Abstract
This rapid review summarizes the latest primary research in temporomandibular joint (TMJ) injection treatment. The final literature searches were conducted on 4 January 2024. Selection was performed systematically following predefined eligibility criteria. Randomized control trials concerning the treatment of TMJ disorders with intra-articular injections were included. Studies on more invasive interventions were excluded. Quality of life, joint pain and range of mandibular mobility were assessed. Ultimately, 12 studies covering a total of 603 patients qualified. They concerned: (1) arthrocentesis (AC) and the administration of, (2) injectable platelet-rich fibrin (I-PRF), (3) platelet-rich plasma (PRP), (4) hyaluronic acid (HA), (5) non-steroidal anti-inflammatory drugs (NSAIDs), and (6) hypertonic dextrose (HD) with a local anesthetic. The dominant approach was to perform arthrocentesis before administering the appropriate injection substance (I-PRF, PRP, HA, or NSAID). Two current studies on the intra-articular administration of NSAIDs, specifically tenoxicam and piroxicam, are noteworthy. A mixture of PRP and HA was injected in another two trials. These two innovative approaches may prove to be significant directions for further research on injection treatment of TMJs.
1. Introduction
1.1. Background
Temporomandibular disorders (TMDs) are a group of conditions manifesting themselves, among others, with temporomandibular joint (TMJ) pain and limited mandibular abduction [1]. TMDs affect between 7% and 31% of the population and it is very possible that this percentage will only increase in the coming years [2,3,4,5]. In addition to somatic ailments, TMDs may also result in social exclusion, chronic anxiety and stress, and depression [6]. People suffering from TMDs take sick leave from work and a disability pension two to three times more often [7].
Current methods of treating TMD include pharmacotherapy, physiotherapy of the masticatory muscles, splint therapy, arthrocentesis (AC), and administration of substances to the TMJ cavity in the form of intra-articular injections [8,9,10]. However, none of the available methods meets the criteria of a golden mean, hence it is necessary to adapt treatment to a specific case, and if there is no response, implement a different protocol. Intra-articular injections prove effective in articular pain treatment and associated limitation of jaw mobility [11]. Therefore, they are performed primarily in osteoarthritis and internal derangement.
1.2. Rationale
Minimally invasive treatment of temporomandibular joints (TMJs) involves inserting an injection needle or needles intra-articularly [11]. Possible interventions include: (1) lavage of the joint cavity, (2) viscosupplementation of hyaluronic acid (HA) as the main component of synovial fluid, (3) administration of drugs, i.e., corticosteroids (CS), (4) injection of blood-derived or adipose-derived autografts, i.e., platelet-rich plasma (PRP) or injectable platelet-rich fibrin (I-PRF), or (5) deposition of an irritant agent, i.e., hypertonic dextrose (HD) [8,12]. Injection techniques are used for the painful limitation of jaw mobility and its hypermobility, although different substances and places of deposition are used depending on the indications [11].
The dynamic development of injection techniques is manifested by the growing number of published clinical studies, the widening range of substances administered, and the increasingly thorough verification of the validity of sticking to established practices. Within the current injection technique development, the issue of the potential possibility of regenerating articular cartilage using autografts is raised [13]. On the other hand, there is increasing attention on the adverse effects of CS and local anesthetics administered intra-articularly [10]. Moreover, it has been considered to replace routine injections into the upper TMJ compartment with lower-compartment ones [14].
Regularly publishing systematic reviews on minimally invasive TMJ treatments allows for collecting and verifying scientific evidence on the effectiveness of individual treatment methods. These are complemented by scoping reviews, summarizing knowledge about specific emerging techniques [9,11,14]. The only mapping review covered the period until March 2023 and has revealed general trends in recent years [8]. The literature lacks synthetic works presenting more recent findings, which we decided to supplement with this rapid review.
1.3. Objective
This rapid review aims to identify and summarize the latest primary research on intra-articular injections into the temporomandibular joints, particularly emphasizing novel solutions.
2. Materials and Methods
The content and layout of this paper is based on the Virginia Commonwealth University Rapid Review Protocol [15]. This review was registered in the Open Science Framework Register under the following number: J9GTC.
2.1. Eligibility Criteria
Randomized clinical trials were included. Dysfunctions limiting the range of motion of the mandible, articular pain without changes in the range of abduction, as well as habitual dislocation were allowed (Table 1). The condition for inclusion in the study was treatment using intra-articular injections. Less invasive co-interventions were allowed, e.g., psychotherapy, physiotherapy, pharmacotherapy, or splint therapy. Studies that performed more invasive procedures, such as arthroscopy or open TMJ surgery, were excluded. Injection of a placebo or other substance, including AC, was accepted as an eligible control. Various co-interventions in the study and control groups excluded a given report from further processing due to the impossibility of objectively comparing the injection component of complex therapy. The change in quality of life, articular pain intensity, and mandibular range of motion was assessed. A minimum follow-up period of 4 weeks was required. Reports available in full text in English were included, following the aim of the work, and only those published in 2023.

Table 1.
Eligibility criteria.
2.2. Review Protocol
Based on the above eligibility criteria, the following search query was developed: “temporomandibular AND (injection OR intra-articular OR arthrocentesis OR lavage OR rinsing)”. Sources indexed by the Elsevier Scopus, Cochrane Library, and United States National Library of Medicine PubMed search engines were searched.
The main search was conducted on 18 December 2023, and was supplemented by follow-up searches on 4 January 2024 and 20 June 2024. A search engine filter was used to preselect reports published only in 2023. Two researchers (K.C. and M.C.) conducted the selection process. In the first stage, titles and abstracts were screened, then a full-text eligibility assessment was performed. In discrepancies between independent assessments, doubts were discussed until a consensus was reached. The quality of the trials presented in the source reports was appraised (F.B. and K.L.) in terms of the level of evidence according to The Oxford Levels of Evidence 2 scale. In the next step, data from the content of the articles were collected (F.B. and K.L.) and tabulated. If necessary, mathematical transformations and changes in units were made. Missing data were noted and no attempt was made to provide estimates.
3. Results
3.1. Selection Process
The flow diagram in Figure 1 illustrates the subsequent selection stages. Of the 176 records that were identified, 1 of them was removed manually as a duplicate, 135 were excluded at the abstract screening stage. During the full-text evaluation, 29 reports were rejected. The reasons for excluding individual papers are summarized in Table A1. The main reason for rejection was ineligible intervention, including procedures that were more invasive than intra-articular injections, especially arthroscopy. Uncontrolled studies, ones with an inadequate control group, and non-randomized ones were also excluded. Ultimately, 12 reports on randomized controlled trials were included.
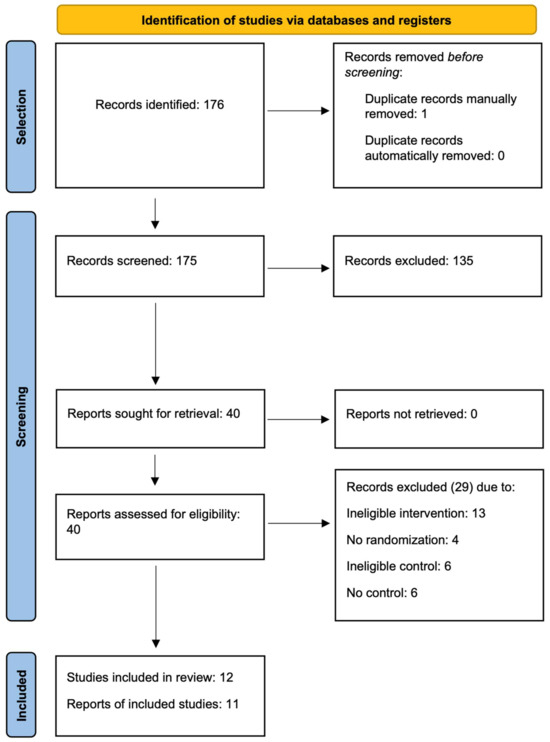
Figure 1.
Flow diagram.
3.2. Characteristics and Outcomes of Included Studies
Twelve studies on 603 patients in total were included in the synthesis (Table 2 and Table 3). All these studies were of level 2 evidence by The Oxford Levels of Evidence 2 scale. The sample size ranged from 14 to 91 and had a mean value of 50.3, and a median of 60. Diagnoses were highly heterogeneous, from general ones such as TMDs, to specific ones such as disc displacement with or without reduction. The qualified reports also included the treatment of recurrent TMJ dislocation. In this diagnosis, injection into the TMJ was intended to limit jaw mobility, which opposed the effect expected in the other studies. Follow-up time in various studies ranged from a month up to 12 months.

Table 2.
Study characteristics.

Table 3.
Outcomes.
The collected material included research on substances routinely used for articular pain and mandibular hypomobility. These were rinsing solutions, HA, and centrifuged autologous blood products. A hypertonic dextrose (HD) solution was used in recurrent TMJ dislocation.
Several less common treatment protocols were reported, i.e., intra-articular administration of tenoxicam, piroxicam, and a mixture of HA with PRP. The first two substances are non-steroidal anti-inflammatory drugs. Administering drugs inside the TMJ is not a unique idea in itself. CS have been particularly popular and are gradually being abandoned in favor of other approaches [8]. A predominant contribution of CS was observed in the reported adverse events following intra-articular administration to the TMJ [9,10]. However, non-steroidal anti-inflammatory drugs are still poorly researched in the intra-articular route of drug administration.
Both HA and PRP are recognized substances used for TMJ articular pain and limitation of mandibular abduction. Their combination is not routinely used and may prove beneficial. The research on this topic is innovative and therefore fits into the purpose of this review.
4. Discussion
Injection treatment is a dynamically developing group of therapeutic methods in TMD management [8]. This is due to its minimal invasiveness and proven quick effectiveness in relieving joint pain and limited jaw mobility [26,27]. The only mapping review in this field showed an increased reporting of studies on autologous preparations [8]. In connection with the results of this rapid review, current interest in non-steroidal anti-inflammatory drug administration is also noticeable [8].
AC as a monotherapy is a recognized method of treating articular pain and hypomobility [26]. It involves injecting the TMJ cavity and depositing the irrigation fluid within [26]. Several types of AC have been described and used, including the double-needle technique, single-needle technique (using a double-lumen needle), and pumping technique [19,26]. The first two involve puncturing the TMJ cavity and gently rinsing it with physiological saline or a ringer lactate flow. The pumping technique consists of repeated fluid administration and removal through the same route. AC is intended to rinse out the inflamed synovial fluid from the joint cavity. The pumping technique additionally exerts pressure on the joint surfaces, which causes the disintegration of inflammatory tissue and facilitates TMJ function [26]. In recent years, it was often proposed to administer various substances to the TMJ after arthrocentesis. These substances were, among others, HA, autologous blood products, or CS. However, sole rinsing of the joint has therapeutic significance. Some clinicians administer injectables into the TMJ without prior AC [11,27]. This matter is still a subject for future scientific research.
4.1. Main Findings
There is a scientific dispute as to whether AC itself is crucial for the treatment of pain and immobility of TMJs, or whether it should be followed by the administration of one of the known injection substances, e.g., HA or a blood derivative. The collected material identified four randomized clinical trials on this topic. The synthesized data is inconsistent and does not prove one specific thesis. Bayramoglu et al. stated that tenoxicam in addition to AC neither relieves pain nor increases mandibular mobility [16]. In turn, Gupta et al. [18] proved that piroxicam combined with AC reduced pain and increased jaw mobility in patients with anterior disc displacement without reduction. Despite the similarity of piroxicam and tenoxicam in terms of structure and pharmacokinetics, the results of the studies are inconsistent. In the context of the lack of previous randomized clinical trials on the administration of piroxicam to the TMJ, further research on this topic is needed [8]. The differences may be due to diagnoses varying between the groups treated in both trials. Another study supporting the claim that adding an injectable increases the effectiveness of AC is the report of Işık et al. [25]. These authors observed that adding I-PRF reduces pain and increases mandibular abduction even further than lavage alone. Sait et al. proved that following AC with HA administration does not influence pain relief, but enhances jaw mobility [12]. Despite the need to perform further randomized studies, it can be noted that a promising direction in the use of additional substances following AC is to consider the use of I-PRF or piroxicam in disk displacement without reduction. Whether the effectiveness of injection AC supplementation results from the diagnosis of disk displacement without reduction, or the use of I-PRF or piroxicam requires clarification in the future. Nevertheless, AC alone is an effective medical procedure that relieves pain and increases mandibular mobility.
Injecting nonsteroidal anti-inflammatory drugs into the TMJ cavities has been gaining popularity in recent years, as reflected in a growing number of clinical trials. However, the validity of such a procedure and the detailed selection of pharmaceuticals are issues that still require stronger scientific support. The included two randomized clinical trials present conflicting results of intra-articular nonsteroidal anti-inflammatory drug injections. Representatives of this drug group, i.e., tenoxicam and piroxicam are similar in structure and pharmacokinetics. Bayramoglu et al. concluded that tenoxicam administration is not beneficial for the clinical effects of AC, neither in terms of changing pain severity nor increasing jaw mobility [16]. Gupta et al. state that piroxicam increases the outcomes of AC in both domains mentioned above [18]. Based on the collected material, it cannot be concluded that combining nonsteroidal anti-inflammatory drugs with AC has a positive or negative effect on its clinical effects. As mentioned earlier, the key here may be the diagnosis, not the specific representative of the group of non-steroidal anti-inflammatory drugs. Further clinical trials and their meta-analytic comparison are needed.
The results of experimental studies suggest the possibility of regeneration of the cartilage covering the TMJ surfaces. The greatest hopes for the actual clinical use of this potential are associated with autologous transplants. This group of substances includes self-derived preparations of adipose tissue, rich in mesenchymal stem cells, and, easier to obtain in an outpatient setting, centrifuged preparations from the patient’s blood. The latter group has many representatives, including primarily plasma rich in growth factors (PRGF), PRP, and I-PRF. The multitude of preparations differing in their composition makes scientific syntheses difficult. Treated separately, they are represented by rather scanty clinical material, albeit collective meta-analyses seem pointless given the significant differences between individual representatives of this group. Clinical comparison of these substances is desirable in the current state of knowledge because it could lead to the displacement of weaker-effect compositions by those with superior therapeutic efficacy. Sharma et al. proved the superiority of I-PRF over PRP in reducing articular pain and increasing maximum mouth opening [23]. These results indicate the advantage of one substance over the other, which, in connection with future similarly designed studies, may contribute to replacing the less effective composition with a better one.
4.2. Novel Solutions
In the systematic review, we identified articles describing innovative solutions in temporomandibular disorder treatment. In the paper of Hegab et al., the study group treatment was based on the use of AC + PRP + HA and compared with AC + PRP and AC + HA controls [20]. The administration of PRP in combination with HA after AC is novel and deserves further attention. The study’s results were noteworthy as the use of PRP + HA in combination with AC resulted better in: (1) increasing maximum voluntary mouth opening, (2) reducing pain, and (3) improving joint sounds, compared to AC + HA. This conclusion was based on a 12-month follow-up, considered a long-term observation in injectable TMJ treatment trials.
Attia et al. compared a group treated with a combination of HA and PRP with HA and CS controls [22]. Hence, the cited study has a major limitation i.e., no reference point of a well-studied TMD treatment technique when comparing two innovative approaches. It would be beneficial to conduct subsequent clinical trials comparing HA and PRP with a well-known and widely used injection treatment for TMD. Attia et al. concluded that HA and PRP relieved TMJ pain better than HA and CS over a 6-month perspective [22]. It was noteworthy that in the 1-week perspective, the combination of HA and CS gave better outcomes, which may be due to the strong anti-inflammatory effect of the drug.
4.3. Limitations of the Evidence
The selected primary studies vary in specific diagnoses, sample sizes, injectables, and treatment protocols.
4.4. Limitations of the Review Process
Due to the rapid nature of this review, it supplemented the state of knowledge with the latest findings. The results of older studies were intentionally not included. Therefore, the syntheses conducted in this review cannot be treated as a contribution to therapeutic guidelines (classical systematic reviews are used for this purpose). Instead, the conducted syntheses indicate the current directions of scientific research and summarize their results. Furthermore, this review was limited to English-language and well-indexed sources.
5. Conclusions
This rapid review complemented the only mapping review on injection treatment of temporomandibular joints. Clinical trials on injection treatment of TMJs are still highly heterogeneous in terms of the substances used. Currently, the choice of autologous blood products or HA as the active injection substance is the standard. Innovative research on mixing the above injectables is promising. The hitherto poorly studied intracavitary administration of nonsteroidal anti-inflammatory drugs has been evaluated in subsequent clinical trials. The presence of new randomized clinical trials on the use of this group of substances encourages a systematic review. Most of the intra-articular administrations are currently preceded by AC.
Author Contributions
Conceptualization, K.L., M.C. and M.S.; Methodology, K.C. and M.C.; Software, K.C. and F.B.; Validation, N.T., I.R. and A.M.; Formal Analysis, K.L. and F.B.; Investigation, K.L., K.C., F.B. and M.C.; Resources, K.C. and M.C.; Data Curation, K.L. and F.B.; Writing—Original Draft Preparation, K.L., K.C., F.B. and M.C.; Writing—Review and Editing, M.C., N.T., I.R, A.M., D.C. and M.S.; Visualization, K.L. and F.B.; Supervision, D.C. and M.S.; Project Administration, D.C. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Excluded reports.
Table A1.
Excluded reports.
| First Author | Digital Object Identifier | Exclusion Reason |
|---|---|---|
| Speculand [28] | 10.1016/j.bjoms.2022.10.001 | Exclusion reason |
| Raveggi [29] | 10.23736/S2724-6329.22.04653-8 | No control |
| Dinsdale [30] | 10.1016/j.msksp.2023.102756 | No control |
| Vingender [31] | 10.1016/j.jcms.2023.01.017 | Ineligible control |
| Bilgir [32] | 10.1080/08869634.2020.1853889 | Ineligible control |
| Keshavamurthy [33] | 10.1097/phm.0000000000002118 | Ineligible control |
| Gaete [34] | 10.1007/s10006-023-01158-2 | No control |
| Rajpoot [35] | 10.1007/s12663-021-01627-9 | Ineligible intervention |
| Araidy [36] | 10.3290/j.qi.b4007423 | Ineligible intervention |
| Ângelo [37] | 10.1016/j.ijom.2023.06.008 | Ineligible intervention |
| Rodrigues [38] | 10.1016/j.jcms.2023.01.006 | Ineligible intervention |
| Wu [39] | 10.3390/jcm12041657 | Ineligible intervention |
| Schabrun [40] | 10.1016/j.ynpai.2023.100117 | Ineligible intervention |
| Li [41] | 10.1007/s11282-022-00621-2 | Ineligible intervention |
| Ali [42] | 10.1097/scs.0000000000009820 | Ineligible intervention |
| Erdil [43] | 10.1016/j.jormas.2023.101438 | Ineligible intervention |
| Martins [44] | 10.1016/j.jcms.2023.02.001 | Ineligible intervention |
| Ângelo [45] | 10.1016/j.jcms.2023.09.010 | Ineligible intervention |
| Navaneetham [46] | 10.1007/s12070-023-03890-3 | Ineligible intervention |
| Mohammed [47] | 10.26355/eurrev_202306_32605 | Ineligible intervention |
| Symanski [48] | 10.1007/s00256-022-04225-z | No control |
| Navaneetham [49] | 10.1007/s12663-023-01986-5 | No control |
| Pohranychna [50] | 10.36740/wlek202301121 | No randomization |
| Tang [51] | 10.1016/j.ijom.2022.08.018 | No control |
| de Almeida [52] | 10.3390/jcm12093228 | Ineligible control |
| Cömert Kiliç [53] | 10.1016/j.joms.2022.12.023 | Ineligible control |
| Fayed [54] | 10.1016/j.jormas.2023.101644 | Ineligible control |
| Hassan [55] | 10.1097/scs.0000000000009085 | No randomization |
| Taşkesen [56] | 10.1080/08869634.2020.1861887 | No randomization |
References
- Iturriaga, V.; Bornhardt, T.; Velasquez, N. Temporomandibular Joint. Dent. Clin. North Am. 2023, 67, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Christidis, N.; Lindström Ndanshau, E.; Sandberg, A.; Tsilingaridis, G. Prevalence and Treatment Strategies Regarding Temporomandibular Disorders in Children and Adolescents—A Systematic Review. J. Oral Rehabil. 2019, 46, 291–301. [Google Scholar] [CrossRef]
- Carlsson, G.E.; Ekbäck, G.; Johansson, A.; Ordell, S.; Unell, L. Is There a Trend of Decreasing Prevalence of TMD-Related Symptoms with Ageing among the Elderly? Acta Odontol. Scand. 2014, 72, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Loster, J.E.; Osiewicz, M.A.; Groch, M.; Ryniewicz, W.; Wieczorek, A. The Prevalence of TMD in Polish Young Adults. J. Prosthodont. 2017, 26, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; De Souza, B.D.M. Prevalence of Temporomandibular Joint Disorders: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Namvar, M.A. The Relationship between Depression and Anxiety with Temporomandibular Disorder Symptoms in Dental Students. Maedica 2021, 16, 590. [Google Scholar] [CrossRef] [PubMed]
- Salinas Fredricson, A.; Krüger Weiner, C.; Adami, J.; Rosén, A.; Lund, B.; Hedenberg-Magnusson, B.; Fredriksson, L.; Svedberg, P.; Naimi-Akbar, A. Sick Leave and Disability Pension among TMD Patients with Musculoskeletal Diseases, Mental and Behavioural Disorders—A SWEREG-TMD Population-Based Cohort Study. BMC Public Health 2023, 23, 852. [Google Scholar] [CrossRef] [PubMed]
- Chęciński, M.; Chęcińska, K.; Turosz, N.; Brzozowska, A.; Chlubek, D.; Sikora, M. Current Clinical Research Directions on Temporomandibular Joint Intra-Articular Injections: A Mapping Review. JCM 2023, 12, 4655. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. IJMS 2021, 22, 7405. [Google Scholar] [CrossRef] [PubMed]
- Turosz, N.; Chęcińska, K.; Chęciński, M.; Michcik, A.; Chlubek, D.; Sikora, M. Adverse Events of Intra-Articular Temporomandibular Joint Injections: A Systematic Search and Review. Pomeranian J. Life Sci. 2023, 69, 48–54. [Google Scholar] [CrossRef]
- Turosz, N.; Chęcińska, K.; Chęciński, M.; Lubecka, K.; Bliźniak, F.; Chlubek, D.; Olszowski, T.; Sikora, M. Temporomandibular Joint Injections and Lavage: An Overview of Reviews. JCM 2024, 13, 2855. [Google Scholar] [CrossRef] [PubMed]
- Sait, A.I.; Sequiera, J.P.; Chandra, J. Efficacy of Arthrocentesis with and Without Sodium Hyaluronate Injection for Temporomandibular Joint Disorders: A Comparative Study. J. Maxillofac. Oral Surg. 2023, 22, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Van Bellinghen, X.; Idoux-Gillet, Y.; Pugliano, M.; Strub, M.; Bornert, F.; Clauss, F.; Schwinté, P.; Keller, L.; Benkirane-Jessel, N.; Kuchler-Bopp, S.; et al. Temporomandibular Joint Regenerative Medicine. IJMS 2018, 19, 446. [Google Scholar] [CrossRef] [PubMed]
- Chęciński, M.; Chęcińska, K.; Turosz, N.; Sikora, M.; Chlubek, D. Intra-Articular Injections into the Inferior versus Superior Compartment of the Temporomandibular Joint: A Systematic Review and Meta-Analysis. JCM 2023, 12, 1664. [Google Scholar] [CrossRef] [PubMed]
- Cyrus, J. Research Guides: Rapid Review Protocol: Steps: Rapid Review. Available online: https://guides.library.vcu.edu/c.php?g=240398&p=1598530 (accessed on 6 May 2024).
- Bayramoglu, Z.; Yavuz, G.Y.; Keskinruzgar, A.; Koparal, M.; Kaya, G.S. Does Intra-Articular Injection of Tenoxicam after Arthrocentesis Heal Outcomes of Temporomandibular Joint Osteoarthritis? A Randomized Clinical Trial. BMC Oral Health 2023, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Jaiswara, C.; Hirani, M.; Chauhan, N.; Mahajan, A.; Krishnan, A. Efficacy of Arthrocentesis with Intra-Articular Injection of Hyaluronic Acid and Corticosteroid in the Treatment of Internal Derangement of Temporomandibular Joint. Natl. J. Maxillofac. Surg. 2023, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Ali, I.; Zeeshan, M.; Singh, S.; Kumar, A.; Adil, A. Role of Intra-Articular Piroxicam in the Temporomandibular Joint After Arthrocentesis for Anterior Disc Displacement Without Reduction. Cureus 2023, 15, e34580. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, D. Is Single Puncture Superior to Double Puncture Arthrocentesis in Patients With Wilkes III Internal Derangement? J. Oral Maxillofac. Surg. 2023, 81, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Hegab, A.F.; Hameed, H.I.A.A.; Hassaneen, A.M.; Hyder, A. Synergistic Effect of Platelet Rich Plasma with Hyaluronic Acid Injection Following Arthrocentesis to Reduce Pain and Improve Function in TMJ Osteoarthritis. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101340. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, D.; Sivakumar, B.; Bhargava, P.G. A Comparative Preliminary Randomized Clinical Study to Evaluate Heavy Bupivacaine Dextrose Prolotherapy (HDP) and Autologous Blood Injection (ABI) for Symptomatic Temporomandibular Joint Hypermobility Disorder. J. Maxillofac. Oral Surg. 2023, 22, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.A.M.M.; Awad, S.S. Hyaluronic Acid and Platelet-Rich Plasma Mixture Versus Hyaluronic Acid and Corticosteroid in the Treatment of Temporomandibular Joint Internal Derangement: A Comparative Randomized Study. J. Maxillofac. Oral Surg. 2024, 23, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Aurora, J.K.; Dubey, K.N.; Tandon, P.; Tiwari, S. A Comparative Analysis between Intra Articular Injections of Injectable Platelet Rich Fibrin versus Platelet Rich Plasma in the Management of Temporomandibular Disorders: A Randomized Control Trial. Natl. J. Maxillofac. Surg. 2023, 14, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-S.; Xu, L.-L.; Liu, L.-K.; Lu, S.-J.; Cai, B. Platelet-Rich Plasma Therapy for Temporomandibular Joint Osteoarthritis: A Randomized Controlled Trial. J. Cranio-Maxillofac. Surg. 2023, 51, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Işık, G.; Kenç, S.; Özveri Koyuncu, B.; Günbay, S.; Günbay, T. Does the Use of Injectable Platelet-Rich Fibrin After Arthrocentesis for Disc Displacement Without Reduction Improve Clinical Outcomes? J. Oral Maxillofac. Surg. 2023, 81, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Siewert-Gutowska, M.; Pokrowiecki, R.; Kamiński, A.; Zawadzki, P.; Stopa, Z. State of the Art in Temporomandibular Joint Arthrocentesis—A Systematic Review. JCM 2023, 12, 4439. [Google Scholar] [CrossRef] [PubMed]
- Chęciński, M.; Chęcińska, K.; Nowak, Z.; Sikora, M.; Chlubek, D. Treatment of Mandibular Hypomobility by Injections into the Temporomandibular Joints: A Systematic Review of the Substances Used. JCM 2022, 11, 2305. [Google Scholar] [CrossRef]
- Speculand, B. Clinical Negligence in Temporomandibular Joint Surgery. Br. J. Oral. Maxillofac. Surg. 2023, 61, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Raveggi, E.; Ramieri, G.; Bosco, G.F.; Zavattero, E. Temporomandibular Joint Arthrocentesis: A Single-Center Experience and Review of the Literature. Minerva Dent. Oral Sci. 2023, 72, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, A.; Thomas, L.; Forbes, R.; Treleaven, J. Do Intra-Articular Temporomandibular Disorders Show an Association between Physical Bite Function, Self-Perceived Bite Limitation and Kinesiophobia? A Case-Control Study. Musculoskelet. Sci. Pract. 2023, 65, 102756. [Google Scholar] [CrossRef] [PubMed]
- Vingender, S.; Dőri, F.; Schmidt, P.; Hermann, P.; Vaszilkó, M.T. Evaluation of the Efficiency of Hyaluronic Acid, PRP and I-PRF Intra-Articular Injections in the Treatment of Internal Derangement of the Temporomandibular Joint: A Prospective Study. J. Cranio-Maxillofac. Surg. 2023, 51, 1–6. [Google Scholar] [CrossRef]
- Bilgir, E.; Yıldırım, D.; Șenturk, M.F.; Orhan, H. Clinical and Ultrasonographic Evaluation of Ultrasound-Guided Single Puncture Temporomandibular Joint Arthrocentesis. CRANIO 2023, 41, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Keshavamurthy, C.; Bansal, P. Pneumarthrosis After Diagnostic Arthrocentesis. Am. J. Phys. Med. Rehabil. 2023, 102, e73. [Google Scholar] [CrossRef]
- Gaete, C.; Droguett, C.; Sáez, F.; Astorga, P. Clinical and Demographic Factors Associated with the Effectiveness of Temporomandibular Joint Arthroscopy. Oral Maxillofac. Surg. 2023, 28, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, D.; Anchlia, S.; Bhatt, U.; Dhuvad, J.; Patel, H.; Mansuri, Z. Arthrocentesis versus Level 1 Arthroscopy in Internal Derangement of Temporomandibular Joint. J. Maxillofac. Oral Surg. 2023, 22, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Araidy, S. TMJ Arthroscopic Level 1 vs. Arthrocentesis in the Management of Internal Derangement of the Temporomandibular Joint. Quintessence Int. 2023, 54, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Ângelo, D.F.; Mota, B.; Sanz, D.; Pimentel, J. Septic Arthritis of the Temporomandibular Joint Managed with Arthroscopy: A Case Report. Int. J. Oral. Maxillofac. Surg. 2023, 52, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.L.P.; Cardoso, H.J.; Ângelo, D.F. Patient Experience and Satisfaction with Different Temporomandibular Joint Treatments: A Retrospective Study. J. Cranio-Maxillofac. Surg. 2023, 51, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-B.; Sun, H.-J.; Sun, N.-N.; Zhou, Q. Analysis of the Curative Effect of Temporomandibular Joint Disc Release and Fixation Combined with Chitosan Injection in the Treatment of Temporomandibular Joint Osteoarthrosis. JCM 2023, 12, 1657. [Google Scholar] [CrossRef]
- Schabrun, S.M.; Si, E.; Millard, S.K.; Chiang, A.K.I.; Chen, S.; Chowdhury, N.S.; Seminowicz, D.A. Intramuscular Injection of Nerve Growth Factor as a Model of Temporomandibular Disorder: Nature, Time-Course, and Sex Differences Characterising the Pain Experience. Neurobiol. Pain 2023, 13, 100117. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, J.; Yu, L.; He, S.; Li, F.; Lin, Y.; Xu, J.; Chen, S. Disc–Condyle Relationship Alterations Following Stabilization Splint Therapy or Arthrocentesis plus Hyaluronic Acid Injection in Patients with Anterior Disc Displacement: A Retrospective Cohort Study. Oral Radiol. 2023, 39, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.A.; Hadi, H.A. Can the Autogenous Nanofat Injection Improve the Symptoms of Patients With Temporomandibular Joint Internal Derangement? A Prospective Observational Clinical Study. J. Craniofacial Surg. 2024, 35, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Erdil, A.; Demirsoy, M.S.; Tümer, M.K. Evaluation of the Effects of Arthrocentesis Combined with Occlusal Stabilization Splint on Disc Displacement without Reduction-Induced Acute and Closed Lock. A Prospective Cohort Study. J. Stomatol. Oral. Maxillofac. Surg. 2023, 124, 101438. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.S.; Radaic, P.; Marchi, L.; Barreto, G.; Pastore, G.P. Assessment of Postoperative Pain in Patients Undergoing Temporomandibular Joint Arthroscopy with Infiltration of Dexamethasone Disodium Phosphate in Different Concentrations. A Randomized Controlled Trial. J. Cranio-Maxillofac. Surg. 2023, 51, 89–97. [Google Scholar] [CrossRef]
- Ângelo, D.F.; Sanz, D.; Cardoso, H.J. Effectiveness of Double-Puncture Temporomandibular Joint Arthrocentesis with Viscosupplementation in Different Categories of Severity—A Prospective Study. J. Cranio-Maxillofac. Surg. 2023, 51, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Navaneetham, A.; Vaibhav, N.; Navaneetham, R.; Balaraj, B.V.; Roy, N.P.; Madhusudan, S. Efficacy of Arthrocentesis and Anterior Repositioning Splints in Treatment of Internal Derangement of TMJ: A Prospective Clinical Study. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 3116–3129. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.R.; Hamad, S.A.; Al-Dawoody, A.D.; Shehab, A.A.; Ahmed, O.S. Effect of Dextrose Prolotherapy on Internal Derangement of the Temporomandibular Joint. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4883–4889. [Google Scholar] [CrossRef] [PubMed]
- Symanski, J.S.; Gimarc, D.; Chan, B.; Stephenson, J.; Markhardt, B.K.; Ross, A.B. Ultrasound-Guided Temporomandibular Joint Aspiration: Technique and Results in Six Cases of Suspected Septic Arthritis. Skeletal Radiol. 2023, 52, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Navaneetham, R.; Navaneetham, A.; Nagaraj, V.; Gnapika, N.; Sankarnarayan, G. Single-Puncture Versus Double-Puncture Technique Arthrocentesis in the Treatment of Internal Derangement of TM Joint—A Comparative Clinical Study. J. Maxillofac. Oral Surg. 2023, 22, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Pohranychna, K.; Ohonovskyi, R.; Rybert, Y.; Minko, L.; Hlova, O. Efficacy of Arthrocentesis for Treatment of Internal Post-Traumatic Temporomandibular Joint Disorders. Wiad Lek 2023, 76, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.H.; Vos, L.M.; Tuin, A.J.; Huddleston Slater, J.J.R.; Gareb, B.; Van Bakelen, N.B.; Spijkervet, F.K.L. Arthrocentesis versus Non-Surgical Intervention as Initial Treatment for Temporomandibular Joint Arthralgia: A Randomized Controlled Trial with Long-Term Follow-Up. Int. J. Oral Maxillofac. Surg. 2023, 52, 595–603. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.M.; Botelho, J.; Machado, V.; Mendes, J.J.; Manso, C.; González-López, S. Comparison of the Efficacy of Two Protocol Treatments in Patients with Symptomatic Disc Displacement without Reduction: A Randomized Controlled Trial. JCM 2023, 12, 3228. [Google Scholar] [CrossRef] [PubMed]
- Cömert Kiliç, S.; Kiliç, N.; Güngörmüş, M. Botulinum Toxin Versus Dextrose Prolotherapy: Which Is More Effective for Temporomandibular Joint Subluxation? A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2023, 81, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Fayed, H.M.; Khairy, M.A.; Eldahshan, D.; Sabry, D.; Ahmed, W.A. Bone Marrow Aspirate Concentrate—A Novel Approach to Alter the Course of Temporomandibular Joint Osteoarthritis (a Clinical Study). J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101644. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.A.; Suhail, Z.A. The Efficacy of Ozonized Water Versus Ringer Lactate Arthrocentesis for the Treatment of Temporomandibular Joint Internal Derangement. J. Craniofacial Surg. 2023, 34, e238–e241. [Google Scholar] [CrossRef] [PubMed]
- Taşkesen, F.; Cezairli, B. Efficacy of Prolotherapy and Arthrocentesis in Management of Temporomandibular Joint Hypermobility. CRANIO 2023, 41, 423–431. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).