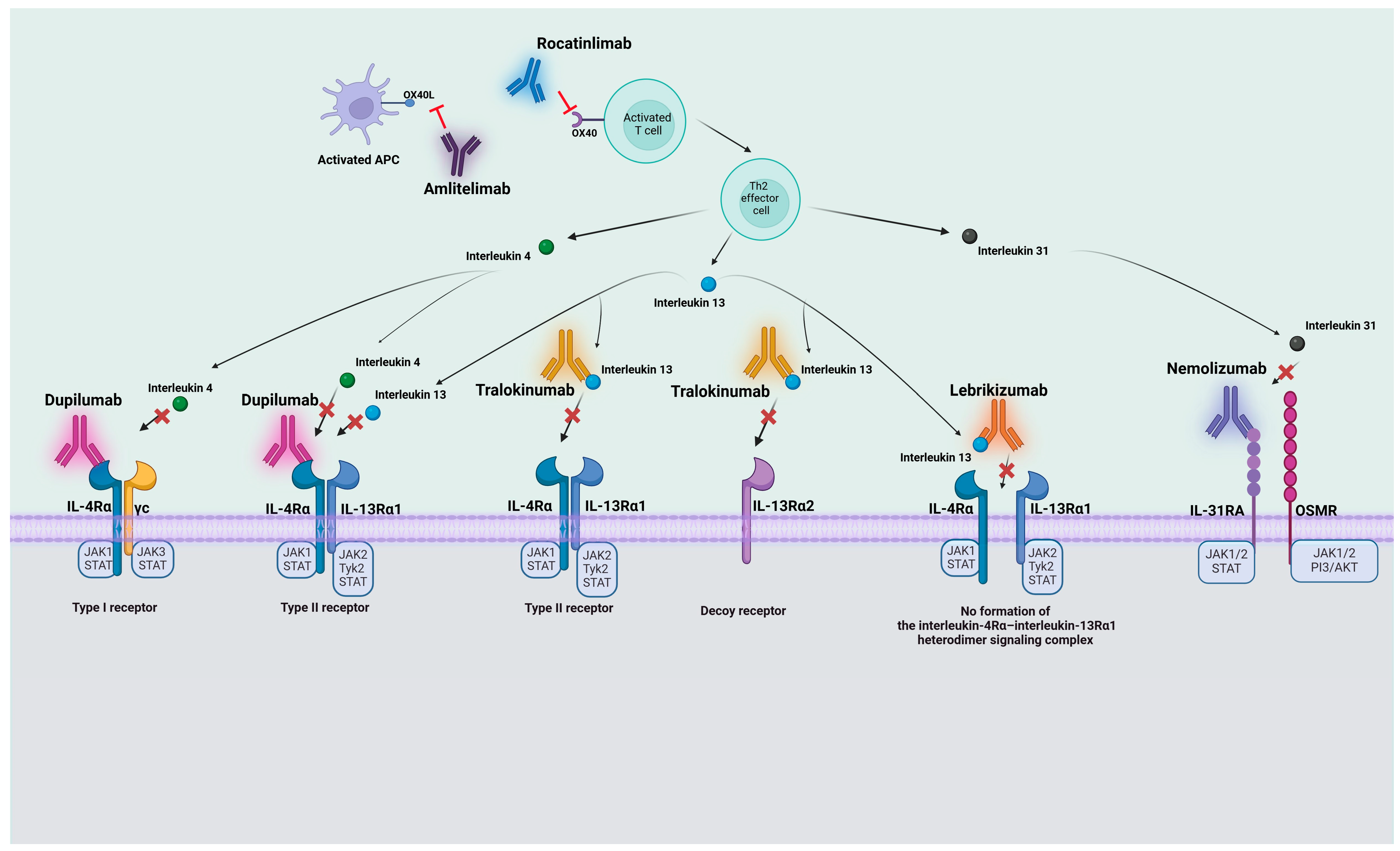
3.1. Anti-IL-4Rα Antibodies
3.1.1. Dupilumab
3.1.2. CM310
3.1.3. MG-K10 (Comekibart) and 611
3.2. Anti-IL-13 Antibodies
3.2.1. Tralokinumab
3.2.2. Lebrikizumab
3.3. Anti-IL31RA Antibodies
Nemolizumab
3.4. Anti-OX40 Antibodies
3.4.1. Rocatinlimab
3.4.2. Amlitelimab
4. Discussion
5. Conclusions and Future Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, G.; Huang, Y.; Chu, M. Clinical trials of antibody drugs in the treatments of atopic dermatitis. Front. Med. 2023, 10, 1229539. [Google Scholar] [CrossRef] [PubMed]
- Puar, N.; Chovatiya, R.; Paller, A.S. New treatments in atopic dermatitis. Ann. Allergy Asthma Immunol. 2021, 126, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. THE IL-4 RECEPTOR: Signaling Mechanisms and Biologic Functions. Ann. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, M.G.; Mentink-Kane, M.; Jacobson, B.A.; Cheever, A.W.; Whitters, M.J.; Goad, M.E.; Wong, A.; Collins, M.; Donaldson, D.D.; Grusby, M.J.; et al. Regulation and Function of the Interleukin 13 Receptor α 2 during a T Helper Cell Type 2-dominant Immune Response. J. Exp. Med. 2003, 197, 687–701. [Google Scholar] [CrossRef]
- Harb, H.; Chatila, T.A. Mechanisms of Dupilumab. Clin. Exp. Allergy 2020, 50, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Welch, A.E.; Hanson, E.M.; Boothby, M.R.; Keegan, A.D. Interleukin-4 and Interleukin-13 Signaling Connections Maps. Science 2003, 300, 1527–1528. [Google Scholar] [CrossRef] [PubMed]
- Merola, J.F.; Chiou, A.S.; During, E.; Costanzo, A.; Foley, P.; Alfalasi, A.; Gogate, S.; Pinter, A.; Dodiuk-Gad, R.; Simon, D.; et al. Dupilumab significantly improves sleep in adults with atopic dermatitis: Results from the 12-week placebo-controlled period of the 24-week phase IV randomized double-blinded placebo-controlled DUPISTAD study. Br. J. Dermatol. 2023, 189, 685–694. [Google Scholar] [CrossRef]
- Siegfried, E.C.; Cork, M.J.; Katoh, N.; Zhang, H.; Chuang, C.C.; Thomas, R.B.; Rossi, A.B.; Cyr, S.L.; Zhang, A. Dupilumab Provides Clinically Meaningful Responses in Children Aged 6–11 Years with Severe Atopic Dermatitis: Post Hoc Analysis Results from a Phase III Trial. Am. J. Clin. Dermatol. 2023, 24, 787–798. [Google Scholar] [CrossRef]
- Kamal, M.A.; Kovalenko, P.; Kosloski, M.P.; Srinivasan, K.; Zhang, Y.; Rajadhyaksha, M.; Lai, C.H.; Kanamaluru, V.; Xu, C.; Sun, X.; et al. The Posology of Dupilumab in Pediatric Patients With Atopic Dermatitis. Clin. Pharmacol. Ther. 2021, 110, 1318–1328. [Google Scholar] [CrossRef]
- Paller, A.S.; Wollenberg, A.; Siegfried, E.; Thaçi, D.; Cork, M.J.; Arkwright, P.D.; Gooderham, M.; Sun, X.; O’Malley, J.T.; Khokhar, F.A.; et al. Laboratory Safety of Dupilumab in Patients Aged 6–11 Years with Severe Atopic Dermatitis: Results from a Phase III Clinical Trial. Paediatr. Drugs 2021, 23, 515–527. [Google Scholar] [CrossRef]
- Paller, A.S.; Siegfried, E.C.; Thaçi, D.; Wollenberg, A.; Cork, M.J.; Arkwright, P.D.; Gooderham, M.; Beck, L.A.; Boguniewicz, M.; Sher, L.; et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J. Am. Acad. Dermatol. 2020, 83, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Siegfried, E.C.; Cork, M.J.; Wollenberg, A.; Arkwright, P.D.; Gonzalez, M.E.; Lockshin, B.; Chen, Z.; Bansal, A.; Levit, N.A.; et al. Laboratory Safety from a Randomized 16-Week Phase III Study of Dupilumab in Children Aged 6 Months to 5 Years with Moderate-to-Severe Atopic Dermatitis. Paediatr. Drugs 2023, 25, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Simpson, E.L.; Siegfried, E.C.; Cork, M.J.; Wollenberg, A.; Arkwright, P.D.; Soong, W.; Gonzalez, M.E.; Schneider, L.C.; Sidbury, R.; et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2022, 400, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Guttman-Yassky, E.; Paller, A.S.; Simpson, E.L.; Cork, M.J.; Weisman, J.; Browning, J.; Soong, W.; Sun, X.; Chen, Z.; et al. Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE). Am. J. Clin. Dermatol. 2022, 23, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Cork, M.J.; Thaçi, D.; Eichenfield, L.F.; Arkwright, P.D.; Hultsch, T.; Davis, J.D.; Zhang, Y.; Zhu, X.; Chen, Z.; Li, M.; et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: Results from a phase IIa open-label trial and subsequent phase III open-label extension. Br. J. Dermatol. 2020, 182, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, L.; Lu, Q.; Gao, X.; Zhu, X.; Yao, X.; Li, L.; Li, W.; Ding, Y.; Song, Z.; et al. The efficacy and safety of dupilumab in Chinese patients with moderate-to-severe atopic dermatitis: A randomized, double-blind, placebo-controlled study*. Br. J. Dermatol. 2022, 186, 633–641. [Google Scholar] [CrossRef]
- Thaçi, D.; Simpson, E.L.; Deleuran, M.; Kataoka, Y.; Chen, Z.; Gadkari, A.; Eckert, L.; Akinlade, B.; Graham, N.M.H.; Pirozzi, G.; et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: A pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J. Dermatol. Sci. 2019, 94, 266–275. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Barbarot, S.; Wollenberg, A.; Silverberg, J.I.; Deleuran, M.; Pellacani, G.; Armario-Hita, J.C.; Chen, Z.; Shumel, B.; Eckert, L.; Gadkari, A.; et al. Dupilumab provides rapid and sustained improvement in SCORAD outcomes in adults with moderate-to-severe atopic dermatitis: Combined results of four randomized phase 3 trials. J. Dermatol. Treat. 2022, 33, 266–277. [Google Scholar] [CrossRef]
- Cork, M.J.; Eckert, L.; Simpson, E.L.; Armstrong, A.; Barbarot, S.; Puig, L.; Girolomoni, G.; de Bruin-Weller, M.; Wollenberg, A.; Kataoka, Y.; et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: Analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J. Dermatol. Treat. 2020, 31, 606–614. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Yosipovitch, G.; Simpson, E.L.; Kim, B.S.; Wu, J.J.; Eckert, L.; Guillemin, I.; Chen, Z.; Ardeleanu, M.; Bansal, A.; et al. Dupilumab treatment results in early and sustained improvements in itch in adolescents and adults with moderate to severe atopic dermatitis: Analysis of the randomized phase 3 studies SOLO 1 and SOLO 2, AD ADOL, and CHRONOS. J. Am. Acad. Dermatol. 2020, 82, 1328–1336. [Google Scholar] [CrossRef]
- Alexis, A.F.; Rendon, M.; Silverberg, J.I.; Pariser, D.M.; Lockshin, B.; Griffiths, C.E.; Weisman, J.; Wollenberg, A.; Chen, Z.; Davis, J.D.; et al. Efficacy of Dupilumab in Different Racial Subgroups of Adults With Moderate-to-Severe Atopic Dermatitis in Three Randomized, Placebo-Controlled Phase 3 Trials. J. Drugs Dermatol. 2019, 18, 804–813. [Google Scholar]
- Silverberg, J.I.; Simpson, E.L.; Ardeleanu, M.; Thaçi, D.; Barbarot, S.; Bagel, J.; Chen, Z.; Eckert, L.; Chao, J.; Korotzer, A.; et al. Dupilumab provides important clinical benefits to patients with atopic dermatitis who do not achieve clear or almost clear skin according to the Investigator’s Global Assessment: A pooled analysis of data from two phase III trials. Br. J. Dermatol. 2019, 181, 80–87. [Google Scholar] [CrossRef]
- de Bruin-Weller, M.; Thaçi, D.; Smith, C.H.; Reich, K.; Cork, M.J.; Radin, A.; Zhang, Q.; Akinlade, B.; Gadkari, A.; Eckert, L.; et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: A placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br. J. Dermatol. 2018, 178, 1083–1101. [Google Scholar]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Merola, J.F.; Sidbury, R.; Wollenberg, A.; Chen, Z.; Zhang, A.; Shumel, B.; Rossi, A.B. Dupilumab prevents flares in adults with moderate to severe atopic dermatitis in a 52-week randomized controlled phase 3 trial. J. Am. Acad. Dermatol. 2021, 84, 495–497. [Google Scholar] [CrossRef]
- Deleuran, M.; Marcoux, D.; Bruin-Weller, M.; Irvine, A.D.; Baselga, E.; Ahn, K.; Castro, A.P.; Bansal, A.; Chao, J.; Bégo-Le-Bagousse, G.; et al. Dupilumab Provides Significant Clinical Benefit in a Phase 3 Trial in Adolescents with Uncontrolled Atopic Dermatitis Irrespective of Prior Systemic Immunosuppressant Use. Acta Derm. Venereol. 2021, 101, adv00504. [Google Scholar] [CrossRef]
- Siegfried, E.C.; Bieber, T.; Simpson, E.L.; Paller, A.S.; Beck, L.A.; Boguniewicz, M.; Schneider, L.C.; Khokhar, F.A.; Chen, Z.; Prescilla, R.; et al. Effect of Dupilumab on Laboratory Parameters in Adolescents with Atopic Dermatitis: Results from a Randomized, Placebo-Controlled, Phase 3 Clinical Trial. Am. J. Clin. Dermatol. 2021, 22, 243–255. [Google Scholar] [CrossRef]
- Paller, A.S.; Bansal, A.; Simpson, E.L.; Boguniewicz, M.; Blauvelt, A.; Siegfried, E.C.; Guttman-Yassky, E.; Hultsch, T.; Chen, Z.; Mina-Osorio, P.; et al. Clinically Meaningful Responses to Dupilumab in Adolescents with Uncontrolled Moderate-to-Severe Atopic Dermatitis: Post-hoc Analyses from a Randomized Clinical Trial. Am. J. Clin. Dermatol. 2020, 21, 119–131. [Google Scholar] [CrossRef]
- Simpson, E.L.; Paller, A.S.; Siegfried, E.C.; Boguniewicz, M.; Sher, L.; Gooderham, M.J.; Beck, L.A.; Guttman-Yassky, E.; Pariser, D.; Blauvelt, A.; et al. Efficacy and Safety of Dupilumab in Adolescents With Uncontrolled Moderate to Severe Atopic Dermatitis. JAMA Dermatol. 2020, 156, 44. [Google Scholar] [CrossRef]
- Beck, L.A.; Thaçi, D.; Deleuran, M.; Blauvelt, A.; Bissonnette, R.; de Bruin-Weller, M.; Hide, M.; Sher, L.; Hussain, I.; Chen, Z.; et al. Dupilumab Provides Favorable Safety and Sustained Efficacy for up to 3 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis. Am. J. Clin. Dermatol. 2020, 21, 567–577. [Google Scholar] [CrossRef]
- Deleuran, M.; Thaçi, D.; Beck, L.A.; de Bruin-Weller, M.; Blauvelt, A.; Forman, S.; Bissonnette, R.; Reich, K.; Soong, W.; Hussain, I.; et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J. Am. Acad. Dermatol. 2020, 82, 377–388. [Google Scholar] [CrossRef]
- Worm, M.; Simpson, E.L.; Thaçi, D.; Bissonnette, R.; Lacour, J.P.; Beissert, S.; Kawashima, M.; Ferrándiz, C.; Smith, C.H.; Beck, L.A.; et al. Efficacy and Safety of Multiple Dupilumab Dose Regimens After Initial Successful Treatment in Patients with Atopic Dermatitis. JAMA Dermatol. 2020, 156, 131. [Google Scholar] [CrossRef]
- Simpson, E.; Silverberg, J.; Worm, M.; Honari, G.; Masuda, K.; Sygula, E.; Maloney, J.; Mannent, L.; Xiao, J.; Dubost-Brama, A.; et al. Dupilumab treatment in patients with atopic hand and foot dermatitis: Results from a phase 3, randomized, double-blind, placebo-controlled trial. J. Investig. Dermatol. 2023, 143, S350. [Google Scholar] [CrossRef]
- Baselga, E.; Ramien, M.; Marcoux, D.; De Graaf, M.; Irvine, A.; Carvalho, V.; Ardusso, L. Dupilumab improves disease severity in children <12 years of age with moderate-severe AD: Interim results from PEDISTAD real-world registry. Pediatr. Dermatol. 2023, 40, 10–89. [Google Scholar]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2)*. Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef]
- Simpson, E.L.; Merola, J.F.; Silverberg, J.I.; Reich, K.; Warren, R.B.; Staumont-Sallé, D.; Girolomoni, G.; Papp, K.; de Bruin-Weller, M.; Thyssen, J.P.; et al. Safety of tralokinumab in adult patients with moderate-to-severe atopic dermatitis: Pooled analysis of five randomized, double-blind, placebo-controlled phase II and phase III trials. Br. J. Dermatol. 2022, 187, 888–899. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Adam, D.N.; Zirwas, M.; Kalia, S.; Gutermuth, J.; Pinter, A.; Pink, A.E.; Chiricozzi, A.; Barbarot, S.; Mark, T.; et al. Tralokinumab Plus Topical Corticosteroids as Needed Provides Progressive and Sustained Efficacy in Adults with Moderate-to-Severe Atopic Dermatitis Over a 32-Week Period: An ECZTRA 3 Post Hoc Analysis. Am. J. Clin. Dermatol. 2022, 23, 547–559. [Google Scholar] [CrossRef]
- Paller, A.S.; Flohr, C.; Cork, M.; Bewley, A.; Blauvelt, A.; Hong, H.C.; Imafuku, S.; Schuttelaar ML, A.; Simpson, E.L.; Soong, W.; et al. Efficacy and Safety of Tralokinumab in Adolescents with Moderate to Severe Atopic Dermatitis. JAMA Dermatol. 2023, 159, 596. [Google Scholar] [CrossRef]
- Gutermuth, J.; Pink, A.E.; Worm, M.; Soldbro, L.; Bjerregård Øland, C.; Weidinger, S. Tralokinumab plus topical corticosteroids in adults with severe atopic dermatitis and inadequate response to or intolerance of ciclosporin A: A placebo-controlled, randomized, phase III clinical trial (ECZTRA 7)*. Br. J. Dermatol. 2022, 186, 440–452. [Google Scholar] [CrossRef]
- Blauvelt, A.; Langley, R.G.; Lacour, J.-P.; Toth, D.; Laquer, V.; Beissert, S.; Wollenberg, A.; Herranz, P.; Pink, A.E.; Peris, K.; et al. Long-term 2-year safety and efficacy of tralokinumab in adults with moderate-to-severe atopic dermatitis: Interim analysis of the ECZTEND open-label extension trial. J. Am. Acad. Dermatol. 2022, 87, 815–824. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Guttman-Yassky, E.; Thaçi, D.; Irvine, A.D.; Stein Gold, L.; Blauvelt, A.; Simpson, E.L.; Chu, C.Y.; Liu, Z.; Gontijo Lima, R.; et al. Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2023, 388, 1080–1091. [Google Scholar] [CrossRef]
- Blauvelt, A.; Thyssen, J.P.; Guttman-Yassky, E.; Bieber, T.; Serra-Baldrich, E.; Simpson, E.; Rosmarin, D.; Elmaraghy, H.; Meskimen, E.; Natalie, C.R.; et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br. J. Dermatol. 2023, 188, 740–748. [Google Scholar] [CrossRef]
- Simpson, E.L.; Gooderham, M.; Wollenberg, A.; Weidinger, S.; Armstrong, A.; Soung, J.; Ferrucci, S.; Lima, R.G.; Witte, M.M.; Xu, W.; et al. Efficacy and Safety of Lebrikizumab in Combination with Topical Corticosteroids in Adolescents and Adults with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial (ADhere). JAMA Dermatol. 2023, 159, 182–191. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Weidinger, S.; Silverberg, J.; Gooderham, M.; Thyssen, J.; Irvine, A.; Elmaraghy, H.; Natalie, C.; Hu, C.; Pierce, E.; et al. Maintenance of efficacy and safety with lebrikizumab up to one year of treatment in patients with moderate-to-severe atopic dermatitis with or without topical corticosteroids. J. Investig. Dermatol. 2023, 143, S348. [Google Scholar] [CrossRef]
- Lilly Investors. Nearly 80% of Patients with Moderate-to-Severe Atopic Dermatitis Maintained Clear or Almost Clear Skin with Lilly’s Lebrikizumab Monthly Maintenance Dosing at Two Years. 2023. Available online: http://investor.lilly.com/news-releases/news-release-details/nearly-80-patients-moderate-severe-atopic-dermatitis-maintained (accessed on 19 January 2024).
- Soung, J.; Laquer, V.; Merola, J.F.; Forman, S.; Elmaraghy, H.; Meskimen, E.; Hu, C.; Natalie, C.; Pierce, E.; Torisu-Itakura, H.; et al. Lebrikizumab does not impact vaccine-induced immune responses: Results from a phase 3 study in adult patients with moderate-to-severe atopic dermatitis. J. Investig. Dermatol. 2023, 143, B15. [Google Scholar] [CrossRef]
- Paller, A.S.; Flohr, C.; Eichenfield, L.F.; Irvine, A.D.; Weisman, J.; Soung, J.; Pinto Correia, A.; Natalie, C.R.; Rodriguez Capriles, C.; Pierce, E.; et al. Safety and Efficacy of Lebrikizumab in Adolescent Patients with Moderate-to-Severe Atopic Dermatitis: A 52-Week, Open-Label, Phase 3 Study. Dermatol. Ther. 2023, 13, 1517–1534. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: Results from two phase III, long-term studies. Br. J. Dermatol. 2022, 186, 642–651. [Google Scholar] [CrossRef]
- Liang, J.; Hu, F.; Dan, M.; Sang, Y.; Abulikemu, K.; Wang, Q.; Hong, Y.; Kang, X. Safety and Efficacy of Nemolizumab for Atopic Dermatitis with Pruritus: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Front. Immunol. 2022, 13, 825312. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Hayakawa, Y.; Kawashima, M. Clinically meaningful improvements in cutaneous lesions and quality of life measures in patients with atopic dermatitis with greater pruritus reductions after treatment with 60 mg nemolizumab subcutaneously every 4 weeks: Subgroup analysis from a phase 3, randomized, controlled trial. J. Dermatol. Treat. 2023, 34, 2177096. [Google Scholar]
- Igarashi, A.; Katsunuma, T.; Matsumura, T.; Komazaki, H.; Nemolizumab-JP04 Study Group. Efficacy and safety of nemolizumab in paediatric patients aged 6-12 years with atopic dermatitis with moderate-to-severe pruritus: Results from a phase III, randomized, double-blind, placebo-controlled, multicentre study. Br. J. Dermatol. 2023, 190, 20–28. [Google Scholar] [CrossRef]
- Silverberg, J.; Thaçi, D.; Papp, K.; Legat, F.; Reich, A.; Wollenberg, A. Nemolizumab improves skin lesions, itch and sleep disturbance in patients with moderate-to-severe atopic dermatitis: Results from two identical phase 3 multinational studies (ARCADIA 1 and ARCADIA 2). In Proceedings of the Late-Breaking Abstract Presented at EADV, Berlin, Germany, 11–14 October 2023. [Google Scholar]
- Del Rosso, J.Q. MONOCLONAL ANTIBODY THERAPIES for Atopic Dermatitis: Where Are We Now in the Spectrum of Disease Management? J. Clin. Aesthet. Dermatol. 2019, 12, 39–41. [Google Scholar]
- Thibodeaux, Q.; Smith, M.P.; Ly, K.; Beck, K.; Liao, W.; Bhutani, T. A review of dupilumab in the treatment of atopic diseases. Hum. Vaccines Immunother. 2019, 15, 2129–2139. [Google Scholar] [CrossRef]
- Shirley, M. Dupilumab: First Global Approval. Drugs 2017, 77, 1115–1121. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, B.; Shen, S.; Song, X.; Jiang, Y.; Shi, L.; Zhao, C.; Yang, Y.; Jiang, L.; Li, J.; et al. Efficacy and safety of CM310 in severe eosinophilic chronic rhinosinusitis with nasal polyps (CROWNS-1): A multicentre, randomised, double-blind, placebo-controlled phase 2 clinical trial. EClinicalMedicine 2023, 61, 102076. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Yang, B.; Li, J.; Ding, Y.; Wu, L.; Zhang, L.; Wang, J.; Zhu, X.; Zhang, F.; et al. Efficacy and safety of CM310 in moderate-to-severe atopic dermatitis: A multicenter, randomized, double-blind, placebo-controlled phase 2b trial. Chin. Med. J. 2024, 137, 200–208. [Google Scholar] [CrossRef]
- Study of MG-K10 Humanized Monoclonal Antibody Injection in Patients with Atopic Dermatitis. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06026891 (accessed on 7 September 2023).
- Chandriani, S.; DePianto, D.J.; N’Diaye, E.N.; Abbas, A.R.; Jackman, J.; Bevers, J., 3rd; Ramirez-Carrozzi, V.; Pappu, R.; Kauder, S.E.; Toy, K.; et al. Endogenously Expressed IL-13Rα2 Attenuates IL-13-Mediated Responses but Does Not Activate Signaling in Human Lung Fibroblasts. J. Immun. 2014, 193, 111–119. [Google Scholar] [CrossRef]
- Popovic, B.; Breed, J.; Rees, D.G.; Gardener, M.J.; Vinall, L.M.; Kemp, B.; Spooner, J.; Keen, J.; Minter, R.; Uddin, F.; et al. Structural Characterisation Reveals Mechanism of IL-13-Neutralising Monoclonal Antibody Tralokinumab as Inhibition of Binding to IL-13Rα1 and IL-13Rα2. J. Mol. Biol. 2017, 429, 208–219. [Google Scholar] [CrossRef]
- Ultsch, M.; Bevers, J.; Nakamura, G.; Vandlen, R.; Kelley, R.F.; Wu, L.C.; Eigenbrot, C. Structural Basis of Signaling Blockade by Anti-IL-13 Antibody Lebrikizumab. J. Mol. Biol. 2013, 425, 1330–1339. [Google Scholar] [CrossRef]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef]
- Bieber, T. Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy 2020, 75, 54–62. [Google Scholar] [CrossRef]
- Duggan, S. Tralokinumab: First Approval. Drugs 2021, 81, 1657–1663. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Gooderham, M. Targeting Interleukin 13 for the Treatment of Atopic Dermatitis. Pharmaceutics 2023, 15, 568. [Google Scholar] [CrossRef]
- Stein Gold, L.; Thaçi, D.; Thyssen, J.P.; Gooderham, M.; Laquer, V.; Moore, A.; Natalie, C.R.; Zhao, F.; Meskimen, E.; Elmaraghy, H.; et al. Safety of Lebrikizumab in Adults and Adolescents with Moderate-to-Severe Atopic Dermatitis: An Integrated Analysis of Eight Clinical Trials. Am. J. Clin. Dermatol. 2023, 24, 595–607. [Google Scholar] [CrossRef]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar] [CrossRef]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef]
- Cornelissen, C.; Marquardt, Y.; Czaja, K.; Wenzel, J.; Frank, J.; Lüscher-Firzlaff, J.; Lüscher, B.; Baron, J.M. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J. Allergy Clin. Immunol. 2012, 129, 426–433.e8. [Google Scholar] [CrossRef]
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008, 19, 347–356. [Google Scholar] [CrossRef]
- Cornelissen, C.; Lüscher-Firzlaff, J.; Baron, J.M.; Lüscher, B. Signaling by IL-31 and functional consequences. Eur. J. Cell Biol. 2012, 91, 552–566. [Google Scholar] [CrossRef]
- Datsi, A.; Steinhoff, M.; Ahmad, F.; Alam, M.; Buddenkotte, J. Interleukin-31: The “itchy” cytokine in inflammation and therapy. Allergy 2021, 76, 2982–2997. [Google Scholar] [CrossRef]
- Kabashima, K.; Irie, H. Interleukin-31 as a Clinical Target for Pruritus Treatment. Front. Med. 2021, 8, 638325. [Google Scholar] [CrossRef]
- Keam, S.J. Nemolizumab: First Approval. Drugs 2022, 82, 1143–1150. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Nemolizumab Improves Patient-Reported Symptoms of Atopic Dermatitis with Pruritus: Post Hoc Analysis of a Japanese Phase III Randomized Controlled Trial. Dermatol. Ther. 2023, 13, 997–1011. [Google Scholar] [CrossRef]
- Saeki, H.; Akiyama, M.; Abe, M.; Igarashi, A.; Imafuku, S.; Ohya, Y.; Katoh, N.; Kameda, H.; Kabashima, K.; Tsunemi, Y.; et al. English version of Japanese guidance for biologics in treating atopic dermatitis. J. Dermatol. 2023, 50, e311–e322. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef]
- Nemoto, O.; Furue, M.; Nakagawa, H.; Shiramoto, M.; Hanada, R.; Matsuki, S.; Imayama, S.; Kato, M.; Hasebe, I.; Taira, K.; et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-co. Br. J. Dermatol. 2016, 174, 296–304. [Google Scholar] [CrossRef]
- Ruzicka, T.; Hanifin, J.M.; Furue, M.; Pulka, G.; Mlynarczyk, I.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Yoshida, H.; et al. Anti–Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 2017, 376, 826–835. [Google Scholar] [CrossRef]
- Kinugasa, E.; Igawa, K.; Shimada, H.; Kondo, M.; Funakoshi, S.; Imada, N.; Itami, N.; Fukazawa, N.; Takubo, R.; Kawata, Y.; et al. Anti-pruritic effect of nemolizumab in hemodialysis patients with uremic pruritus: A phase II, randomized, double-blind, placebo-controlled clinical study. Clin. Exp. Nephrol. 2021, 25, 875–884. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Pinter, A.; Pulka, G.; Poulin, Y.; Bouaziz, J.D.; Wollenberg, A.; Murrell, D.F.; Alexis, A.; Lindsey, L.; Ahmad, F.; et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 2020, 145, 173–182. [Google Scholar] [CrossRef]
- Ständer, S.; Yosipovitch, G.; Legat, F.J.; Lacour, J.P.; Paul, C.; Narbutt, J.; Bieber, T.; Misery, L.; Wollenberg, A.; Reich, A.; et al. Trial of Nemolizumab in Moderate-to-Severe Prurigo Nodularis. N. Engl. J. Med. 2020, 382, 706–716. [Google Scholar] [CrossRef]
- Furue, M.; Furue, M. OX40L–OX40 Signaling in Atopic Dermatitis. J. Clin. Med. 2021, 10, 2578. [Google Scholar] [CrossRef] [PubMed]
- Lé, A.M.; Torres, T. OX40-OX40L Inhibition for the Treatment of Atopic Dermatitis—Focus on Rocatinlimab and Amlitelimab. Pharmaceutics 2022, 14, 2753. [Google Scholar] [CrossRef] [PubMed]
- Elsner, J.; Carlsson, M.; Stougaard, J.; Nygaard, U.; Buchner, M.; Fölster-Holst, R.; Hvid, M.; Vestergaard, C.; Deleuran, M.; Deleuran, B. The OX40 Axis is Associated with Both Systemic and Local Involvement in Atopic Dermatitis. Acta Derm. Venereol. 2020, 100, adv00099-5. [Google Scholar] [CrossRef] [PubMed]
- Jember, A.G.-H.; Zuberi, R.; Liu, F.-T.; Croft, M. Development of Allergic Inflammation in a Murine Model of Asthma Is Dependent on the Costimulatory Receptor Ox40. J. Exp. Med. 2001, 193, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Seshasayee, D.; Lee, W.P.; Zhou, M.; Shu, J.; Suto, E.; Zhang, J.; Diehl, L.; Austin, C.D.; Meng, Y.G.; Tan, M.; et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Investig. 2007, 117, 3868–3878. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2021, 48, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J.L. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Liu, Y.-J. OX40-OX40L interactions: A promising therapeutic target for allergic diseases? J. Clin. Investig. 2007, 117, 3655–3657. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Simpson, E.L.; Reich, K.; Kabashima, K.; Igawa, K.; Suzuki, T.; Mano, H.; Matsui, T.; Esfandiari, E.; Furue, M. An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: A multicentre, double-blind, placebo-controlled phase 2b study. Lancet 2023, 401, 204–214. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Simpson, E.; Reich, K.; Kabashima, K.; Igawa, K.; Takahashi, H.; Matsuo, K. Efficacy and Safety Results of KHK4083/AMG 451 (anti-OX40 mAb) in Subjects with Moderate to Severe Atopic Dermatitis: A Phase 2, Multicentre, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study. In Proceedings of the 30th European Academy of Dermatology and Venereology (EADV) Congress, Vienna, Austria, 29 September–2 October 2021. [Google Scholar]
- Croft, M.; Esfandiari, E.; Chong, C.; Hsu, H.; Kabashima, K.; Kricorian, G.; Warren, R.B.; Wollenberg, A.; Guttman-Yassky, E. OX40 in the Pathogenesis of Atopic Dermatitis—A New Therapeutic Target. Am. J. Clin. Dermatol. 2024, 25, 447–461, Correction in Am. J. Clin. Dermatol. 2024, 25, 463. [Google Scholar] [CrossRef]
| Study No. | Clinical Trial Phase | No. of Patients | Treatment | Results | Ref. No. |
|---|---|---|---|---|---|
| Anti-IL-4Rα antibodies | |||||
| DUPILUMAB | |||||
| NCT04033367 (DUPISTAD) | Phase IV | 188; Adults | Dupilumab 300 mg Q2W or placebo Q2W for 12 weeks; Patients then entered an open-label phase and received dupilumab 300 mg Q2W for 12 weeks. TCS permitted | Week 12 results: Dupilumab demonstrated a substantial enhancement in sleep quality as opposed to a placebo (with a LSMD of −15.5%), and no unfavorable alterations were observed in routine laboratory parameters. | [7] |
| NCT03345914 (LIBERTY AD PEDS) | Phase III | 367; Children aged 6–11 | Dupilumab 300 mg (Q4W) + TCS, or a weight-based regimen of dupilumab 100 mg (baseline weight < 30 kg) or 200 mg (baseline weight ≥ 30 kg) Q2W + TCS, or placebo + TCS | Week 16 results:
| [8] [9] [10] [11] |
| NCT03346434 (Liberty AD PRESCHOOL) | Phase III | 162; Children aged 6 months to <6 years | Dupilumab (5 kg to <15 kg: 200 mg; 15 kg to <30 kg: 300 mg) Q4W + TCS or placebo + TCS | Week 16 results:
| [12,13] |
| NCT02612454 (LIBERTY AD PED-OLE) | Phase III | 294; Adolescents aged ≥12 to <18 years | A weight-based regimen of dupilumab (2 or 4 mg/kg every week). Following protocol amendment: dupilumab 300 mg Q4W. Patients with an inadequate clinical response: dose regimens of 200 or 300 mg Q2W. Concomitant TCS permitted. | Week 52 results:
| [14] [15] |
| NCT03912259 | Phase III | 165; Adults | Dupilumab 300 mg or placebo Q2W for 16 weeks. (monotherapy) | Week 16 results:
| [16] |
| NCT02277743 (SOLO1) NCT02277769 (SOLO2) pooled data | Phase III | 1379; Adults | Dupilumab 300 mg Q2W or Dupilumab 300 mg QW or Placebo QW (monotherapy) | Week 16 results:
| [17] [18] [19] [20] [21] [22] [23] |
| NCT02755649 (CAFÉ) | Phase III | 325; Adults with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable | Dupilumab 300 mg Q2W + TCS or Dupilumab 300 mg QW + TCS or Placebo QW + TCS | Week 16 results:
| [19] [24] |
| NCT02260986 (CHRONOS) | Phase III | 740; Adults | Dupilumab 300 mg Q2W + TCS or Dupilumab 300 mg QW + TCS or Placebo QW + TCS | Week 16 results:
Notably, significant clinical improvement and favorable tolerability were observed across White, Asian, and Black/African American racial subgroups. | [25] [19] [26] [21] [22] |
| NCT03054428 (LIBERTY AD ADOL) | Phase III | 251; Adolescents aged ≥12 to <18 years | Dupilumab 300 mg Q4W, or Dupilumab 200 or 300 mg Q2W (<60 kg or ≥60 kg, respectively) or placebo; (monotherapy) | Week 16 results:
EASI-75 at week 16: 33.3% with prior use of systemic immunosuppressant (SIS); 51.4% without prior SIS. Posology was proposed for adolescents: (200/300 mg Q2W; when weight 30–<60 kg/≥60 kg) No clinically meaningful changes in laboratory parameters were seen in adolescents. Positive effect on itch observed. | [9] [27] [28] [21] [29] [30] |
| NCT01949311 (LIBERTY AD OLE) | Phase III | Of 2677 patients enrolled, 347 reached week 148; Adults | Dupilumab 200 mg QW. Following protocol amendment: Dupilumab 300 mg QW TCS permitted | Week 148 results:
| [31] [32] |
| NCT02395133 (LIBERTY AD SOLO-CONTINUE) | Phase III | 422; Adults | Responding patients treated with dupilumab in SOLO were rerandomized 2:1:1:1 to: - original regimen of dupilumab, - 300 mg QW or Q2W - 300 mg, Q4W or Q8W - placebo for 36 weeks; (monotherapy) | More patients taking dupilumab QW or Q2W (71.6%) maintained EASI-75 response than those taking dupilumab Q4W (58.3%) or Q8W (54.9%) or those taking placebo (30.4%) | [33] |
| NCT04417894 (LIBERTY-AD-HAFT) | Phase III | 133; Adults and adolescents (≥12 years) | Dupilumab 300 mg Q2W in adults; 200/300 mg Q2W in adolescents, or placebo (monotherapy) | Week 16 results:
| [34] |
| NCT03687359 (PEDISTAD) | Phase III | 214 (dupilumab); Children aged 6 months to 11 years | Dose at discretion of study investigator (real-world registry) | 3 years results: The mean (± SE) EASI score decreased with dupilumab from 19.7 ± 1.0 at start to 6.1 ± 0.8 at last observation | [35] |
| Anti-IL-13 antibodies | |||||
| TRALOKINUMAB | |||||
| NCT03131648 (ECZTRA 1) | Phase III | 802; Adults | Tralokinumab 300 mg (Q2W) or placebo (monotherapy) After 16 weeks, 185 patients were rerandomized 2:2:1 to: - continue tralokinumab Q2W, - tralokinumab Q4W, - placebo Q2W | Week 16 results:
In patients who achieved IGA 0 or 1 with tralokinumab at week 16,
| [36] |
| NCT03160885 (ECZTRA 2) | Phase III | 794; Adults | Tralokinumab 300 mg (Q2W) (monotherapy) After 16 weeks, 227 patients were rerandomized 2:2:1 to: - continue tralokinumab Q2W, - tralokinumab Q4W, - placebo Q2W | Week 16 results:
In patients who achieved IGA 0 or 1 with tralokinumab at week 16,
| [36] |
| NCT03363854 (ECZTRA 3) | Phase III | 380; Adults | Tralokinumab 300 mg or placebo Q2W for 16 weeks. At Week 16, patients who achieved IGA 0-1 and/or EASI-75 were re-randomized 1:1 to tralokinumab Q2W or Q4W. TCS allowed as needed. Patients not achieving the clinical response criteria: tralokinumab Q2W + TCS from week 16 | Week 32 results:
| [37] [38] |
| NCT03526861 (ECZTRA 6) | Phase III | 289; Adolescentsaged 12 to 17 years | Tralokinumab, 150 or 300 mg, or placebo Q2W (monotherapy) | Week 16 results:
| [39] |
| NCT03761537 (ECZTRA 7) | Phase III | 277; Adults with inadequate response to or intolerance of ciclosporin A | Tralokinumab 300 mg or placebo Q2W + TCS as needed | Week 16 results:
| [40] |
| NCT03587805 (ECZTEND) | Phase III | 345; Adults Inclusion of participants regardless of prior level of response. Nevertheless, participants with a good response might be more likely to enroll. | Tralokinumab 300 mg + TCS/TCI, Q2W | 2 years results:
| [41] |
| LEBRIKIZUMAB | |||||
| NCT04146363 (ADvocate1) | Phase III | 424; Adults and adolescents (12 to <18 years of age, weighing ≥40 kg) | Lebrikizumab 250 mg or placebo, Q2W (monotherapy) for 16 weeks Patients who responded at week 16 were re-randomized to receive lebrikizumab 250 mg Q2W, lebrikizumab 250 mg Q4W or placebo Q2W for 36 additional weeks. | Week 16 results:
| [42] [43] |
| NCT04178967 (ADvocate2) | Phase III | 427; Adults and adolescents (12 to <18 years of age, weighing ≥40 kg) | Lebrikizumab 250 mg or placebo, Q2W (monotherapy) for 16 weeks Patients who responded at week 16 were re-randomized to receive lebrikizumab 250 mg Q2W, lebrikizumab 250 mg Q4W or placebo Q2W for 36 additional weeks. | Week 16 results:
| [42] [43] |
| NCT04250337 (ADhere) | Phase III | 211 Adults; adolescents (aged ≥12 to <18 years weighing ≥40 kg) | Lebrikizumab 250 mg Q2W or placebo Q2W in combination with TCS for 16 weeks. | Week 16 results:
| [44] |
| NCT04392154 (ADjoin) | Phase III | 267; Patients who achieved IGA 0,1 or EASI-75 at 16 weeks in ADvocate 1/2 and ADhere enrolled in ADjoin | Lebrikizumab 250 mg Q2W or Q4W + TCS | Week 40 results:
| [45] [46] |
| NCT04626297 (ADopt-VA) | Phase III | 188; Adults | Lebrikizumab 250 mg Q2W | Lebrikizumab did not negatively impact immune responses for Tdap or MCV vaccines. | [47] |
| NCT04250350 (ADore) | Phase III | 206; Adolescents (≥12 to <18 years old, weighing ≥ 40 kg) | Lebrikizumab 250 mg Q2W through week 52 TCS, TCI, topical PDE-4 inhibitor allowed as rescue treatment. | Week 16 results:
| [48] |
| Anti-IL31RA antibodies | |||||
| NEMOLIZUMAB | |||||
| JapicCTI-183894 (Study-JP02) | Phase III | 88; Adults and adolescents (aged ≥ 13 years, weighing ≥ 30.0 kg) | Nemolizumab 60 mg Q4W Patients completing 16 weeks could enter a 52-week extension period; no additional selection criteria imposed. All patients received nemolizumab 60 mg Q4W up to week 64. | Week 16 results:
| [49] |
| JapicCTI-173740 (Study-JP01) | Phase III | 215; Adults and adolescents (aged ≥ 13 years, weighing ≥ 30.0 kg) | Nemolizumab 60 mg or placebo Q4W. Previous medications for AD (TCS, TCI, oral antihistamines) unchanged; 16 weeks. Patients completing 16 weeks could enter a 52-week extension period; no additional selection criteria imposed. All patients received nemolizumab 60 mg Q4W up to week 64 (nemolizumab/nemolizumab and placebo/nemolizumab groups). | Week 16 results:
| [50] [51] [49] |
| jRCT2080225289 | Phase III | 89; Children aged ≥6 and <13 years | Nemolizumab 30 mg Q4W or placebo Q4W; TCS allowed | Week 16 results:
| [52] |
| NCT03985943 (ARCADIA 1) | Phase III | 941; Adults and adolescents (aged ≥ 12 years) | Nemolizumab 30 mg Q4W + TCS/TCI or placebo Q4W + TCS/TCI | Week 16 results:
| [53] |
| NCT03989349 (ARCADIA 2) | Phase III | 787; Adults and adolescents (aged ≥ 12 years) | Nemolizumab 30 mg Q4W +TCS/TCI or placebo Q4W + TCS/TCI | Week 16 results:
| [53] |
| Anti-OX40 antibodies | |||||
| ROCATINLIMAB | |||||
| NCT05899816 NCT05398445 NCT05651711 NCT05882877 NCT05704738 NCT05724199 NCT05633355 | Phase III | Trials in progress | Diverse treatment regimens | Phase III trial results have not yet been published. Results from the Phase II trial are detailed in the text. | |
| AMLITELIMAB | |||||
| NCT06130566 NCT06181435 | Phase III | Trials in progress | Diverse treatment regimens | Phase III trial results have not yet been published. Results from the Phase II trial are detailed in the text. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waligóra-Dziwak, K.; Dańczak-Pazdrowska, A.; Jenerowicz, D. A Comprehensive Review of Biologics in Phase III and IV Clinical Trials for Atopic Dermatitis. J. Clin. Med. 2024, 13, 4001. https://doi.org/10.3390/jcm13144001
Waligóra-Dziwak K, Dańczak-Pazdrowska A, Jenerowicz D. A Comprehensive Review of Biologics in Phase III and IV Clinical Trials for Atopic Dermatitis. Journal of Clinical Medicine. 2024; 13(14):4001. https://doi.org/10.3390/jcm13144001
Chicago/Turabian StyleWaligóra-Dziwak, Katarzyna, Aleksandra Dańczak-Pazdrowska, and Dorota Jenerowicz. 2024. "A Comprehensive Review of Biologics in Phase III and IV Clinical Trials for Atopic Dermatitis" Journal of Clinical Medicine 13, no. 14: 4001. https://doi.org/10.3390/jcm13144001
APA StyleWaligóra-Dziwak, K., Dańczak-Pazdrowska, A., & Jenerowicz, D. (2024). A Comprehensive Review of Biologics in Phase III and IV Clinical Trials for Atopic Dermatitis. Journal of Clinical Medicine, 13(14), 4001. https://doi.org/10.3390/jcm13144001







