Myocardial Work Indices in Patients Recently Recovered from Mild-to-Moderate COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Transthoracic Echocardiography
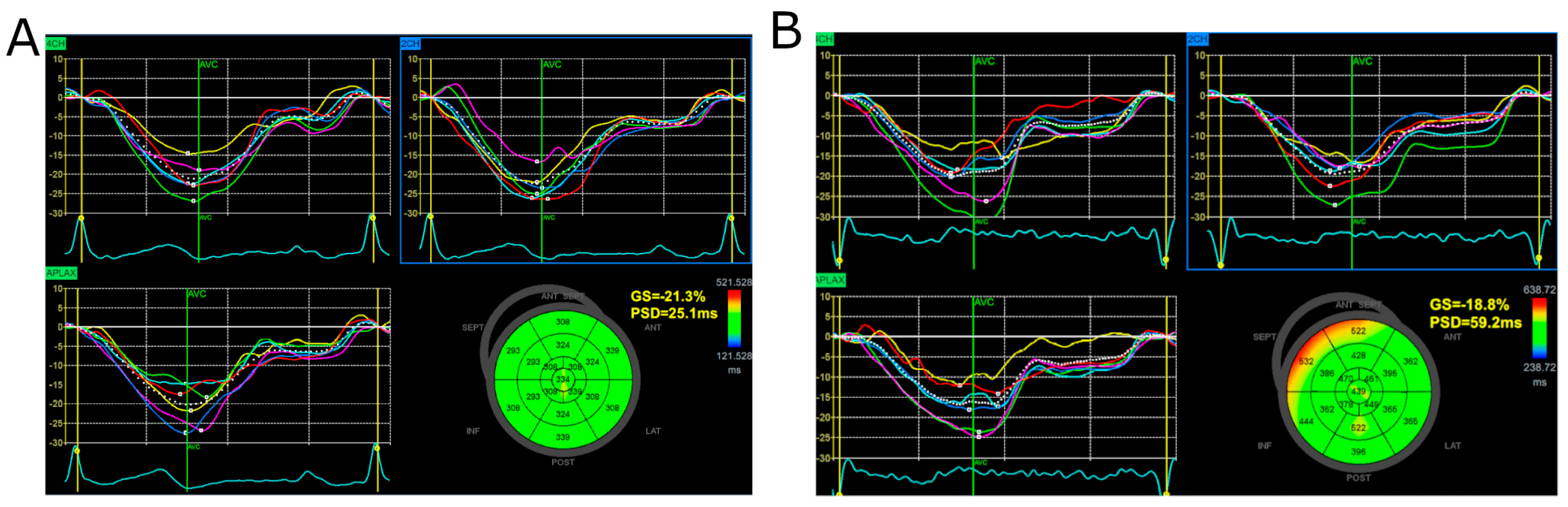
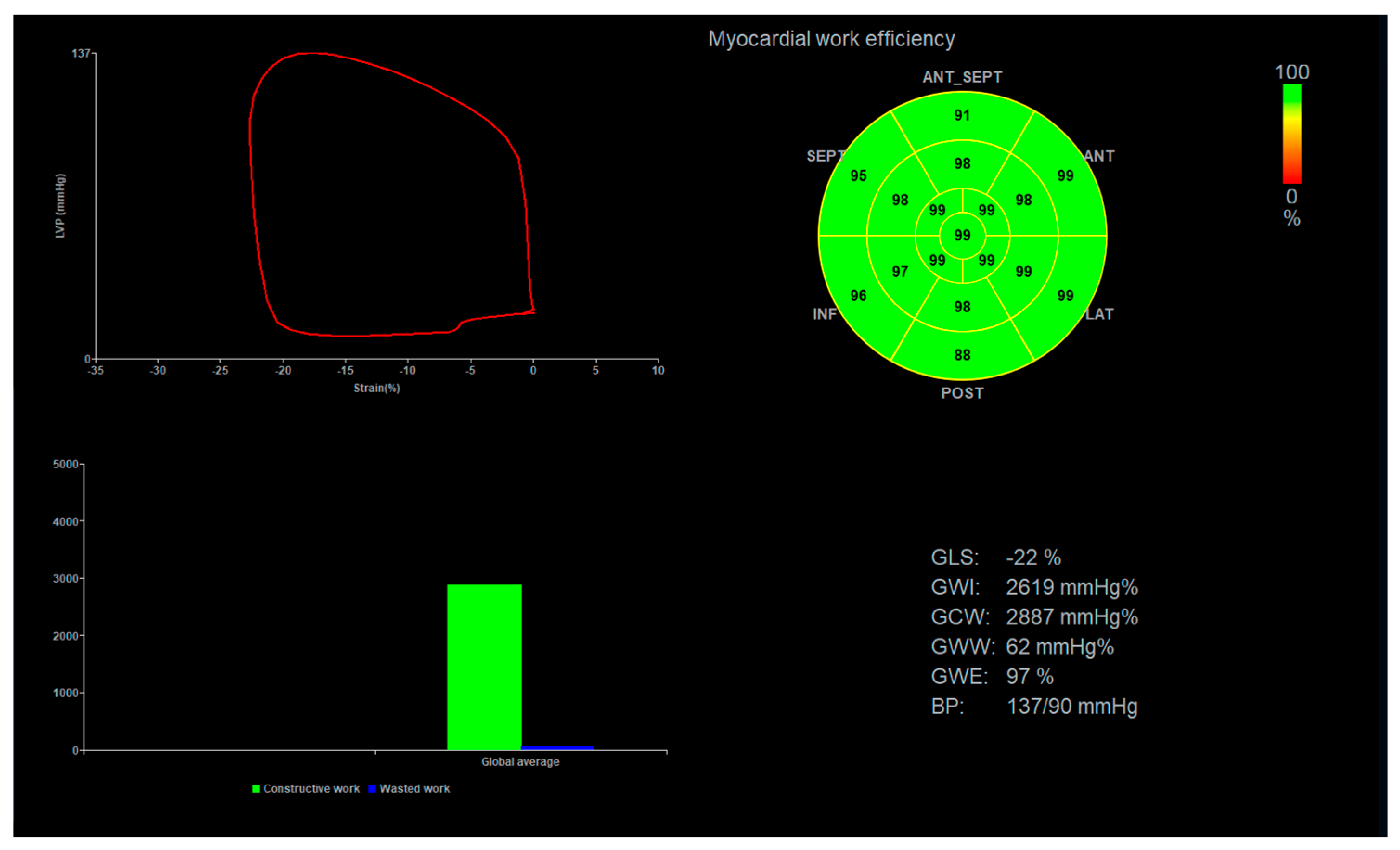
2.2. Global Longitudinal Strain and Myocardial Work Analysis
2.3. Statistical Analysis
3. Results
3.1. Comparison of Groups with Normal and Elevated Troponin I Levels
3.2. Global Longitudinal Strain, Peak Systolic Dispersion, and Myocardial Work Indices
4. Discussion
4.1. Summary of Findings
4.2. High-Sensitivity Troponin I in Our Study Group
4.3. Importance of Myocardial Work Indices
4.4. PSD as an Indicator of Left Ventricular Contraction Inhomogeneity
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, H.; Li, J.; Li, J.; Li, H.; Xin, Y. COVID-19 and Acute Cardiac Injury: Clinical Manifestations, Biomarkers, Mechanisms, Diagnosis, and Treatment. Curr. Cardiol. Rep. 2023, 25, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Croft, L.B.; Stefanini, G.G.; Bragato, R.; Silbiger, J.J.; Vicenzi, M.; Danilov, T.; Kukar, N.; Shaban, N.; Kini, A.; et al. Characterization of Myocardial Injury in Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Vazirani, R.; Feltes, G.; Hoyo, R.S.-D.; Viana-Llamas, M.C.; Raposeiras-Roubín, S.; Romero, R.; Alfonso-Rodríguez, E.; Uribarri, A.; Santoro, F.; Becerra-Muñoz, V.; et al. Elevated Troponins after COVID-19 Hospitalization and Long-Term COVID-19 Symptoms: Incidence, Prognosis, and Clinical Outcomes-Results from a Multi-Center International Prospective Registry (HOPE-2). J. Clin. Med. 2024, 13, 2596. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Deng, K.-Q.; Li, C.; Yang, Z.; Hu, H.; Cai, H.; Zhang, C.; He, T.; Zheng, F.; Wang, H.; et al. Cardiac Involvement in Recovered Patients from COVID-19: A Preliminary 6-Month Follow-Up Study. Front. Cardiovasc. Med. 2021, 8, 654405. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, P.; Śmiarowski, M.; Przyborska, W.; Zemlik, K.; Małecka-Giełdowska, M.; Leszczyńska, A.; Garley, M.; Ciepiela, O. Mild-to-Moderate COVID-19 Convalescents May Present Pro-Longed Endothelium Injury. J. Clin. Med. 2022, 11, 6461. [Google Scholar] [CrossRef] [PubMed]
- Gherbesi, E.; Bergamaschi, L.; Cusmano, I.; Tien, T.T.; Paolisso, P.; Foà, A.; Pizzi, C.; Barosi, A. The Usefulness of Speckle Tracking Echocardiography in Identifying Subclinical Myocardial Dysfunction in Young Adults Recovered from Mild COVID-19. Echocardiogr. Mt. Kisco N 2022, 39, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Rácz, G.; Takács, H.; Kormányos, Á.; Polestyuk, B.; Borbás, J.; Gyenes, N.; Schvartz, N.; Németh, G.; Kincses, Z.T.; Sepp, R.; et al. Screening for Myocardial Injury after Mild SARS-CoV-2 Infection with Advanced Transthoracic Echocardiography Modalities. Diagnostics 2022, 12, 1941. [Google Scholar] [CrossRef]
- Ardahanli, I.; Akhan, O.; Sahin, E.; Akgun, O.; Gurbanov, R. Myocardial Performance Index Increases at Long-Term Follow-up in Patients with Mild to Moderate COVID-19. Echocardiogr. Mt. Kisco N 2022, 39, 620–625. [Google Scholar] [CrossRef]
- Nour, A.; Fouad, M.; Salam, Z.A. Evaluation of Cardiovascular Autonomic Dysfunction in Symptomatic Post-COVID-19 Patients Using the Heart Rate Variability (HRV) and Detection of Subtle LV Dysfunction Using 2D Global Longitudinal Strain (GLS). Int. J. Cardiovasc. Imaging 2023, 39, 2107–2118. [Google Scholar] [CrossRef]
- Malesevic, S.; Sievi, N.A.; Baumgartner, P.; Roser, K.; Sommer, G.; Schmidt, D.; Vallelian, F.; Jelcic, I.; Clarenbach, C.F.; Kohler, M. Impaired Health-Related Quality of Life in Long-COVID Syndrome after Mild to Moderate COVID-19. Sci. Rep. 2023, 13, 7717. [Google Scholar] [CrossRef]
- Tsai, E.J.; Čiháková, D.; Tucker, N.R. Cell-Specific Mechanisms in the Heart of COVID-19 Patients. Circ. Res. 2023, 132, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A Novel Clinical Method for Quantification of Regional Left Ventricular Pressure-Strain Loop Area: A Non-Invasive Index of Myocardial Work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.; Özden Tok, Ö.; Mitrousi, K.; Ikonomidis, I. Myocardial Work: Methodology and Clinical Applications. Diagnostics 2021, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, F.; D’Andrea, A.; D’Ascenzi, F.; Bandera, F.; Benfari, G.; Esposito, R.; Malagoli, A.; Mandoli, G.E.; Santoro, C.; Russo, V.; et al. Myocardial Work by Echocardiography: Principles and Applications in Clinical Practice. J. Clin. Med. 2021, 10, 4521. [Google Scholar] [CrossRef] [PubMed]
- Italia, L.; Ingallina, G.; Napolano, A.; Boccellino, A.; Belli, M.; Cannata, F.; Rolando, M.; Ancona, F.; Melillo, F.; Stella, S.; et al. Subclinical Myocardial Dysfunction in Patients Recovered from COVID-19. Echocardiography 2021, 38, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Katogiannis, K.; Thymis, J.; Korakas, E.; Pavlidis, G.; Kazakou, P.; Panagopoulos, G.; et al. Myocardial Work and Vascular Dysfunction Are Partially Improved at 12 Months after COVID-19 Infection. Eur. J. Heart Fail. 2022, 24, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.S.; Gilotra, N.A.; Goerlich, E.; Metkus, T.; Garibaldi, B.T.; Sharma, G.; Bavaro, N.; Phillip, S.; Michos, E.D.; Hays, A.G. Myocardial Work Efficiency, A Novel Measure of Myocardial Dysfunction, Is Reduced in COVID-19 Patients and Associated with In-Hospital Mortality. Front. Cardiovasc. Med. 2021, 8, 667721. [Google Scholar] [CrossRef]
- Luchian, M.-L.; Motoc, A.; Lochy, S.; Magne, J.; Belsack, D.; De Mey, J.; Roosens, B.; Van den Bussche, K.; Boeckstaens, S.; Chameleva, H.; et al. Subclinical Myocardial Dysfunction in Patients with Persistent Dyspnea One Year after COVID-19. Diagn. Basel Switz. 2021, 12, 57. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vivid E95/E90/E80, EchoPAC Software Only, EchoPAC Plug-in Reference Manual 2023; GE HealthCare: Chicago, IL, USA, 2023.
- Smiseth, O.A.; Donal, E.; Penicka, M.; Sletten, O.J. How to Measure Left Ventricular Myocardial Work by Pressure–Strain Loops. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 259–261. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.Covid19treatmentguidelines.Nih.Gov (accessed on 15 April 2024).
- Truong, V.T.; Vo, H.Q.; Ngo, T.N.M.; Mazur, J.; Nguyen, T.T.H.; Pham, T.T.M.; Le, T.K.; Phan, H.; Palmer, C.; Nagueh, S.F.; et al. Normal Ranges of Global Left Ventricular Myocardial Work Indices in Adults: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2022, 35, 369–377.e8. [Google Scholar] [CrossRef]
- D’Ávila, L.B.O.; Milani, M.; Le Bihan, D.C.S.; de Lima, A.C.G.B.; Milani, J.G.P.O.; Cipriano, G.F.B.; da Silva, V.Z.M.; Cipriano, G. Longitudinal Strain and Myocardial Work in Symptomatic Patients Having Recovered from COVID-19 and Possible Associations with the Severity of the Disease. Int. J. Cardiovasc. Imaging 2024, 40, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Olsen, F.J.; Lassen, M.C.H.; Skaarup, K.G.; Christensen, J.; Davidovski, F.S.; Alhakak, A.S.; Sengeløv, M.; Nielsen, A.B.; Johansen, N.D.; Graff, C.; et al. Myocardial Work in Patients Hospitalized with COVID-19: Relation to Biomarkers, COVID-19 Severity, and All-Cause Mortality. J. Am. Heart Assoc. 2022, 11, e026571. [Google Scholar] [CrossRef] [PubMed]
- Alsagaff, M.Y.; Wardhani, L.F.K.; Nugraha, R.A.; Putra, T.S.; Khrisna, B.P.D.; Al-Farabi, M.J.; Gunadi, R.I.; Azmi, Y.; Budianto, C.P.; Fagi, R.A.; et al. Quantification of Hs-Troponin Levels and Global Longitudinal Strain among Critical COVID-19 Patients with Myocardial Involvement. J. Clin. Med. 2024, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Than, M.P.; Aldous, S.J.; Troughton, R.W.; Pemberton, C.J.; Richards, A.M.; Frampton, C.M.A.; Florkowski, C.M.; George, P.M.; Bailey, S.; Young, J.M.; et al. Detectable High-Sensitivity Cardiac Troponin within the Population Reference Interval Conveys High 5-Year Cardiovascular Risk: An Observational Study. Clin. Chem. 2018, 64, 1044–1053. [Google Scholar] [CrossRef]
- Hayama, H.; Ide, S.; Moroi, M.; Kitami, Y.; Bekki, N.; Kubota, S.; Uemura, Y.; Hara, H.; Kutsuna, S.; Ohmagari, N.; et al. Elevated High-Sensitivity Troponin Is Associated with Subclinical Cardiac Dysfunction in Patients Recovered from Coronavirus Disease 2019. Glob. Health Med. 2021, 3, 95–101. [Google Scholar] [CrossRef]
- Olsen, F.J.; Skaarup, K.G.; Lassen, M.C.H.; Johansen, N.D.; Sengeløv, M.; Jensen, G.B.; Schnohr, P.; Marott, J.L.; Søgaard, P.; Gislason, G.; et al. Normal Values for Myocardial Work Indices Derived from Pressure-Strain Loop Analyses: From the CCHS. Circ. Cardiovasc. Imaging 2022, 15, e013712. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic Reference Ranges for Normal Non-Invasive Myocardial Work Indices: Results from the EACVI NORRE Study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, X.; Huang, K.; Tang, Y.; Gao, Q.; Chen, X.; Jing, B.; Wang, X.; Lin, B.; Su, M. Echocardiographic Reference Ranges for Noninvasive Left Ventricular 18-Segment Myocardial Work Index and Work Efficiency in a Healthy Asian Population. Cardiovasc. Ultrasound 2023, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Morbach, C.; Sahiti, F.; Tiffe, T.; Cejka, V.; Eichner, F.A.; Gelbrich, G.; Heuschmann, P.U.; Störk, S. STAAB consortium Myocardial Work—Correlation Patterns and Reference Values from the Population-Based STAAB Cohort Study. PLoS ONE 2020, 15, e0239684. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, T.; Smiseth, O.A. Assessment of Wasted Myocardial Work: A Novel Method to Quantify Energy Loss Due to Uncoordinated Left Ventricular Contractions. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H996–H1003. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Tersalvi, G.; Vicenzi, M.; Calabretta, D.; Biasco, L.; Pedrazzini, G.; Winterton, D. Elevated Troponin in Patients with Coronavirus Disease 2019: Possible Mechanisms. J. Card. Fail. 2020, 26, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Guo, Y.; Wang, X.; Yang, C.; Li, Y.; Meng, X.; Pei, Z.; Zhang, R.; Zhong, Y.; Wang, F. Myocardial Work by Speckle Tracking Echocardiography Accurately Assesses Left Ventricular Function of Coronary Artery Disease Patients. Front. Cardiovasc. Med. 2021, 8, 727389. [Google Scholar] [CrossRef]
- Tudoran, M.; Tudoran, C.; Lazureanu, V.; Marinescu, A.; Pop, G.; Pescariu, A.; Enache, A.; Cut, T. Alterations of Left Ventricular Function Persisting during Post-Acute COVID-19 in Subjects without Previously Diagnosed Cardiovascular Pathology. J. Pers. Med. 2021, 11, 225. [Google Scholar] [CrossRef]
- Caiado, L.D.C.; Azevedo, N.C.; Azevedo, R.R.C.; Caiado, B.R. Cardiac Involvement in Patients Recovered from COVID-19 Identified Using Left Ventricular Longitudinal Strain. J. Echocardiogr. 2022, 20, 51–56. [Google Scholar] [CrossRef]
- Mornoş, C.; Muntean, D.; Mornoş, A.; Crişan, S.; Petrescu, L.; Ionac, A.; Sosdean, R.; Cozma, D. Risk Stratification in Patients with Heart Failure: The Value of Considering Both Global Longitudinal Left Ventricular Strain and Mechanical Dispersion. Can. J. Physiol. Pharmacol. 2017, 95, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yuan, M.; Li, K.; Bai, W.; Rao, L. Value of Peak Strain Dispersion in Discovering Left Ventricular Dysfunction in Diabetes Mellitus. Sci. Rep. 2020, 10, 21437. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Peng, Q.; Yin, L.; Li, C. Evaluation of Exercise Tolerance in Non-Obstructive Hypertrophic Cardiomyopathy with Myocardial Work and Peak Strain Dispersion by Speckle-Tracking Echocardiography. Front. Cardiovasc. Med. 2022, 9, 927671. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hu, C.; Wang, Y.; Cheng, Y.; Zhao, Y.; Liu, Y.; Zheng, S.; Chen, H.; Shu, X. Mechanical Synchrony and Myocardial Work in Heart Failure Patients with Left Bundle Branch Area Pacing and Comparison with Biventricular Pacing. Front. Cardiovasc. Med. 2021, 8, 727611. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of Myocardial Injury in Recovered Troponin-Positive COVID-19 Patients Assessed by Cardiovascular Magnetic Resonance. Eur. Heart J. 2021, 42, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Zidar, D.A.; Bristow, M.R.; Cameron, S.J.; Chan, T.; Harding, C.V.; Kwon, D.H.; Singh, T.; Tilton, J.C.; Tsai, E.J.; et al. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ. Res. 2021, 128, 1214–1236. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Fogarty, H.; Dyer, A.; Martin-Loeches, I.; Bannan, C.; Nadarajan, P.; Bergin, C.; O’Farrelly, C.; Conlon, N.; Bourke, N.M.; et al. Prolonged Elevation of D-dimer Levels in Convalescent COVID-19 Patients Is Independent of the Acute Phase Response. J. Thromb. Haemost. 2021, 19, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Prosch, H.; Zehetmayer, S.; Gysan, M.R.; Bernitzky, D.; Vonbank, K.; Idzko, M.; Gompelmann, D. Impact of Persistent D-Dimer Elevation Following Recovery from COVID-19. PLoS ONE 2021, 16, e0258351. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and Its Implications for Thrombosis and Anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Izzo, C.; Visco, V.; Gambardella, J.; Ferruzzi, G.J.; Rispoli, A.; Rusciano, M.R.; Toni, A.L.; Virtuoso, N.; Carrizzo, A.; Di Pietro, P.; et al. Cardiovascular Implications of microRNAs in Coronavirus Disease 2019. J. Pharmacol. Exp. Ther. 2023, 384, 102–108. [Google Scholar] [CrossRef]

| Number of Patients | 102 |
|---|---|
| Gender: Male/Female (%) | 45 (44.1)/57 (55.9) |
| Age (years) | 52 (43.0–63.0) |
| BMI (kg/m2) | 27.2 (23.4–29.9) |
| Systolic blood pressure (mmHg) | 131.5 (122.0–143.0) |
| Diastolic blood pressure (mmHg) | 82.0 (75.0–89.0) |
| Resting heart rate (beats/min) | 76.0 (69.0–85.0) |
| Days since COVID-19 diagnosis | 56.0 (42.0–71.0) |
| Number of pts hospitalized due to COVID-19 (%) | 26 (26.0) |
| Comorbidities (%) | |
| Hypertension | 36 (35) |
| Diabetes mellitus | 10 (10) |
| Asthma/Chronic obstructive pulmonary disease | 9 (9) |
| Ischemic heart disease | 3 (3) |
| Laboratory data (n = 99) | |
| White blood cells (109/L) | 6.9 (5.8–8.4) |
| Red blood cells (1012/L) | 4.6 (4.3–4.9) |
| Hemoglobin (mmol/L) | 8.9 (8.2–9.5) |
| Hematocrit (%) | 42.6 (39.8–45.6) |
| Platelet (109/L) | 253.0 (223.0–282.0) |
| D-Dimer (ng/mL) | 334.2 (247.2–529.6) |
| Creatinine (mg/dL) | 0.8 (0.8–0.9) |
| eGFR (mL/min/1.73 m2) | 88.3 (79.6–97.4) |
| C-reactive protein (mg/L) | 1.1 (0.6–2.8) |
| High-sensitive troponin I (pg/mL) | 1.6 (1.6–2.7) |
| NT-proBNP (pg/mL) | 60.4 (34.0–97.6) |
| Electrocardiographic Parameters (n = 104) | |
| PR interval (ms) | 157 (140–173) |
| QRS duration (ms) | 97 (90–105) |
| QTc interval (ms) | 429 (412–443) |
| Echocardiographic data (n = 102) | |
| Interventricular septum thickness (cm) | 0.9 (0.9–1.1) |
| Left ventricular internal diameter (cm) | 4.8 (4.6–5.1) |
| Left ventricular posterior wall thickness (cm) | 0.8 (0.7–0.9) |
| Left atrium dimension (cm) | 3.7 (3.2–3.8) |
| Mitral E-wave velocity (m/s) | 0.68 (0.6–0.78) |
| Mitral valve deceleration time (ms) | 183 (150–208) |
| Mitral A-wave velocity (m/s) | 0.73 (0.61–0.84) |
| Mitral valve E/A ratio | 0.94 (0.75–1.14) |
| E′ (m/s) | 0.12 (0.09–0.14) |
| E/E′ | 5.73 (4.72–7.32) |
| Left atrium volume index (mL/m2) | 28.2 (23.1–31.4) |
| Left Ventricular Ejection fraction (%) | 61 (58–64) |
| LVEDV (mL) | 104 (86–123) |
| LVESV (mL) | 41 (33–49) |
| Normal Levels of Troponin I (n = 57) | Increased Level of Troponin I (n = 42) | p Value | |
|---|---|---|---|
| Gender: Male/Female (%) | 26 (45.6)/31 (54.4) | 18 (42.9)/24 (57.1) | 0.78 |
| Age (years) | 50.0 (38.0–64.0) | 54.0 (43.0–61.0) | 0.40 |
| BMI (kg/m2) | 27.3 (23.9–29.3) | 26.7 (23.3–31.6) | 0.56 |
| Systolic blood pressure (mmHg) | 130.0 (123.0–143.0) | 133.5 (119.0–143.0) | 0.73 |
| Diastolic blood pressure (mmHg) | 80.0 (74.0–87.0) | 85.0 (78.0–90.0) | 0.05 |
| Resting heart rate (beats/min) | 75.0 (68.0–85.0) | 77.0 (70.0–83.0) | 0.59 |
| Days since COVID-19 diagnosis | 56.0 (42.0–67.0) | 57.5 (41.0–71.0) | 0.75 |
| Number of pts hospitalized due to COVID-19 (%) | 13 (22.8) | 11 (26.2) | 0.70 |
| Comorbidities (%) | |||
| Hypertension | 20 (35.1) | 15 (35.7) | 0.95 |
| Diabetes mellitus | 5 (8.8) | 5 (11.1) | 0.69 |
| Asthma/Chronic obstructive pulmonary disease | 6 (10.5) | 3 (7.1) | 0.56 |
| Ischemic heart disease | 2 (3.5) | 1 (2.4) | 0.75 |
| Normal Levels of Troponin I (n = 57) | Increased Level of Troponin I (n = 42) | p Value | |
|---|---|---|---|
| Interventricular Septum thickness (cm) | 0.9 (0.8–1.0) | 1.0 (0.9–1.1) | 0.002 |
| Left Ventricular Internal Diameter (cm) | 4.7 (4.5–5.0) | 4.9 (4.7–5.2) | 0.009 |
| Left Ventricular Posterior Wall thickness (cm) | 0.8 (0.7–0.9) | 0.9 (0.8–1.0) | 0.002 |
| Left Atrium Dimension (cm) | 3.5 (3.1–3.8) | 3.7 (3.4–3.9) | 0.09 |
| Mitral E-wave Velocity (m/s) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.64 |
| Mitral Valve Deceleration Time (ms) | 178.0 (154.0–197.0) | 201.0 (150.0–221.0) | 0.14 |
| Mitral A-wave Velocity (m/s) | 0.7 (0.6–0.8) | 0.8 (0.7–0.9) | 0.004 |
| Mitral Valve E/A Ratio | 1.0 (0.8–1.2) | 0.8 (0.7–1.0) | 0.01 |
| E′ (cm/s) | 12 (10–15) | 10 (8–13) | 0.002 |
| E/E′ | 5.4 (4.6–6.6) | 6.3 (5.0–7.7) | 0.07 |
| Left Atrial Volume Index (mL/m2) | 26.0 (22.4–30.4) | 28.8 (24.4–35.9) | 0.11 |
| Left Ventricular Ejection fraction (%) | 62.0 (59.0–65.0) | 60.0 (55.0–64.0) | 0.20 |
| LVEDV(mL) | 101 (83–116) | 109 (96–130) | 0.04 |
| LVESV (mL) | 38 (32–47) | 45 (38–52) | 0.01 |
| Entire Group (n = 97) | Normal Levels of Troponin I (n = 57) | Increased Level of Troponin I (n = 37) | p Value | |
|---|---|---|---|---|
| GLS (%) | −19.0 (−20.0–−17.0) | −19.0 (−21.0–−18.0) | −18.0 (−19.0–−16.0) | 0.008 |
| PSD (ms) | 45 (39–56) | 43 (36–49) | 54 (43–64) | <0.001 |
| GWI (mmHg%) | 1834 (1168–2054) | 1857 (1867–2045) | 1867 (1641–2060) | 0.53 |
| GCW (mmHg%) | 2130 (2010–2398) | 2120 (1976–2035) | 2182 (2030–2460) | 0.67 |
| GWW (mmHg%) | 119 (78–175) | 97 (69–132) | 168 (121–196) | <0.005 |
| GWE (%) | 94 (92–96) | 95 (94–96) | 93 (90–94) | <0.005 |
| Parameter | Intraobserver ICC (95% CI) | Interobserver ICC (95% CI) |
|---|---|---|
| PSD | 0.93 (0.82–0.97) | 0.90 (0.76–0.96) |
| GLS | 0.97 (0.93–0.99) | 0.89 (0.75–0.96) |
| GCW | 0.98 (0.96–0.99) | 0.94 (0.84–0.98) |
| GWE | 0.83 (0.57–0.93) | 0.81 (0.51–0.93) |
| GWW | 0.85 (0.62–0.94) | 0.84 (0.60–0.94) |
| GWI | 0.96 (0.88–0.98) | 0.88(0.62–0.95) |
| GWI | GCW | GWW | GWE | |
|---|---|---|---|---|
| Troponin I | ns | ns | 0.40; p < 001 | −0.41; p < 001 |
| PSD | −0.20; p = 0.05 | ns | 0.63; p < 0.001 | −0.64; p < 0.001 |
| LVEDd | ns | ns | 0.24; p = 0.02 | ns |
| LVEF | 0.35; p < 001 | 0.32; p = 0.001 | ns | 0.25; p = 0.02 |
| E wave | 0.25; p = 0.01 | ns | ns | |
| E/A | ns | ns | ns | 0.22; p = 0.03 |
| Independent Variable | β (Beta) | SE (Standard Error) | t | p | R2 | |
|---|---|---|---|---|---|---|
| GWW | Age | −0.02 | 0.08 | −0.25 | 0.80 | 0.51 |
| BMI | 0.03 | 0.08 | 0.32 | 0.75 | ||
| Time since infection | 0.07 | 0.08 | 0.89 | 0.38 | ||
| hs-TnI | 0.09 | 0.09 | 1.02 | 0.31 | ||
| D-Dimer | 0.09 | 0.08 | 1.09 | 0.28 | ||
| Creatinine | −0.14 | 0.08 | −1.70 | 0.09 | ||
| LVIDd | 0.08 | 0.08 | 1.05 | 0.30 | ||
| LVEF | −0.06 | 0.08 | −0.78 | 0.44 | ||
| E/A ratio | 0.14 | 0.09 | 1.60 | 0.11 | ||
| PSD | 0.67 | 0.09 | 7.82 | <0.001 | ||
| GWE | Age | 0.01 | 0.08 | 0.08 | 0.94 | 0.53 |
| BMI | 0.02 | 0.08 | 0.24 | 0.81 | ||
| Time since infection | −0.08 | 0.08 | −0.98 | 0.33 | ||
| hs-TnI | −0.04 | 0.09 | −0.52 | 0.60 | ||
| D-Dimer | −0.12 | 0.08 | −1.51 | 0.13 | ||
| Creatinine | 0.14 | 0.08 | 1.66 | 0.10 | ||
| LVIDd | 0.00 | 0.08 | 0.04 | 0.97 | ||
| LVEF | 0.16 | 0.08 | 1.99 | 0.05 | ||
| E/A ratio | −0.08 | 0.09 | −0.89 | 0.38 | ||
| PSD | −0.67 | 0.08 | −7.96 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dankowski, R.; Sacharczuk, W.; Fedorowicz, J.; Małek-Elikowska, M.; Ożegowski, S.; Baszko, A. Myocardial Work Indices in Patients Recently Recovered from Mild-to-Moderate COVID-19. J. Clin. Med. 2024, 13, 4090. https://doi.org/10.3390/jcm13144090
Dankowski R, Sacharczuk W, Fedorowicz J, Małek-Elikowska M, Ożegowski S, Baszko A. Myocardial Work Indices in Patients Recently Recovered from Mild-to-Moderate COVID-19. Journal of Clinical Medicine. 2024; 13(14):4090. https://doi.org/10.3390/jcm13144090
Chicago/Turabian StyleDankowski, Rafał, Wioletta Sacharczuk, Julita Fedorowicz, Małgorzata Małek-Elikowska, Stefan Ożegowski, and Artur Baszko. 2024. "Myocardial Work Indices in Patients Recently Recovered from Mild-to-Moderate COVID-19" Journal of Clinical Medicine 13, no. 14: 4090. https://doi.org/10.3390/jcm13144090
APA StyleDankowski, R., Sacharczuk, W., Fedorowicz, J., Małek-Elikowska, M., Ożegowski, S., & Baszko, A. (2024). Myocardial Work Indices in Patients Recently Recovered from Mild-to-Moderate COVID-19. Journal of Clinical Medicine, 13(14), 4090. https://doi.org/10.3390/jcm13144090







