Comparison of Conventional versus Modified Preperitoneal Pelvic Packing in Patients with Bleeding Pelvic Fractures: A Single-Center Retrospective Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Data Collection
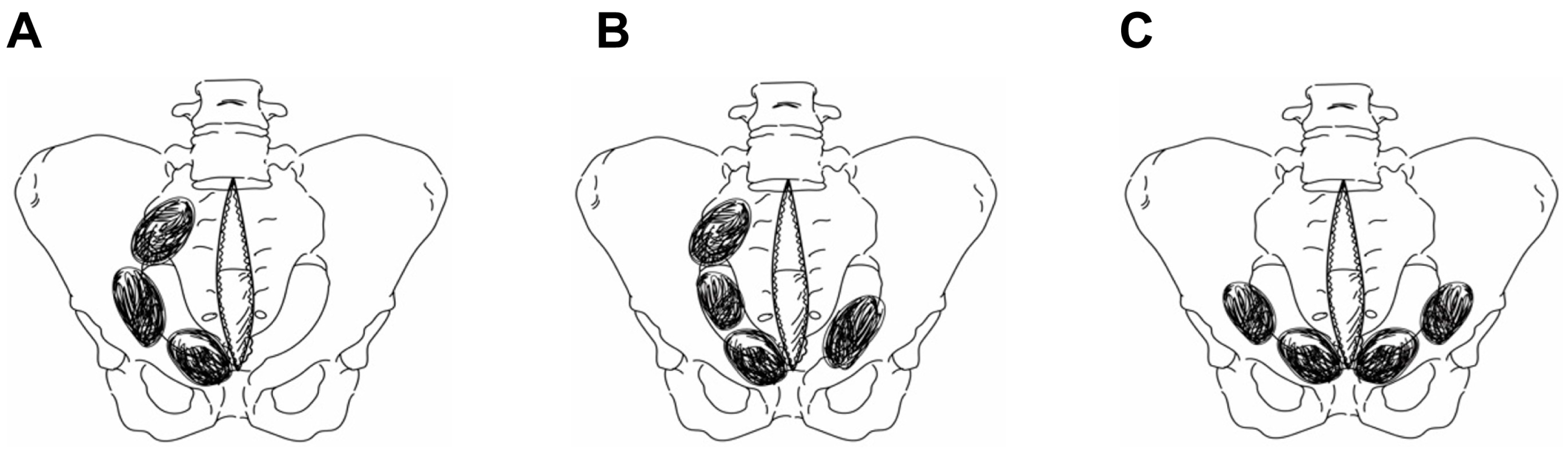
2.2. Preperitoneal Pelvic Packing Techniques
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valisena, S.; Abboud, A.E.; Andereggen, E.; Ansorge, A.; Gamulin, A. Management of high-energy blunt pelvic ring injuries: A retrospective cohort study evaluating an institutional protocol. Injury 2022, 53, 4054–4061. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.J.; Hsu, J.R.M. The road to survival for haemodynamically unstable patients with open pelvic fractures. Front. Surg. 2020, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Costantini, T.W.; Coimbra, R.; Holcomb, J.B.; Podbielski, J.M.; Catalano, R.; Blackburn, A.; Scalea, T.M.; Stein, D.M.; Williams, L.; Conflitti, J.L.; et al. Current management of hemorrhage from severe pelvic fractures: Results of an American Association for the Surgery of Trauma multi-institutional trial. J. Trauma Acute Care Surg. 2016, 80, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Purghel, F.; Jemna, C.; Ciuvica, R. Retroperitoneal hematoma in pelvic fractures. Chirurgia 2011, 106, 23–31. [Google Scholar] [PubMed]
- Schweigkofler, U.; Wohlrath, B.; Trentzsch, H.; Horas, K.; Hoffmann, R.; Wincheringer, D. Is there any benefit in the pre-hospital application of pelvic binders in patients with suspected pelvic injuries? Eur. J. Trauma Emerg. Surg. 2021, 47, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Hundersmarcka, D.; Hietbrink, F.; Leenen, L.P.H.; Heng, M. Pelvic packing and angio-embolization after blunt pelvic trauma: A retrospective 18-year analysis. Injury 2021, 52, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Magee, G.A.; Fox, C.J.; Moore, E.E. Resuscitative endovascular balloon occlusion of the aorta in pelvic ring fractures: The Denver Health protocol. Injury 2021, 52, 2702–2706. [Google Scholar] [CrossRef]
- Jang, J.Y.; Bae, K.S.; Chang, S.W.; Jung, K.; Kim, D.H.; Kang, B.H. Current management and clinical outcomes for patients with haemorrhagic shock due to pelvic fracture in Korean regional trauma centres: A multi-institutional trial. Injury 2022, 53, 488–495. [Google Scholar] [CrossRef]
- Höch, A.; Zeidler, S.; Pieroh, P.; Josten, C.; Stuby, F.M.; Herath, S.C.; German Pelvic Trauma Registry. Trends and efficacy of external emergency stabilization of pelvic ring fractures: Results from the German Pelvic Trauma Registry. Eur. J. Trauma Emerg. Surg. 2021, 47, 523–531. [Google Scholar] [CrossRef]
- Lustenberger, T.; Meier, C.; Benninger, E.; Lenzlinger, P.M.; Keel, M.J. C-clamp and pelvic packing for control of hemorrhage in patients with pelvic ring disruption. J. Emerg. Trauma Shock 2011, 4, 477–482. [Google Scholar] [CrossRef]
- Magnone, S.; Coccolini, F.; Manfredi, R.; Piazzalunga, D.; Agazzi, R.; Arici, C.; Barozzi, M.; Bellanova, G.; Belluati, A.; Berlot, G.; et al. Management of hemodynamically unstable pelvic trauma: Results of the first Italian consensus conference (cooperative guidelines of the Italian Society of Surgery, the Italian Association of Hospital Surgeons, the Multi-specialist Italian Society of Young Surgeons, the Italian Society of Emergency Surgery and Trauma, the Italian Society of Anesthesia, Analgesia, Resuscitation and Intensive Care, the Italian Society of Orthopaedics and Traumatology, the Italian Society of Emergency Medicine, the Italian Society of Medical Radiology -Section of Vascular and Interventional Radiology- and the World Society of Emergency Surgery). World J. Emerg. Surg. 2014, 9, 18. [Google Scholar] [PubMed]
- Coccolini, F.; Stahel, P.F.; Montori, G.; Biffl, W.; Horer, T.M.; Catena, F.; Kluger, Y.; Moore, E.E.; Peitzman, A.B.; Ivatury, R.; et al. Pelvic trauma: WSES classification and guidelines. World J. Emerg. Surg. 2017, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Gänsslen, A.; Hildebrand, F.; Pohlemann, T. Management of hemodynamic unstable patients “in extremis” with pelvic ring fractures. Acta Chir. Orthop. Traumatol. Cech. 2012, 79, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Smith, W.R.; Moore, E.E. Pelvic packing or angiography: Competitive or complementary? Injury 2009, 40, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Matsushima, K.; Matsumoto, S. Angioembolization versus preperitoneal packing for severe pelvic fractures: A propensity matched analysis. Am. J. Surg. 2023, 225, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.R.; Moore, E.E.; Osborn, P.; Agudelo, J.F.; Morgan, S.J.; Parekh, A.A.; Cothren, C. Retroperitoneal packing as a resuscitation technique for hemodynamically unstable patients with pelvic fractures: Report of two representative cases and a description of technique. J. Trauma Acute Care Surg. 2005, 59, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Cothren, C.C.; Osborn, P.M.; Moore, E.E.; Morgan, S.J.; Johnson, J.L.; Smith, W.R. Preperitonal pelvic packing for hemodynamically unstable pelvic fractures: A paradigm shift. J. Trauma Acute Care Surg. 2007, 62, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Lovisetto, F.; Zonta, S.; Rota, E.; Bottero, L.; Faillace, G.; Turra, G.; Fantini, A.; Longoni, M. Laparoscopic transabdominal preperitoneal (TAPP) hernia repair: Surgical phases and complications. Surg. Endosc. 2007, 21, 646–652. [Google Scholar] [CrossRef]
- Poredos, P.; Gams, P.; Dolinar, D. Massive preperitoneal hemorrhage from unknown source after revision total hip arthroplasty, case report. Clin. Surg. 2020, 3, 1–3. [Google Scholar]
- Logothetopulos, K. Eine absolut sichere Blutstillungsmethode bei vaginalen und abdominalen gynäkologischen operationen. Zentralbl. Gynäkol. 1926, 50, 3202. [Google Scholar]
- Pohlemann, T.; Gänsslen, A.; Bosch, U.; Tscherne, H. The technique of packing for control of hemorrhage in complex pelvic fractures. Tech. Orthop. 1994, 9, 267–270. [Google Scholar] [CrossRef]
- Burlew, C.C.; Moore, E.E.; Stahel, P.F.; Geddes, A.E.; Wagenaar, A.E.; Pieracci, F.M.; Fox, C.J.; Campion, E.M.; Johnson, J.L.; Mauffrey, C. Preperitoneal pelvic packing reduces mortality in patients with life-threatening hemorrhage due to unstable pelvic fractures. J. Trauma Acute Care Surg. 2017, 82, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Shim, H.; Jung, P.Y.; Kim, S.; Bae, K.S. Preperitoneal pelvic packing in patients with hemodynamic instability due to severe pelvic fracture: Early experience in a Korean trauma center. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.K.C.; Li, W.H.; Lee, K.Y.; Cheng, M.N.; Lee, K.B.; Tang, L.F.; Lai, A.K.; Ho, H.F.; Cheung, M.T. Retroperitoneal pelvic packing in the management of hemodynamically unstable pelvic fractures: A level I trauma center experience. J. Trauma Acute Care Surg. 2011, 71, E79–E86. [Google Scholar] [CrossRef] [PubMed]
- Bittner, R.; Arregui, M.E.; Bisgaard, T.; Dudai, M.; Ferzli, G.S.; Fitzgibbons, R.J.; Fortelny, R.H.; Klinge, U.; Kockerling, F.; Kuhry, E.; et al. Guidelines for laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal Hernia [International Endohernia Society (IEHS)]. Surg. Endosc. 2011, 25, 2773–2843. [Google Scholar] [CrossRef]
- Duchesne, J.; Costantini, T.W.; Khan, M.; Taub, E.; Rhee, P.; Morse, B.; Namias, N.; Schwarz, A.; Graves, J.; Kim, D.Y.; et al. The effect of hemorrhage control adjuncts on outcome in severe pelvic fracture: A multi-institutional study. J. Trauma Acute Care Surg. 2019, 87, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Perkins, Z.B.; Maytham, G.D.; Koers, L.; Bates, P.; Brohi, K.; Tai, N.R.M. Impact on outcome of a targeted performance improvement programme in haemodynamically unstable patients with a pelvic fracture. Bone Jt. J. 2014, 96, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Osborn, P.M.; Smith, W.R.; Moore, E.E.; Cothren, C.C.; Morgan, S.J.; Williams, A.E.; Stahel, P.F. Direct retroperitoneal pelvic packing versus pelvic angiography: A comparison of two management protocols for haemodynamically unstable pelvic fractures. Injury 2009, 40, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.T.; Wier, J.; Gary, J.L. Preperitoneal pelvic packing for hypotension has a greater risk of venous thromboembolism than angioembolization management of refractory hypotension in closed pelvic ring injury. J. Bone Jt. Surg. Am. 2022, 104, 1821–1829. [Google Scholar] [CrossRef]
- Heelan, A.A.; Freedberg, M.; Moore, E.E.; Platnick, B.K.; Pieracci, F.M.; Cohen, M.J.; Lawless, R.; Campion, E.M.; Coleman, J.J.; Hoehn, M.; et al. Worth looking! venous thromboembolism in patients who undergo preperitoneal pelvic packing warrants screening duplex. Am. J. Surg. 2020, 220, 1395–1399. [Google Scholar] [CrossRef]
- Shim, H.; Jang, J.Y.; Kim, J.W.; Ryu, H.; Jung, P.Y.; Kim, S.; Kwon, H.Y.; Kim, K.M.; Chung, H.; Bae, K.S. Effectiveness and postoperative wound infection of preperitoneal pelvic packing in patients with hemodynamic instability caused by pelvic fracture. PLoS ONE 2018, 13, e0206991. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Kim, M.J.; Chung, J.S.; Ko, J.W.; Choi, Y.U.; Shim, H.; Jang, J.Y.; Bae, K.S.; Kim, K. Determination of risk factors associated with surgical site infection in patients undergoing preperitoneal pelvic packing for unstable pelvic fracture. Acute Crit. Care 2022, 37, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Ertel, W.; Oberholzer, A.; Platz, A.; Stocker, R.; Trentz, O. Incidence and clinical pattern of the abdominal compartment syndrome after “damage-control” laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit. Care Med. 2000, 28, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Lustenberger, T.; Wutzler, S.; Störmann, P.; Marzi, I. The role of pelvic packing for hemodynamically unstable pelvic ring injuries. Clin. Med. Insights Trauma Intensive Med. 2015, 6, CMTIM-S12257. [Google Scholar] [CrossRef]

| Characteristics | Conventional PPP (n = 19) | Modified PPP (n = 28) | p Value |
|---|---|---|---|
| Age, years, mean ± SD | 49.5 ± 21.6 | 54.3 ± 16.3 | 0.412 |
| Gender | 0.589 | ||
| Male, n (%) | 13 (68.4) | 17 (60.7) | |
| Mechanism, n (%) | 0.01 | ||
| MVC | 1 (5.3) | 1 (3.6) | |
| MCC | 1 (5.3) | 0 (0.0) | |
| AVP | 8 (42.1) | 2 (7.1) | |
| Fall | 4 (21.1) | 19 (67.9) | |
| Others | 5 (26.3) | 6 (21.4) | |
| Vital signs at ED | |||
| SBP, mmHg, mean ± SD | 79.5 ± 24.6 | 77.7 ± 37.1 | 0.854 |
| HR, bpm median [IQR] | 98.0 [90.0–140.0] | 119.0 [103.0–125.0] | 0.704 |
| GCS, median [IQR] | 12 [9–15] | 5 [3–12] | 0.008 |
| Lactate, mmol/L, mean ± SD | 6.6 ± 3.6 | 8.7 ± 4.4 | 0.084 |
| AIS ≥ 3, n (%) | |||
| Head ≥ 3 | 3 (15.8) | 13 (46.4) | 0.030 |
| Chest ≥ 3 | 12 (63.2) | 19 (67.9) | 0.739 |
| Abdomen ≥ 3 | 14 (73.7) | 15 (53.6) | 0.164 |
| Extremity ≥ 3 | 19 (100.0) | 22 (78.6) | 0.031 |
| ISS, mean ± SD | 39.4 ± 11.7 | 38.6 ± 12.7 | 0.828 |
| ISS > 25, n (%) | 18 (94.7%) | 23 (82.1%) | 0.378 |
| Pelvic fracture type (Young–Burgess classification), n (%) | 0.463 | ||
| APC type II | 1 (5.3) | 2 (7.1) | |
| APC type III | 4 (21.1) | 4 (14.3) | |
| LC type I | 1 (5.3) | 6 (21.4) | |
| LC type II | 7 (36.8) | 5 (17.9) | |
| LC type III | 3 (15.8) | 7 (25.0) | |
| VS type | 1 (5.3) | 3 (10.7) | |
| Combined | 2 (10.5) | 1 (3.6) | |
| Pelvic AE, n (%) | 11 (57.9%) | 22 (78.6%) | 0.128 |
| REBOA, n (%) | 2 (10.5%) | 8 (28.6%) | 0.138 |
| Conventional PPP (n = 19) | Modified PPP (n = 28) | p Value | |
|---|---|---|---|
| Mortality, n (%) | |||
| 24 h mortality | 5 (26.3%) | 12 (42.9%) | 0.247 |
| 30-day mortality | 9 (47.4%) | 17 (60.7%) | 0.366 |
| Length of stay, days, median [IQR] | |||
| ICU LOS | 8 [2–17] | 4 [1–19] | 0.262 |
| Hospital LOS | 26 [2–61] | 4 [1–42] | 0.095 |
| Pelvic complications, n (%) | 6 (31.6) | 8 (28.6) | 0.975 |
| SSI | 4 (21.2%) | 3 (10.7%) | 0.417 |
| Repacking | 2 (10.5%) | 5 (17.9%) | 0.685 |
| Non-pelvic complications, n (%) | 12 (63.1) | 8 (28.6) | 0.061 |
| Pneumonia | 7 (36.8%) | 5 (17.9%) | 0.143 |
| CLABSIs | 4 (21.2%) | 2 (7.1%) | 0.204 |
| VTE | 1 (5.3%) | 1 (3.6%) | 1.000 |
| Transfusion | |||
| pRBCs, units/4 h, mean ± SD | 11.2 ± 9.6 | 12.6 ± 7.2 | 0.563 |
| Plasma, units/4 h, mean ± SD | 4.6 ± 4.0 | 5.1 ± 4.6 | 0.737 |
| pRBCs, units/24 h, median [IQR] | 9 [3–26] | 4 [0–10] | 0.011 |
| Plasma, units/24 h, median [IQR] | 8 [3–21] | 5 [1–12] | 0.187 |
| Factors | Univariate (24-Day Mortality) | Multivariate (24-Day Mortality) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age > 55 | 1.60 | 0.47–5.47 | 0.450 | |||
| Sex (male/female) | 1.40 | 0.41–4.78 | 0.591 | |||
| SBP < 90 mmHg | 2.33 | 0.54–10.05 | 0.255 | |||
| HR > 120 bpm | 1.68 | 0.51–5.60 | 0.393 | |||
| Lactate | 1.18 | 1.01–1.38 | 0.037 | 1.13 | 0.94–1.34 | 0.191 |
| GCS ≤ 9 | 4.87 | 1.28–18.57 | 0.020 | 2.06 | 0.42–10.09 | 0.373 |
| ISS > 25 | 1.70 | 0.38–7.50 | 0.486 | |||
| Pelvic AE (Y/N) | 0.18 | 0.05–0.69 | 0.012 | 0.10 | 0.97–16.77 | 0.054 |
| REBOA (Y/N) | 2.08 | 0.50–8.60 | 0.310 | |||
| Pelvic complications (Y/N) | 6.85 | 0.78–59.81 | 0.081 | |||
| pRBCs > 10 | 1.83 | 0.53–6.24 | 0.332 | |||
| Modified PPP | 2.10 | 0.59–7.44 | 0.251 | 4.81 | 0.89–26.04 | 0.068 |
| Factors | Univariate (30-Day Mortality) | Multivariate (30-Day Mortality) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age > 55 | 1.70 | 0.53–5.50 | 0.376 | |||
| Sex (male/female) | 2.40 | 0.68–8.50 | 0.175 | |||
| SBP < 90 mmHg | 1.88 | 0.52–6.85 | 0.336 | |||
| HR > 120 bpm | 1.39 | 0.43–4.49 | 0.579 | |||
| Lactate | 1.17 | 1.01–1.37 | 0.042 | 1.14 | 0.96–1.36 | 0.138 |
| GCS < 9 | 5.54 | 1.56–19.61 | 0.008 | 4.07 | 1.07–15.39 | 0.038 |
| ISS > 25 | 1.17 | 0.30–4.54 | 0.824 | |||
| Pelvic AE (Y/N) | 0.02 | 0.03–0.72 | 0.019 | 0.20 | 0.04–1.12 | 0.067 |
| REBOA (Y/N) | 1.98 | 1.44–8.89 | 0.371 | |||
| Pelvic complications (Y/N) | 2.46 | 0.59–10.28 | 0.216 | |||
| pRBCs > 10 | 2.08 | 0.64–6.74 | 0.258 | |||
| Modified PPP | 2.00 | 0.61–6.56 | 0.252 | 2.07 | 0.38–11.36 | 0.404 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.; Yu, B.; Lee, G.J.; Lee, M.A.; Lee, J.; Choi, K.K. Comparison of Conventional versus Modified Preperitoneal Pelvic Packing in Patients with Bleeding Pelvic Fractures: A Single-Center Retrospective Pilot Study. J. Clin. Med. 2024, 13, 4062. https://doi.org/10.3390/jcm13144062
Jeon S, Yu B, Lee GJ, Lee MA, Lee J, Choi KK. Comparison of Conventional versus Modified Preperitoneal Pelvic Packing in Patients with Bleeding Pelvic Fractures: A Single-Center Retrospective Pilot Study. Journal of Clinical Medicine. 2024; 13(14):4062. https://doi.org/10.3390/jcm13144062
Chicago/Turabian StyleJeon, Sebeom, Byungchul Yu, Gil Jae Lee, Min A Lee, Jungnam Lee, and Kang Kook Choi. 2024. "Comparison of Conventional versus Modified Preperitoneal Pelvic Packing in Patients with Bleeding Pelvic Fractures: A Single-Center Retrospective Pilot Study" Journal of Clinical Medicine 13, no. 14: 4062. https://doi.org/10.3390/jcm13144062
APA StyleJeon, S., Yu, B., Lee, G. J., Lee, M. A., Lee, J., & Choi, K. K. (2024). Comparison of Conventional versus Modified Preperitoneal Pelvic Packing in Patients with Bleeding Pelvic Fractures: A Single-Center Retrospective Pilot Study. Journal of Clinical Medicine, 13(14), 4062. https://doi.org/10.3390/jcm13144062





