Charting Alzheimer’s Disease and Dementia: Epidemiological Insights, Risk Factors and Prevention Pathways
Abstract
1. Introduction
2. Methods
3. Is the Incidence of Dementia on the Rise or Declining?
4. Risk Factors
4.1. Genetic Risk Factors
4.2. Early Risk Factors
4.3. Preventable vs. Non-Preventable Risk Factors
4.4. Risk Factors: Individual vs. Population Factors
4.5. Individual Factors
4.5.1. Education and Cognitive Stimulating Activities
4.5.2. Medical Conditions
- Cardiovascular Diseases (CVDs)
- Diabetes mellitus
- Hearing impairment
- Neurological diseases
- Epilepsy
- Depression and Anxiety
- Sleep disorders
- Frailty/Poor health
4.6. Lifestyle/Environment Preventable Risk Factors
4.6.1. Alcohol Consumption
4.6.2. Smoking
4.6.3. Dietary Patterns
4.6.4. Physical Activity (PA)
4.6.5. Social Isolation
4.7. Population Factors
4.7.1. Air Pollution
4.7.2. Other Population Risk Factors
5. Implications of Modifiable Risk Factors for Intervention Programs
6. Discussion
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Aβ | Amyloid Beta |
| AD | Alzheimer’s Disease |
| ApoE | Apolipoprotein E |
| CHAP | Chicago Health and Aging Project |
| CFAS | Cognitive Function and Ageing Study |
| CVD | Cardiovascular Disease |
| DM | Diabetes Mellitus |
| DOHaD | Developmental Origins of Health and Disease |
| GWAS | Genome-Wide Association Studies |
| IIDP | Indianapolis-Ibadan Dementia Project |
| MCI | Mild Cognitive Impairment |
| NICE | National Institute for Health and Care Excellence |
| PA | Physical Activity |
| PRS | Polygenic Risk Score |
| RF | Risk Factors |
| SAS | Survey of Aging Shanghai |
| SESD | Shanghai Epidemiological Survey of Dementia |
| SNPs | Single Nucleotide Polymorphisms |
| TBI | Traumatic Brain Injury |
| VaD | Vascular Dementia |
| VRFs | Vascular Risk Factors |
| WHO | World Health Organization |
References
- Wimo, A.; Seeher, K.; Cataldi, R.; Cyhlarova, E.; Dielemann, J.L.; Frisell, O.; Guerchet, M.; Jönsson, L.; Malaha, A.K.; Nichols, E.; et al. The Worldwide Costs of Dementia in 2019. Alzheimers Dement. 2023, 19, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global Estimates on the Number of Persons across the Alzheimer’s Disease Continuum. Alzheimers Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Satizabal, C.L.; Beiser, A.S.; Chouraki, V.; Chêne, G.; Dufouil, C.; Seshadri, S. Incidence of Dementia over Three Decades in the Framingham Heart Study. N. Engl. J. Med. 2016, 374, 523–532. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switzerland, 2017.
- World Health Organization. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2019.
- Arnsten, A.F.T.; Datta, D.; Del Tredici, K.; Braak, H. Hypothesis: Tau Pathology Is an Initiating Factor in Sporadic Alzheimer’s Disease. Alzheimers Dement. 2021, 17, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the Pathologic Process in Alzheimer Disease: Age Categories from 1 to 100 Years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. Where, When, and in What Form Does Sporadic Alzheimer’s Disease Begin? Curr. Opin. Neurol. 2012, 25, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Khachaturian, Z.S.; Khachaturian, A.S. Politics of Science: Progress toward Prevention of the Dementia–Alzheimer’s Syndrome. Mol. Asp. Med. 2015, 43–44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Roehr, S.; Pabst, A.; Luck, T.; Riedel-Heller, S. Is Dementia Incidence Declining in High-Income Countries? A Systematic Review and Meta-Analysis. Clin. Epidemiol. 2018, 10, 1233–1247. [Google Scholar] [CrossRef]
- Wolters, F.J.; Chibnik, L.B.; Waziry, R.; Anderson, R.; Berr, C.; Beiser, A.; Bis, J.C.; Blacker, D.; Bos, D.; Brayne, C.; et al. Twenty-Seven-Year Time Trends in Dementia Incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology 2020, 95, e519–e531. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Beiser, A.S.; Breteler, M.M.B.; Fratiglioni, L.; Helmer, C.; Hendrie, H.C.; Honda, H.; Ikram, M.A.; Langa, K.M.; Lobo, A.; et al. The Changing Prevalence and Incidence of Dementia over Time—Current Evidence. Nat. Rev. Neurol. 2017, 13, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S. The Enigma of Decreasing Dementia Incidence. JAMA Netw. Open 2020, 3, e2011199. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Puterman, E.; Prather, A.A.; Ware, E.B.; Rehkopf, D.H. A Data-Driven Prospective Study of Dementia among Older Adults in the United States. PLoS ONE 2020, 15, e0239994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.-D.; Deng, Y.-T.; You, J.; He, X.-Y.; Wu, X.-R.; Wu, B.-S.; Yang, L.; Zhang, Y.-R.; Kuo, K.; et al. Identifying Modifiable Factors and Their Joint Effect on Dementia Risk in the UK Biobank. Nat. Hum. Behav. 2023, 7, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 Year Multidomain Intervention of Diet, Exercise, Cognitive Training, and Vascular Risk Monitoring versus Control to Prevent Cognitive Decline in at-Risk Elderly People (FINGER): A Randomised Controlled Trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.; Mangialasche, F.; Ngandu, T.; Solomon, A.; Kivipelto, M. Multidomain Interventions to Prevent Cognitive Impairment, Alzheimer’s Disease and Dementia: From Finger to World-Wide Fingers. J. Prev. Alzheimers Dis. 2020, 7, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dacks, P.A.; Andrieu, S.; Blacker, D.; Carman, A.J.; Green, A.M.; Grodstein, F.; Henderson, V.W.; James, B.D.; Lane, R.F.; Lau, J.; et al. Dementia Prevention: Optimizing the Use of Observational Data for Personal, Clinical, and Public Health Decision-Making. J. Prev. Alzheimers Dis. 2014, 1, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Eggink, E.; Moll Van Charante, E.P.; Van Gool, W.A.; Richard, E. A Population Perspective on Prevention of Dementia. J. Clin. Med. 2019, 8, 834. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Altomare, D.; Ribaldi, F.; Villain, N.; Brayne, C.; Mukadam, N.; Abramowicz, M.; Barkhof, F.; Berthier, M.; Bieler-Aeschlimann, M.; et al. Dementia Prevention in Memory Clinics: Recommendations from the European Task Force for Brain Health Services. Lancet Reg. Health Eur. 2023, 26, 100576. [Google Scholar] [CrossRef]
- Mangialasche, F.; Kivipelto, M.; Solomon, A.; Fratiglioni, L. Dementia Prevention: Current Epidemiological Evidence and Future Perspective. Alzheimers Res. Ther. 2012, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Maresova, P.; Rezny, L.; Bauer, P.; Valko, M.; Kuca, K. Nonpharmacological Intervention Therapies for Dementia: Potential Break-Even Intervention Price and Savings for Selected Risk Factors in the European Healthcare System. BMC Public Health 2024, 24, 1293. [Google Scholar] [CrossRef] [PubMed]
- Manton, K.C.; Gu, X.L.; Ukraintseva, S.V. Declining Prevalence of Dementia in the U.S. Elderly Population. Adv. Gerontol. Uspekhi Gerontol. 2005, 16, 30–37. [Google Scholar]
- Rocca, W.A.; Petersen, R.C.; Knopman, D.S.; Hebert, L.E.; Evans, D.A.; Hall, K.S.; Gao, S.; Unverzagt, F.W.; Langa, K.M.; Larson, E.B.; et al. Trends in the Incidence and Prevalence of Alzheimer’s Disease, Dementia, and Cognitive Impairment in the United States. Alzheimers Dement. 2011, 7, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Remoli, G.; Bacigalupo, I.; Palazzesi, I.; Piscopo, P.; Bellomo, G.; Canevelli, M.; Corbo, M.; Vanacore, N.; Lacorte, E. Decreasing Trend in the Incidence and Prevalence of Dementia: A Systematic Review. Minerva Med. 2021, 112, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Morovatdar, N.; Avan, A.; Azarpazhooh, M.R.; Di Napoli, M.; Stranges, S.; Kapral, M.K.; Rezayat, A.A.; Shariatzadeh, A.; Abootalebi, S.; Mokhber, N.; et al. Secular Trends of Ischaemic Heart Disease, Stroke, and Dementia in High-Income Countries from 1990 to 2017: The Global Burden of Disease Study 2017. Neurol. Sci. 2022, 43, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Ishii, S.; Ishii, T.; Eboshida, A. Prevalence of Dementia in Japan: A Systematic Review. Dement. Geriatr. Cogn. Disord. 2013, 36, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Han, J.W.; So, Y.S.; Seo, J.Y.; Kim, K.Y.; Kim, K.W. Prevalence and Trends of Dementia in Korea: A Systematic Review and Meta-Analysis. J. Korean Med. Sci. 2014, 29, 903. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, H.; Lu, X.-L.; Zhang, B.; Weng, W.; Yang, J.; Zhang, J.; Dong, M.-J. Prevalence of Dementia in the People’s Republic of China from 1985 to 2015: A Systematic Review and Meta-Regression Analysis. BMC Public Health 2019, 19, 578. [Google Scholar] [CrossRef]
- Ribeiro, F.; Teixeira-Santos, A.C.; Caramelli, P.; Leist, A.K. Prevalence of Dementia in Latin America and Caribbean Countries: Systematic Review and Meta-Analyses Exploring Age, Sex, Rurality, and Education as Possible Determinants. Ageing Res. Rev. 2022, 81, 101703. [Google Scholar] [CrossRef]
- Langa, K.M.; Larson, E.B.; Karlawish, J.H.; Cutler, D.M.; Kabeto, M.U.; Kim, S.Y.; Rosen, A.B. Trends in the Prevalence and Mortality of Cognitive Impairment in the United States: Is There Evidence of a Compression of Cognitive Morbidity? Alzheimers Dement. 2008, 4, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Derby, C.A.; Katz, M.J.; Lipton, R.B.; Hall, C.B. Trends in Dementia Incidence in a Birth Cohort Analysis of the Einstein Aging Study. JAMA Neurol. 2017, 74, 1345. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.E.; Bienias, J.L.; Aggarwal, N.T.; Wilson, R.S.; Bennett, D.A.; Shah, R.C.; Evans, D.A. Change in Risk of Alzheimer Disease over Time. Neurology 2010, 75, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Lopez-Anton, R.; Santabárbara, J.; de-la-Cámara, C.; Ventura, T.; Quintanilla, M.A.; Roy, J.F.; Campayo, A.J.; Lobo, E.; Palomo, T.; et al. Incidence and Lifetime Risk of Dementia and Alzheimer’s Disease in a Southern European Population: Incidence and LTR of Dementia and AD. Acta Psychiatr. Scand. 2011, 124, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, E.M.C.; Verhaaren, B.F.J.; Koudstaal, P.J.; Hofman, A.; Ikram, M.A.; Breteler, M.M.B. Is Dementia Incidence Declining? Trends in Dementia Incidence since 1990 in the Rotterdam Study. Neurology 2012, 78, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, P.; Waern, M.; Billstedt, E.; Östling, S.; Skoog, I. Secular Trends in the Prevalence of Dementia and Depression in Swedish Septuagenarians 1976–2006. Psychol. Med. 2013, 43, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, G.O., Jr. Alzheimer’s Disease: Current Trends in Wales. Oman Med. J. 2014, 29, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Grasset, L.; Brayne, C.; Joly, P.; Jacqmin-Gadda, H.; Peres, K.; Foubert-Samier, A.; Dartigues, J.; Helmer, C. Trends in Dementia Incidence: Evolution over a 10-year Period in France. Alzheimers Dement. 2016, 12, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Matthews, F.E.; Stephan, B.C.M.; Robinson, L.; Jagger, C.; Barnes, L.E.; Arthur, A.; Brayne, C.; Cognitive Function and Ageing Studies (CFAS) Collaboration; Comas-Herrera, A.; Wittenberg, R.; et al. A Two Decade Dementia Incidence Comparison from the Cognitive Function and Ageing Studies I and II. Nat. Commun. 2016, 7, 11398. [Google Scholar] [CrossRef]
- Kosteniuk, J.G.; Morgan, D.G.; O’Connell, M.E.; Kirk, A.; Crossley, M.; Teare, G.F.; Stewart, N.J.; Bello-Haas, V.D.; McBain, L.; Mou, H.; et al. Simultaneous Temporal Trends in Dementia Incidence and Prevalence, 2005–2013: A Population-Based Retrospective Cohort Study in Saskatchewan, Canada. Int. Psychogeriatr. 2016, 28, 1643–1658. [Google Scholar] [CrossRef]
- Wimo, A.; Sjölund, B.-M.; Sköldunger, A.; Qiu, C.; Klarin, I.; Nordberg, G.; Von Strauss, E. Cohort Effects in the Prevalence and Survival of People with Dementia in a Rural Area in Northern Sweden. J. Alzheimers Dis. 2016, 50, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Abhari, S.; Guzman-Castillo, M.; Bandosz, P.; Shipley, M.J.; Muniz-Terrera, G.; Singh-Manoux, A.; Kivimäki, M.; Steptoe, A.; Capewell, S.; O’Flaherty, M.; et al. Temporal Trend in Dementia Incidence since 2002 and Projections for Prevalence in England and Wales to 2040: Modelling Study. BMJ 2017, 358, j2856. [Google Scholar] [CrossRef] [PubMed]
- Cerasuolo, J.O.; Cipriano, L.E.; Sposato, L.A.; Kapral, M.K.; Fang, J.; Gill, S.S.; Hackam, D.G.; Hachinski, V. Population-based Stroke and Dementia Incidence Trends: Age and Sex Variations. Alzheimers Dement. 2017, 13, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.M.; Schupf, N.; Manly, J.J.; Andrews, H.; Tang, M.-X.; Mayeux, R. Secular Trends in the Incidence of Dementia in a Multi-Ethnic Community. J. Alzheimers Dis. 2017, 60, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Pérès, K.; Brayne, C.; Matharan, F.; Grasset, L.; Helmer, C.; Letenneur, L.; Foubert-Samier, A.; Baldi, I.; Tison, F.; Amieva, H.; et al. Trends in Prevalence of Dementia in French Farmers from Two Epidemiological Cohorts. J. Am. Geriatr. Soc. 2017, 65, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zissimopoulos, J.M. Racial and Ethnic Differences in Trends in Dementia Prevalence and Risk Factors in the United States. Alzheimers Dement. Transl. Res. Clin. Interv. 2018, 4, 510–520. [Google Scholar] [CrossRef]
- Hendrie, H.C.; Smith-Gamble, V.; Lane, K.A.; Purnell, C.; Clark, D.O.; Gao, S. The Association of Early Life Factors and Declining Incidence Rates of Dementia in an Elderly Population of African Americans. J. Gerontol. Ser. B 2018, 73 (Suppl. 1), S82–S89. [Google Scholar] [CrossRef]
- Seblova, D.; Quiroga, M.; Fors, S.; Johnell, K.; Lövdén, M.; Ponce De Leon, A.; Svensson, A.; Wicks, S.; Lager, A. Thirty-Year Trends in Dementia: A Nationwide Population Study of Swedish Inpatient Records. Clin. Epidemiol. 2018, 10, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; Wilson, R.S.; Evans, D.A. Prevalence and Incidence of Clinically Diagnosed Alzheimer’s Disease Dementia from 1994 to 2012 in a Population Study. Alzheimers Dement. 2019, 15, 1–7. [Google Scholar] [CrossRef]
- Sullivan, K.J.; Dodge, H.H.; Hughes, T.F.; Chang, C.-C.H.; Zhu, X.; Liu, A.; Ganguli, M. Declining Incident Dementia Rates Across Four Population-Based Birth Cohorts. J. Gerontol. Ser. A 2019, 74, 1439–1445. [Google Scholar] [CrossRef]
- Ding, M.; Qiu, C.; Rizzuto, D.; Grande, G.; Fratiglioni, L. Tracing Temporal Trends in Dementia Incidence over 25 Years in Central Stockholm, Sweden. Alzheimers Dement. 2020, 16, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.P.; Zhang, Y.S.; Kim, J.K.; Hayward, M.D.; Crimmins, E.M. Trends in Dementia Prevalence, Incidence, and Mortality in the United States (2000–2016). J. Aging Health 2022, 34, 100–108. [Google Scholar] [CrossRef]
- Van Bussel, E.F.; Richard, E.; Arts, D.L.; Nooyens, A.C.J.; Coloma, P.M.; De Waal, M.W.M.; Van Den Akker, M.; Biermans, M.C.J.; Nielen, M.M.J.; Van Boven, K.; et al. Dementia Incidence Trend over 1992–2014 in the Netherlands: Analysis of Primary Care Data. PLOS Med. 2017, 14, e1002235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bandosz, P.; Stoye, G.; Liu, Y.; Wu, Y.; Lobanov-Rostovsky, S.; French, E.; Kivimaki, M.; Livingston, G.; Liao, J.; et al. Dementia Incidence Trend in England and Wales, 2002–2019, and Projection for Dementia Burden to 2040: Analysis of Data from the English Longitudinal Study of Ageing. Lancet Public Health 2023, 8, e859–e867. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ogunniyi, A.; Hall, K.S.; Baiyewu, O.; Unverzagt, F.W.; Lane, K.A.; Murrell, J.R.; Gureje, O.; Hake, A.M.; Hendrie, H.C. Dementia Incidence Declined in African-Americans but Not in Yoruba. Alzheimers Dement. 2016, 12, 244–251. [Google Scholar] [CrossRef]
- Ohara, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Iwaki, T.; Kitazono, T.; Kanba, S.; Kiyohara, Y.; Ninomiya, T. Trends in Dementia Prevalence, Incidence, and Survival Rate in a Japanese Community. Neurology 2017, 88, 1925–1932. [Google Scholar] [CrossRef]
- Ding, D.; Zhao, Q.; Wu, W.; Xiao, Z.; Liang, X.; Luo, J.; Hong, Z. Prevalence and Incidence of Dementia in an Older Chinese Population over Two Decades: The Role of Education. Alzheimers Dement. 2020, 16, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Mori, T.; Yoshida, T.; Tachibana, A.; Ozaki, T.; Yoshino, Y.; Ochi, S.; Sonobe, N.; Matsumoto, T.; Komori, K.; et al. Secular Trends in the Prevalence of Dementia Based on a Community-based Complete Enumeration in Japan: The Nakayama Study. Psychogeriatrics 2022, 22, 631–641. [Google Scholar] [CrossRef]
- Huang, S.-T.; Loh, C.-H.; Lin, C.-H.; Hsiao, F.-Y.; Chen, L.-K. Trends in Dementia Incidence and Mortality, and Dynamic Changes in Comorbidity and Healthcare Utilization from 2004 to 2017: A Taiwan National Cohort Study. Arch. Gerontol. Geriatr. 2024, 121, 105330. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the Epidemiology of Cardiovascular Disease: A Historical Perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef]
- Brandes, N.; Weissbrod, O.; Linial, M. Open Problems in Human Trait Genetics. Genome Biol. 2022, 23, 131. [Google Scholar] [CrossRef]
- Bergem, A.L.M. The Role of Heredity in Late-Onset Alzheimer Disease and Vascular Dementia: A Twin Study. Arch. Gen. Psychiatry 1997, 54, 264. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, L.T.; Christoffersen, M.; Frikke-Schmidt, R. Shared Risk Factors between Dementia and Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 9777. [Google Scholar] [CrossRef]
- Turnpenny, P.D.; Ellard, S.; Cleaver, R. Emery’s Elements of Medical Genetics and Genomics, 16th ed.; Elsevier: Philadelphia, PA, USA, 2022. [Google Scholar]
- Wingo, T.S. Autosomal Recessive Causes Likely in Early-Onset Alzheimer Disease. Arch. Neurol. 2012, 69, 59. [Google Scholar] [CrossRef]
- Seto, M.; Weiner, R.L.; Dumitrescu, L.; Hohman, T.J. Protective Genes and Pathways in Alzheimer’s Disease: Moving towards Precision Interventions. Mol. Neurodegener. 2021, 16, 29. [Google Scholar] [CrossRef]
- Fortea, J.; Pegueroles, J.; Alcolea, D.; Belbin, O.; Dols-Icardo, O.; Vaqué-Alcázar, L.; Videla, L.; Gispert, J.D.; Suárez-Calvet, M.; Johnson, S.C.; et al. APOE4 Homozygozity Represents a Distinct Genetic Form of Alzheimer’s Disease. Nat. Med. 2024, 30, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Arboleda-Velasquez, J.F.; Lopera, F.; O’Hare, M.; Delgado-Tirado, S.; Marino, C.; Chmielewska, N.; Saez-Torres, K.L.; Amarnani, D.; Schultz, A.P.; Sperling, R.A.; et al. Resistance to Autosomal Dominant Alzheimer’s Disease in an APOE3 Christchurch Homozygote: A Case Report. Nat. Med. 2019, 25, 1680–1683. [Google Scholar] [CrossRef]
- Baker, E.; Leonenko, G.; Schmidt, K.M.; Hill, M.; Myers, A.J.; Shoai, M.; De Rojas, I.; Tesi, N.; Holstege, H.; Van Der Flier, W.M.; et al. What Does Heritability of Alzheimer’s Disease Represent? PLoS ONE 2023, 18, e0281440. [Google Scholar] [CrossRef] [PubMed]
- Gatz, M.; Reynolds, C.A.; Fratiglioni, L.; Johansson, B.; Mortimer, J.A.; Berg, S.; Fiske, A.; Pedersen, N.L. Role of Genes and Environments for Explaining Alzheimer Disease. Arch. Gen. Psychiatry 2006, 63, 168. [Google Scholar] [CrossRef]
- Migliore, L.; Coppedè, F. Gene–Environment Interactions in Alzheimer Disease: The Emerging Role of Epigenetics. Nat. Rev. Neurol. 2022, 18, 643–660. [Google Scholar] [CrossRef]
- Verheijen, J.; Sleegers, K. Understanding Alzheimer Disease at the Interface between Genetics and Transcriptomics. Trends Genet. 2018, 34, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.R.; O’Connell, K.M.S.; Kaczorowski, C.C. Gene-by-Environment Interactions in Alzheimer’s Disease and Parkinson’s Disease. Neurosci. Biobehav. Rev. 2019, 103, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.K.; Escott-Price, V.; Gatz, M.; Hardy, J.; Pedersen, N.L.; Shoai, M.; Reynolds, C.A. Measuring Heritable Contributions to Alzheimer’s Disease: Polygenic Risk Score Analysis with Twins. Brain Commun. 2022, 4, fcab308. [Google Scholar] [CrossRef]
- Andrews, S.J.; Fulton-Howard, B.; Goate, A. Interpretation of Risk Loci from Genome-Wide Association Studies of Alzheimer’s Disease. Lancet Neurol. 2020, 19, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Schmidt, M.; Klein, H.-U.; Naj, A.C.; Hamilton-Nelson, K.L.; Larson, E.B.; Evans, D.A.; De Jager, P.L.; Crane, P.K.; Buxbaum, J.D.; et al. Novel Alzheimer Disease Risk Loci and Pathways in African American Individuals Using the African Genome Resources Panel: A Meta-Analysis. JAMA Neurol. 2021, 78, 102. [Google Scholar] [CrossRef] [PubMed]
- Pimenova, A.A.; Raj, T.; Goate, A.M. Untangling Genetic Risk for Alzheimer’s Disease. Biol. Psychiatry 2018, 83, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Wray, N.R.; Goddard, M.E.; Visscher, P.M. Estimating Missing Heritability for Disease from Genome-Wide Association Studies. Am. J. Hum. Genet. 2011, 88, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.; Tanzi, R.E. Genomic Mechanisms in Alzheimer’s Disease. Brain Pathol. 2020, 30, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New Insights into the Genetic Etiology of Alzheimer’s Disease and Related Dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Gan, J.; Fu, H.; Zhu, X. Relationships between Multiple Dimensions of Insight and Neurocognition, Metacognition, and Social Cognition: A Meta-Analysis. J. Nerv. Ment. Dis. 2022, 210, 577–584. [Google Scholar] [CrossRef]
- Dattani, S.; Howard, D.M.; Lewis, C.M.; Sham, P.C. Clarifying the Causes of Consistent and Inconsistent Findings in Genetics. Genet. Epidemiol. 2022, 46, 372–389. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Mak, T.S.-H.; O’Reilly, P.F. Tutorial: A Guide to Performing Polygenic Risk Score Analyses. Nat. Protoc. 2020, 15, 2759–2772. [Google Scholar] [CrossRef]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Hyppönen, E.; Kuzma, E.; Llewellyn, D.J. Association of Lifestyle and Genetic Risk with Incidence of Dementia. JAMA 2019, 322, 430. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.D.; Ranson, J.M.; Wallace, L.M.K.; Llewellyn, D.J.; Rockwood, K. Frailty, Lifestyle, Genetics and Dementia Risk. J. Neurol. Neurosurg. Psychiatry 2022, 93, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Marden, J.R.; Walter, S.; Tchetgen Tchetgen, E.J.; Kawachi, I.; Glymour, M.M. Validation of a Polygenic Risk Score for Dementia in Black and White Individuals. Brain Behav. 2014, 4, 687–697. [Google Scholar] [CrossRef] [PubMed]
- International Genomics of Alzheimer’s Disease Consortium (IGAP); Jones, L.; Lambert, J.; Wang, L.; Choi, S.; Harold, D.; Vedernikov, A.; Escott-Price, V.; Stone, T.; Richards, A.; et al. Convergent Genetic and Expression Data Implicate Immunity in Alzheimer’s Disease. Alzheimers Dement. 2015, 11, 658–671. [Google Scholar] [CrossRef]
- Naj, A.C.; Schellenberg, G.D.; for the Alzheimer’s Disease Genetics Consortium (ADGC). Genomic Variants, Genes, and Pathways of Alzheimer’s Disease: An Overview. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Ridge, P.G.; Hoyt, K.B.; Boehme, K.; Mukherjee, S.; Crane, P.K.; Haines, J.L.; Mayeux, R.; Farrer, L.A.; Pericak-Vance, M.A.; Schellenberg, G.D.; et al. Assessment of the Genetic Variance of Late-Onset Alzheimer’s Disease. Neurobiol. Aging 2016, 41, 200.e13–200.e20. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Osmond, C.; Winter, P.D.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 334, 577–580. [Google Scholar] [CrossRef]
- Dover, G.J. The Barker Hypothesis: How Pediatricans Will Diagnose and Prevent Common Adult-Onset Diseases. Trans. Am. Clin. Climatol. Assoc. 2009, 120, 199–207. [Google Scholar]
- Heindel, J.J.; Vandenberg, L.N. Developmental Origins of Health and Disease: A Paradigm for Understanding Disease Cause and Prevention. Curr. Opin. Pediatr. 2015, 27, 248–253. [Google Scholar] [CrossRef]
- Borenstein, A.R.; Copenhaver, C.I.; Mortimer, J.A. Early-Life Risk Factors for Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2006, 20, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Pareja, F. Alzheimer: Prevention from Childhood, 1st ed.; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2018. [Google Scholar]
- Bleker, L.S.; De Rooij, S.R.; Painter, R.C.; Ravelli, A.C.; Roseboom, T.J. Cohort Profile: The Dutch Famine Birth Cohort (DFBC)—A Prospective Birth Cohort Study in the Netherlands. BMJ Open 2021, 11, e042078. [Google Scholar] [CrossRef] [PubMed]
- Melrose, R.J.; Brewster, P.; Marquine, M.J.; MacKay-Brandt, A.; Reed, B.; Farias, S.T.; Mungas, D. Early Life Development in a Multiethnic Sample and the Relation to Late Life Cognition. J. Gerontol. B Psychol. Sci. Soc. Sci. 2015, 70, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Moody, L.; Chen, H.; Pan, Y.-X. Early-Life Nutritional Programming of Cognition—The Fundamental Role of Epigenetic Mechanisms in Mediating the Relation between Early-Life Environment and Learning and Memory Process. Adv. Nutr. 2017, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Lövdén, M.; Nilsson, T.; Seblova, D. Very Early-Life Risk Factors for Developing Dementia: Evidence from Full Population Registers. J. Gerontol. Ser. B 2023, 78, gbad142. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Carlson, M.C.; Fitzpatrick, A.L.; Kuller, L.H.; Fried, L.P.; Zandi, P.P. Knee Height and Arm Span: A Reflection of Early Life Environment and Risk of Dementia. Neurology 2008, 70 Pt 2, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Ruisch, I.H.; Dietrich, A.; Glennon, J.C.; Buitelaar, J.K.; Hoekstra, P.J. Maternal Substance Use during Pregnancy and Offspring Conduct Problems: A Meta-Analysis. Neurosci. Biobehav. Rev. 2018, 84, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, A.M.; Venerosi, A.; Calamandrei, G. Early-life toxic insults and onset of sporadic neurodegenerative diseases—An overview of experimental studies. In Neurotoxin Modeling of Brain Disorders—Life-Long Outcomes in Behavioral Teratology; Kostrzewa, R.M., Archer, T., Eds.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2015; Volume 29, pp. 231–264. [Google Scholar] [CrossRef]
- Wang, X.-J.; Xu, W.; Li, J.-Q.; Cao, X.-P.; Tan, L.; Yu, J.-T. Early-Life Risk Factors for Dementia and Cognitive Impairment in Later Life: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2019, 67, 221–229. [Google Scholar] [CrossRef]
- Zuin, M.; Cervellati, C.; Brombo, G.; Trentini, A.; Roncon, L.; Zuliani, G. Elevated Blood Homocysteine and Risk of Alzheimer’s Dementia: An Updated Systematic Review and Meta-Analysis Based on Prospective Studies. J. Prev. Alzheimers Dis. 2021, 8, 1–6. [Google Scholar] [CrossRef]
- De Rooij, S.R.; Wouters, H.; Yonker, J.E.; Painter, R.C.; Roseboom, T.J. Prenatal Undernutrition and Cognitive Function in Late Adulthood. Proc. Natl. Acad. Sci. USA 2010, 107, 16881–16886. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, S.R.; Mutsaerts, H.J.M.M.; Petr, J.; Asllani, I.; Caan, M.W.A.; Groot, P.; Nederveen, A.J.; Schwab, M.; Roseboom, T.J. Late-Life Brain Perfusion after Prenatal Famine Exposure. Neurobiol. Aging 2019, 82, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Gaser, C.; Roseboom, T.J.; Schwab, M.; De Rooij, S.R. Premature Brain Aging in Humans Exposed to Maternal Nutrient Restriction during Early Gestation. NeuroImage 2018, 173, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Wiegersma, A.M.; Boots, A.; Langendam, M.W.; Limpens, J.; Shenkin, S.D.; Korosi, A.; Roseboom, T.J.; De Rooij, S.R. Do Prenatal Factors Shape the Risk for Dementia? A Systematic Review of the Epidemiological Evidence for the Prenatal Origins of Dementia. Soc. Psychiatry Psychiatr. Epidemiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.; Wiegersma, A.M.; Vali, Y.; Van Den Hof, M.; Langendam, M.W.; Limpens, J.; Backhouse, E.V.; Shenkin, S.D.; Wardlaw, J.M.; Roseboom, T.J.; et al. Shaping the Risk for Late-Life Neurodegenerative Disease: A Systematic Review on Prenatal Risk Factors for Alzheimer’s Disease-Related Volumetric Brain Biomarkers. Neurosci. Biobehav. Rev. 2023, 146, 105019. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, F.; Mandolesi, L.; Serra, L.; Sorrentino, G.; Caltagirone, C. The Neuroprotective Effects of Experience on Cognitive Functions: Evidence from Animal Studies on the Neurobiological Bases of Brain Reserve. Neuroscience 2018, 370, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia Prevention, Intervention, and Care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Nithianantharajah, J.; Hannan, A.J. Mechanisms Mediating Brain and Cognitive Reserve: Experience-Dependent Neuroprotection and Functional Compensation in Animal Models of Neurodegenerative Diseases. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 331–339. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Zhao, Q.-F.; Li, J.-Q.; Wang, J.; Yu, J.-T. Meta-Analysis of Modifiable Risk Factors for Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
- Henderson, A.S. The Risk Factors for Alzheimer’s Disease: A Review and a Hypothesis. Acta Psychiatr. Scand. 1988, 78, 257–275. [Google Scholar] [CrossRef]
- Van Duijn, C.M.; Stijnen, T.; Hofman, A.; The Eurodem Risk Factors Research Group. Risk Factors for Alzheimer’s Disease: Overview of the EURODEM Collaborative Re-Analysis of Case-Control Studies. Int. J. Epidemiol. 1991, 20 (Suppl. 2), S4–S12. [Google Scholar] [CrossRef]
- Ballarini, T.; Melo Van Lent, D.; Brunner, J.; Schröder, A.; Wolfsgruber, S.; Altenstein, S.; Brosseron, F.; Buerger, K.; Dechent, P.; Dobisch, L.; et al. Mediterranean Diet, Alzheimer Disease Biomarkers, and Brain Atrophy in Old Age. Neurology 2021, 96, e2920–e2932. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V.; Einhäupl, K.; Ganten, D.; Alladi, S.; Brayne, C.; Stephan, B.C.M.; Sweeney, M.D.; Zlokovic, B.; Iturria-Medina, Y.; Iadecola, C.; et al. Preventing Dementia by Preventing Stroke: The Berlin Manifesto. Alzheimers Dement. 2019, 15, 961–984. [Google Scholar] [CrossRef]
- Lee, C.M.; Woodward, M.; Batty, G.D.; Beiser, A.S.; Bell, S.; Berr, C.; Bjertness, E.; Chalmers, J.; Clarke, R.; Dartigues, J.; et al. Association of Anthropometry and Weight Change with Risk of Dementia and Its Major Subtypes: A Meta-analysis Consisting 2.8 Million Adults with 57 294 Cases of Dementia. Obes. Rev. 2020, 21, e12989. [Google Scholar] [CrossRef]
- Lee, Y.; Back, J.H.; Kim, J.; Kim, S.-H.; Na, D.L.; Cheong, H.-K.; Hong, C.H.; Kim, Y.G. Systematic Review of Health Behavioral Risks and Cognitive Health in Older Adults. Int. Psychogeriatr. 2010, 22, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L. Systematic Review: Factors Associated with Risk for and Possible Prevention of Cognitive Decline in Later Life. Ann. Intern. Med. 2010, 153, 182. [Google Scholar] [CrossRef]
- Seyedsalehi, A.; Warrier, V.; Bethlehem, R.A.I.; Perry, B.I.; Burgess, S.; Murray, G.K. Educational Attainment, Structural Brain Reserve and Alzheimer’s Disease: A Mendelian Randomization Analysis. Brain 2023, 146, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Schwarzinger, M.; Dufouil, C. Forecasting the Prevalence of Dementia. Lancet Public Health 2022, 7, e94–e95. [Google Scholar] [CrossRef]
- Litke, R.; Garcharna, L.C.; Jiwani, S.; Neugroschl, J. Modifiable Risk Factors in Alzheimer Disease and Related Dementias: A Review. Clin. Ther. 2021, 43, 953–965. [Google Scholar] [CrossRef]
- Pope, S.K.; Shue, V.M.; Beck, C. Will a Healthy Lifestyle Help Prevent Alzheimer’s Disease? Annu. Rev. Public Health 2003, 24, 111–132. [Google Scholar] [CrossRef]
- Haan, M.N.; Wallace, R. Can Dementia Be Prevented? Brain Aging in a Population-Based Context. Annu. Rev. Public Health 2004, 25, 1–24. [Google Scholar] [CrossRef]
- Jansson, E.T. Alzheimer Disease Is Substantially Preventable in the United States -- Review of Risk Factors, Therapy, and the Prospects for an Expert Software System. Med. Hypotheses 2005, 64, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Middleton, L.; Yaffe, K. Promising Strategies for the Prevention of Dementia. Arch. Neurol. 2009, 66, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Carriere, I.; Ritchie, C.W.; Berr, C.; Artero, S.; Ancelin, M.-L. Designing Prevention Programmes to Reduce Incidence of Dementia: Prospective Cohort Study of Modifiable Risk Factors. BMJ 2010, 341, c3885. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K. The Projected Impact of Risk Factor Reduction on Alzheimer’s Disease Prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Song, X.; Mitnitski, A.; Rockwood, K. Nontraditional Risk Factors Combine to Predict Alzheimer Disease and Dementia. Neurology 2011, 77, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Anstey, K.J.; Cherbuin, N.; Herath, P.M. Development of a New Method for Assessing Global Risk of Alzheimer’s Disease for Use in Population Health Approaches to Prevention. Prev. Sci. 2013, 14, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, L.Y.; Marzo, A.; Muñoz-Ruiz, M.; Ikram, M.A.; Kivipelto, M.; Ruefenacht, D.; Venneri, A.; Soininen, H.; Wanke, I.; Ventikos, Y.A.; et al. Modifiable Lifestyle Factors in Dementia: A Systematic Review of Longitudinal Observational Cohort Studies. J. Alzheimers Dis. 2014, 42, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Anstey, K.J.; Eramudugolla, R.; Hosking, D.E.; Lautenschlager, N.T.; Dixon, R.A. Bridging the Translation Gap: From Dementia Risk Assessment to Advice on Risk Reduction. J. Prev. Alzheimers Dis. 2015, 2, 189–198. [Google Scholar] [CrossRef]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the Evidence on Modifiable Risk Factors for Cognitive Decline and Dementia: A Population-based Perspective. Alzheimers Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Deckers, K.; Van Boxtel, M.P.J.; Schiepers, O.J.G.; De Vugt, M.; Muñoz Sánchez, J.L.; Anstey, K.J.; Brayne, C.; Dartigues, J.-F.; Engedal, K.; Kivipelto, M.; et al. Target Risk Factors for Dementia Prevention: A Systematic Review and Delphi Consensus Study on the Evidence from Observational Studies: Major Risk Factors for Dementia Prevention. Int. J. Geriatr. Psychiatry 2015, 30, 234–246. [Google Scholar] [CrossRef]
- Hazar, N.; Seddigh, L.; Rampisheh, Z.; Nojomi, M. Population Attributable Fraction of Modifiable Risk Factors for Alzheimer Disease: A Systematic Review of Systematic Reviews. Iran. J. Neurol. 2016, 15, 164–172. [Google Scholar] [PubMed]
- Killin, L.O.J.; Starr, J.M.; Shiue, I.J.; Russ, T.C. Environmental Risk Factors for Dementia: A Systematic Review. BMC Geriatr. 2016, 16, 175. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Fratiglioni, L.; Matthews, F.E.; Lobo, A.; Breteler, M.M.B.; Skoog, I.; Brayne, C. Dementia in Western Europe: Epidemiological Evidence and Implications for Policy Making. Lancet Neurol. 2016, 15, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Middleton, L.T.; Ioannidis, J.P.A.; Evangelou, E. Systematic Evaluation of the Associations between Environmental Risk Factors and Dementia: An Umbrella Review of Systematic Reviews and Meta-Analyses. Alzheimers Dement. 2017, 13, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, G.; Szabo, S.T.; Alexopoulos, G.S.; Zannas, A.S. Strategies for Dementia Prevention: Latest Evidence and Implications. Ther. Adv. Chronic Dis. 2017, 8, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Markus, H.S. Does Treating Vascular Risk Factors Prevent Dementia and Alzheimer’s Disease? A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2018, 64, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Anstey, K.J.; Ee, N.; Eramudugolla, R.; Jagger, C.; Peters, R. A Systematic Review of Meta-Analyses That Evaluate Risk Factors for Dementia to Evaluate the Quantity, Quality, and Global Representativeness of Evidence. J. Alzheimers Dis. 2019, 70 (Suppl. 1), S165–S186. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Risk Factors for Alzheimer’s Disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef]
- Edwards Iii, G.A.; Gamez, N.; Escobedo, G., Jr.; Calderon, O.; Moreno-Gonzalez, I. Modifiable Risk Factors for Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 146. [Google Scholar] [CrossRef]
- Peters, R.; Booth, A.; Rockwood, K.; Peters, J.; D’Este, C.; Anstey, K.J. Combining Modifiable Risk Factors and Risk of Dementia: A Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e022846. [Google Scholar] [CrossRef]
- Rochoy, M.; Bordet, R.; Gautier, S.; Chazard, E. Factors Associated with the Onset of Alzheimer’s Disease: Data Mining in the French Nationwide Discharge Summary Database between 2008 and 2014. PLoS ONE 2019, 14, e0220174. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-T.; Xu, W.; Tan, C.-C.; Andrieu, S.; Suckling, J.; Evangelou, E.; Pan, A.; Zhang, C.; Jia, J.; Feng, L.; et al. Evidence-Based Prevention of Alzheimer’s Disease: Systematic Review and Meta-Analysis of 243 Observational Prospective Studies and 153 Randomised Controlled Trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Lu, L.; Li, J.; Qu, X.; Li, J.; Qian, S.; Wang, Y.; Jia, R.; Wang, C.; Xu, Y. Contributions of Modifiable Risk Factors to Dementia Incidence: A Bayesian Network Analysis. J. Am. Med. Dir. Assoc. 2020, 21, 1592–1599.e13. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Stachiv, I.; Nikolai, T. Association of Late Life Depression, Non-Modifiable Risk and Protective Factors with Dementia and Alzheimer’s Disease: Literature Review on Current Evidences, Preventive Interventions and Possible Future Trends in Prevention and Treatment of Dementia. Int. J. Environ. Res. Public. Health 2020, 17, 7475. [Google Scholar] [CrossRef] [PubMed]
- Rolandi, E.; Zaccaria, D.; Vaccaro, R.; Abbondanza, S.; Pettinato, L.; Davin, A.; Guaita, A. Estimating the Potential for Dementia Prevention through Modifiable Risk Factors Elimination in the Real-World Setting: A Population-Based Study. Alzheimers Res. Ther. 2020, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Ali, M.U.; Kenny, M.; Mayhew, A.; Mokashi, V.; He, H.; Lin, S.; Yavari, E.; Paik, K.; Subramanian, D.; et al. Potentially Modifiable Risk Factors for Dementia and Mild Cognitive Impairment: An Umbrella Review and Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2024, 53, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Stephan, B.C.M.; Cochrane, L.; Kafadar, A.H.; Brain, J.; Burton, E.; Myers, B.; Brayne, C.; Naheed, A.; Anstey, K.J.; Ashor, A.W.; et al. Population Attributable Fractions of Modifiable Risk Factors for Dementia: A Systematic Review and Meta-Analysis. Lancet Healthy Longev. 2024, 5, e406–e421. [Google Scholar] [CrossRef]
- Larsson, S.C.; Traylor, M.; Malik, R.; Dichgans, M.; Burgess, S.; Markus, H.S. Modifiable Pathways in Alzheimer’s Disease: Mendelian Randomisation Analysis. BMJ 2017, 359, j5375. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for Primary Prevention of Alzheimer’s Disease: An Analysis of Population-Based Data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Contador, I.; Bermejo-Pareja, F.; Puertas-Martin, V.; Benito-Leon, J. Childhood and Adulthood Rural Residence Increases the Risk of Dementia: NEDICES Study. Curr. Alzheimer Res. 2015, 12, 350–357. [Google Scholar] [CrossRef]
- Dekhtyar, S.; Wang, H.-X.; Fratiglioni, L.; Herlitz, A. Childhood School Performance, Education and Occupational Complexity: A Life-Course Study of Dementia in the Kungsholmen Project. Int. J. Epidemiol. 2016, 45, dyw008. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y. Cognitive Reserve: Implications for Diagnosis and Prevention of Alzheimer’s Disease. Curr. Neurol. Neurosci. Rep. 2004, 4, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive Reserve and Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2006, 20, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Saraulli, D.; Costanzi, M.; Mastrorilli, V.; Farioli-Vecchioli, S. The Long Run: Neuroprotective Effects of Physical Exercise on Adult Neurogenesis from Youth to Old Age. Curr. Neuropharmacol. 2017, 15, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Wolf, S.A.; Kempermann, G. Adult Neurogenesis and Neurodegenerative Disease. Regen. Med. 2006, 1, 15–28. [Google Scholar] [CrossRef]
- Xu, W.; Yu, J.-T.; Tan, M.-S.; Tan, L. Cognitive Reserve and Alzheimer’s Disease. Mol. Neurobiol. 2015, 51, 187–208. [Google Scholar] [CrossRef]
- He, C.; Tsipis, C.P.; LaManna, J.C.; Xu, K. Environmental enrichment induces increased cerebral capillary density and improved cognitive function in mice. In Oxygen Transport to Tissue XXXIX; Halpern, H.J., LaManna, J.C., Harrison, D.K., Epel, B., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 977, pp. 175–181. [Google Scholar] [CrossRef]
- Ramos-Miguel, A.; Jones, A.A.; Sawada, K.; Barr, A.M.; Bayer, T.A.; Falkai, P.; Leurgans, S.E.; Schneider, J.A.; Bennett, D.A.; Honer, W.G. Frontotemporal Dysregulation of the SNARE Protein Interactome Is Associated with Faster Cognitive Decline in Old Age. Neurobiol. Dis. 2018, 114, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.K.; Herskowitz, J.H. Dendritic Spines: Mediators of Cognitive Resilience in Aging and Alzheimer’s Disease. Neuroscience 2021, 27, 487–505. [Google Scholar] [CrossRef]
- Arenaza-Urquijo, E.M.; Landeau, B.; La Joie, R.; Mevel, K.; Mézenge, F.; Perrotin, A.; Desgranges, B.; Bartrés-Faz, D.; Eustache, F.; Chételat, G. Relationships between Years of Education and Gray Matter Volume, Metabolism and Functional Connectivity in Healthy Elders. NeuroImage 2013, 83, 450–457. [Google Scholar] [CrossRef]
- Bozzali, M.; Dowling, C.; Serra, L.; Spanò, B.; Torso, M.; Marra, C.; Castelli, D.; Dowell, N.G.; Koch, G.; Caltagirone, C.; et al. The Impact of Cognitive Reserve on Brain Functional Connectivity in Alzheimer’s Disease. J. Alzheimers Dis. 2015, 44, 243–250. [Google Scholar] [CrossRef]
- Perani, D.; Farsad, M.; Ballarini, T.; Lubian, F.; Malpetti, M.; Fracchetti, A.; Magnani, G.; March, A.; Abutalebi, J. The Impact of Bilingualism on Brain Reserve and Metabolic Connectivity in Alzheimer’s Dementia. Proc. Natl. Acad. Sci. USA 2017, 114, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Van Balkom, T.D.; Van Den Heuvel, O.A.; Berendse, H.W.; Van Der Werf, Y.D.; Vriend, C. The Effects of Cognitive Training on Brain Network Activity and Connectivity in Aging and Neurodegenerative Diseases: A Systematic Review. Neuropsychol. Rev. 2020, 30, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Varela-López, B.; Cruz-Gómez, Á.J.; Lojo-Seoane, C.; Díaz, F.; Pereiro, A.X.; Zurrón, M.; Lindín, M.; Galdo-Álvarez, S. Cognitive Reserve, Neurocognitive Performance, and High-Order Resting-State Networks in Cognitively Unimpaired Aging. Neurobiol. Aging 2022, 117, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Adesuyan, M.; Jani, Y.H.; Alsugeir, D.; Howard, R.; Wong, I.C.K.; Wei, L.; Brauer, R. Trends in the Incidence of Dementia in People with Hypertension in the UK 2000 to 2021. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12466. [Google Scholar] [CrossRef] [PubMed]
- Lennon, M.J.; Makkar, S.R.; Crawford, J.D.; Sachdev, P.S. Midlife Hypertension and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2019, 71, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Power, M.C.; Weuve, J.; Gagne, J.J.; McQueen, M.B.; Viswanathan, A.; Blacker, D. The Association Between Blood Pressure and Incident Alzheimer Disease: A Systematic Review and Meta-Analysis. Epidemiology 2011, 22, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Rouch, L.; Cestac, P.; Hanon, O.; Cool, C.; Helmer, C.; Bouhanick, B.; Chamontin, B.; Dartigues, J.-F.; Vellas, B.; Andrieu, S. Antihypertensive Drugs, Prevention of Cognitive Decline and Dementia: A Systematic Review of Observational Studies, Randomized Controlled Trials and Meta-Analyses, with Discussion of Potential Mechanisms. CNS Drugs 2015, 29, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.M. Hypertension-Related Risk for Dementia: A Summary Review with Future Directions. Semin. Cell Dev. Biol. 2021, 116, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.M.; Sink, K.M. Hypertension and Its Role in Cognitive Function: Current Evidence and Challenges for the Future. Am. J. Hypertens. 2016, 29, 149–157. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, X.; Shen, J.; Jiao, L.; Tong, J.; Zhao, W.; Du, K.; Gong, S.; Liu, M.; Wei, M. Elevated Serum TC and LDL-C Levels in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis Study. Brain Res. 2020, 1727, 146554. [Google Scholar] [CrossRef]
- Espeland, M.A.; Newman, A.B.; Sink, K.; Gill, T.M.; King, A.C.; Miller, M.E.; Guralnik, J.; Katula, J.; Church, T.; Manini, T.; et al. Associations Between Ankle-Brachial Index and Cognitive Function: Results from the Lifestyle Interventions and Independence for Elders Trial. J. Am. Med. Dir. Assoc. 2015, 16, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Guerchet, M.; Aboyans, V.; Nubukpo, P.; Lacroix, P.; Clément, J.-P.; Preux, P.-M. Ankle-Brachial Index as a Marker of Cognitive Impairment and Dementia in General Population. A Systematic Review. Atherosclerosis 2011, 216, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Anstey, K.J.; Cherbuin, N.; Budge, M.; Young, J. Body Mass Index in Midlife and Late-Life as a Risk Factor for Dementia: A Meta-Analysis of Prospective Studies: BMI and Risk of Dementia. Obes. Rev. 2011, 12, e426–e437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Chen, S. Hyperhomocysteinemia and Risk of Incident Cognitive Outcomes: An Updated Dose-Response Meta-Analysis of Prospective Cohort Studies. Ageing Res. Rev. 2019, 51, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Isiozor, N.M.; Voutilainen, A.; Laukkanen, J.A. Handgrip Strength and Risk of Cognitive Outcomes: New Prospective Study and Meta-Analysis of 16 Observational Cohort Studies. GeroScience 2022, 44, 2007–2024. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Roncon, L.; Passaro, A.; Cervellati, C.; Zuliani, G. Metabolic Syndrome and the Risk of Late Onset Alzheimer’s Disease: An Updated Review and Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; Schnitzler, A.; Empana, J.-P.; Ebmeier, K.P.; Dugravot, A.; Kivimäki, M.; Singh-Manoux, A. Association of Ideal Cardiovascular Health at Age 50 with Incidence of Dementia: 25 Year Follow-up of Whitehall II Cohort Study. BMJ 2019, 366, l4414. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Singh-Manoux, A.; Pentti, J.; Sabia, S.; Nyberg, S.T.; Alfredsson, L.; Goldberg, M.; Knutsson, A.; Koskenvuo, M.; Koskinen, A.; et al. Physical Inactivity, Cardiometabolic Disease, and Risk of Dementia: An Individual-Participant Meta-Analysis. BMJ 2019, 365, l1495. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, J.; Roudsari, A.H.; Bawadi, H.; Clark, C.; Ryan, P.M.; Salehisahlabadi, A.; Rahimi Sakak, F.; Goodarzi, N.; Razaz, J.M. Body Mass Index and Risk of Parkinson, Alzheimer, Dementia, and Dementia Mortality: A Systematic Review and Dose–Response Meta-Analysis of Cohort Studies among 5 Million Participants. Nutr. Neurosci. 2022, 25, 423–431. [Google Scholar] [CrossRef]
- Wang, C.; Fu, W.; Cao, S.; Jiang, H.; Guo, Y.; Xv, H.; Liu, J.; Gan, Y.; Lu, Z. Weight Loss and the Risk of Dementia: A Meta-Analysis of Cohort Studies. Curr. Alzheimer Res. 2021, 18, 125–135. [Google Scholar] [CrossRef]
- Vagelatos, N.T.; Eslick, G.D. Type 2 Diabetes as a Risk Factor for Alzheimer’s Disease: The Confounders, Interactions, and Neuropathology Associated with This Relationship. Epidemiol. Rev. 2013, 35, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Seshadri, S.; Beiser, A.; Au, R.; Himali, J.J.; Palumbo, C.; Wolf, P.A.; DeCarli, C. Midlife Vascular Risk Factor Exposure Accelerates Structural Brain Aging and Cognitive Decline. Neurology 2011, 77, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Lyall, D.M.; Ritchie, S.J.; Bastin, M.E.; Harris, M.A.; Buchanan, C.R.; Fawns-Ritchie, C.; Barbu, M.C.; De Nooij, L.; Reus, L.M.; et al. Associations between Vascular Risk Factors and Brain MRI Indices in UK Biobank. Eur. Heart J. 2019, 40, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Azarpazhooh, M.R.; Avan, A.; Cipriano, L.E.; Munoz, D.G.; Sposato, L.A.; Hachinski, V. Concomitant Vascular and Neurodegenerative Pathologies Double the Risk of Dementia. Alzheimers Dement. 2018, 14, 148–156. [Google Scholar] [CrossRef]
- Bilgel, M.; Bannerjee, A.; Shafer, A.; An, Y.; Resnick, S.M. Vascular Risk Is Not Associated with PET Measures of Alzheimer’s Disease Neuropathology among Cognitively Normal Older Adults. Neuroimage Rep. 2021, 1, 100068. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liu, Z.; Chen, Y.; Xu, Y.; Qin, J.; Guo, S.; Huang, J.; Tao, J. The Prevalence of Mild Cognitive Impairment in Type 2 Diabetes Mellitus Patients: A Systematic Review and Meta-Analysis. Acta Diabetol. 2021, 58, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Kopf, D.; Frölich, L. Risk of Incident Alzheimer’s Disease in Diabetic Patients: A Systematic Review of Prospective Trials. J. Alzheimers Dis. 2009, 16, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Hua, S.; Liao, H.; Wang, M.; Xiong, Y.; Cao, F. An Updated Meta-Analysis of Cohort Studies: Diabetes and Risk of Alzheimer’s Disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef]
- Cooper, C.; Sommerlad, A.; Lyketsos, C.G.; Livingston, G. Modifiable Predictors of Dementia in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Am. J. Psychiatry 2015, 172, 323–334. [Google Scholar] [CrossRef]
- Li, L.; Cavuoto, M.; Biddiscombe, K.; Pike, K.E. Diabetes Mellitus Increases Risk of Incident Dementia in APOE ε4 Carriers: A Meta-Analysis. J. Alzheimers Dis. 2020, 74, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, D.G.; Kelly, M.E.; Kelley, G.A.; Brennan, S.; Lawlor, B.A. Association of Age-Related Hearing Loss with Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-Analysis. JAMA Otolaryngol. Neck Surg. 2018, 144, 115. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Sasaki, T.; Takahashi, K.; Kitazawa, M.; Nonomura, Y.; Yagi, C.; Yamagishi, T.; Ohshima, S.; Izumi, S.; Wakasugi, M.; et al. Age-Related Hearing Loss Is Strongly Associated with Cognitive Decline Regardless of the APOE4 Polymorphism. Otol. Neurotol. 2019, 40, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, T.D.; Lad, M.; Kumar, S.; Holmes, E.; McMurray, B.; Maguire, E.A.; Billig, A.J.; Sedley, W. How Can Hearing Loss Cause Dementia? Neuron 2020, 108, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.M.; An, Y.; Doshi, J.; Erus, G.; Ferrucci, L.; Davatzikos, C.; Deal, J.A.; Lin, F.R.; Resnick, S.M. Association of Midlife Hearing Impairment with Late-Life Temporal Lobe Volume Loss. JAMA Otolaryngol. Neck Surg. 2019, 145, 794. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, S.; Forli, F.; Guglielmi, V.; De Corso, E.; Paludetti, G.; Berrettini, S.; FetonI, A.R. Ipoacusia e Declino Cognitivo: Revisione Della Letteratura. Acta Otorhinolaryngol. ital. 2016, 36, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Sugiura, S.; Nishita, Y.; Saji, N.; Sone, M.; Ueda, H. Age-Related Hearing Loss and Cognitive Decline—The Potential Mechanisms Linking the Two. Auris. Nasus. Larynx 2019, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fleminger, S. Head Injury as a Risk Factor for Alzheimer’s Disease: The Evidence 10 Years on; a Partial Replication. J. Neurol. Neurosurg. Psychiatry 2003, 74, 857–862. [Google Scholar] [CrossRef]
- Gu, D.; Ou, S.; Liu, G. Traumatic Brain Injury and Risk of Dementia and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2022, 56, 4–16. [Google Scholar] [CrossRef]
- Mendez, M.F. What Is the Relationship of Traumatic Brain Injury to Dementia? J. Alzheimers Dis. 2017, 57, 667–681. [Google Scholar] [CrossRef]
- Perry, D.C.; Sturm, V.E.; Peterson, M.J.; Pieper, C.F.; Bullock, T.; Boeve, B.F.; Miller, B.L.; Guskiewicz, K.M.; Berger, M.S.; Kramer, J.H.; et al. Association of Traumatic Brain Injury with Subsequent Neurological and Psychiatric Disease: A Meta-Analysis. J. Neurosurg. 2016, 124, 511–526. [Google Scholar] [CrossRef]
- Crane, P.K.; Gibbons, L.E.; Dams-O’Connor, K.; Trittschuh, E.; Leverenz, J.B.; Keene, C.D.; Sonnen, J.; Montine, T.J.; Bennett, D.A.; Leurgans, S.; et al. Association of Traumatic Brain Injury with Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol. 2016, 73, 1062. [Google Scholar] [CrossRef]
- Cechetto, D.F.; Hachinski, V.; Whitehead, S.N. Vascular Risk Factors and Alzheimer’s Disease. Expert Rev. Neurother. 2008, 8, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Pinho, J.; Quintas-Neves, M.; Dogan, I.; Reetz, K.; Reich, A.; Costa, A.S. Incident Stroke in Patients with Alzheimer’s Disease: Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 16385. [Google Scholar] [CrossRef]
- Barba, R.; Martínez-Espinosa, S.; Rodríguez-García, E.; Pondal, M.; Vivancos, J.; Del Ser, T. Poststroke Dementia: Clinical Features and Risk Factors. Stroke 2000, 31, 1494–1501. [Google Scholar] [CrossRef]
- Barba, R.; Castro, M.D.; Del Mar Morín, M.; Rodriguez-Romero, R.; Rodríguez-García, E.; Cantón, R.; Del Ser, T. Prestroke Dementia. Cerebrovasc. Dis. 2001, 11, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liang, G.-H.; Li, J.-A.; Yu, P.; Dong, M. Migraine and the Risk of Dementia: A Meta-Analysis and Systematic Review. Aging Clin. Exp. Res. 2022, 34, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fu, C.; Li, J.; Peng, S. Late-Onset Epilepsy and the Risk of Dementia: A Systematic Review and Meta-Analysis. Aging Clin. Exp. Res. 2022, 34, 1771–1779. [Google Scholar] [CrossRef]
- Zhao, N.; Chen, H.; Zhang, W.; Yao, J.; Tu, Q.; Yu, X.; Sun, X. Bidirectional Influences between Seizures and Dementia: A Systematic Review and Meta-analysis. Int. J. Geriatr. Psychiatry 2022, 37, gps.5723. [Google Scholar] [CrossRef]
- Altuna, M.; Olmedo-Saura, G.; Carmona-Iragui, M.; Fortea, J. Mechanisms Involved in Epileptogenesis in Alzheimer’s Disease and Their Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 4307. [Google Scholar] [CrossRef]
- Diniz, B.S.; Butters, M.A.; Albert, S.M.; Dew, M.A.; Reynolds, C.F. Late-Life Depression and Risk of Vascular Dementia and Alzheimer’s Disease: Systematic Review and Meta-Analysis of Community-Based Cohort Studies. Br. J. Psychiatry 2013, 202, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Thandavan, S.P.; Mohebbi, M.; Pasco, J.A.; Williams, L.J.; Walder, K.; Ng, B.L.; Gupta, V.B. Depression and Bone Loss as Risk Factors for Cognitive Decline: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2022, 76, 101575. [Google Scholar] [CrossRef] [PubMed]
- Santabárbara Serrano, J.; Sevil Pérez, A.; Olaya, B.; Gracia García, P.; López Antón, R. Depresión tardía clínicamente relevante y riesgo de demencia: Revisión sistemática y metaanálisis de estudios prospectivos de cohortes. Rev. Neurol. 2019, 68, 493. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M. Precision Psychiatry: A Neural Circuit Taxonomy for Depression and Anxiety. Lancet Psychiatry 2016, 3, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.D.; Lim, Y.Y.; Gould, E.; Maruff, P. Amyloid-Beta and Depression in Healthy Older Adults: A Systematic Review. Aust. N. Z. J. Psychiatry 2015, 49, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Disease Neuroimaging Initiative; Cai, W.-J.; Tian, Y.; Ma, Y.-H.; Dong, Q.; Tan, L.; Yu, J.-T. Associations of Anxiety with Amyloid, Tau, and Neurodegeneration in Older Adults without Dementia: A Longitudinal Study. J. Alzheimers Dis. 2021, 82, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Hanseeuw, B.J.; Jonas, V.; Jackson, J.; Betensky, R.A.; Rentz, D.M.; Johnson, K.A.; Sperling, R.A.; Donovan, N.J. Association of Anxiety with Subcortical Amyloidosis in Cognitively Normal Older Adults. Mol. Psychiatry 2020, 25, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.; Orellana Rios, C.L.; Lahmann, C.; Rücker, G.; Bauer, J.; Boeker, M. Anxiety as a Risk Factor of Alzheimer’s Disease and Vascular Dementia. Br. J. Psychiatry 2018, 213, 654–660. [Google Scholar] [CrossRef]
- Luo, J.; Beam, C.R.; Gatz, M. Is Stress an Overlooked Risk Factor for Dementia? A Systematic Review from a Lifespan Developmental Perspective. Prev. Sci. 2023, 24, 936–949. [Google Scholar] [CrossRef]
- Bubu, O.M.; Brannick, M.; Mortimer, J.; Umasabor-Bubu, O.; Sebastião, Y.V.; Wen, Y.; Schwartz, S.; Borenstein, A.R.; Wu, Y.; Morgan, D.; et al. Sleep, Cognitive Impairment, and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Sleep 2017, 40, zsw032. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D.; Tan, Y. A Systematic Review and Dose-Response Meta-Analysis of Sleep Duration and the Occurrence of Cognitive Disorders. Sleep Breath. 2018, 22, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; Van Hees, V.T.; Paquet, C.; Sommerlad, A.; Kivimäki, M.; Dugravot, A.; Singh-Manoux, A. Association of Sleep Duration in Middle and Old Age with Incidence of Dementia. Nat. Commun. 2021, 12, 2289. [Google Scholar] [CrossRef] [PubMed]
- López-García, S.; Lage, C.; Pozueta, A.; García-Martínez, M.; Kazimierczak, M.; Fernández-Rodríguez, A.; Bravo, M.; Reyes-González, L.; Irure, J.; López-Hoyos, M.; et al. Sleep Time Estimated by an Actigraphy Watch Correlates with CSF Tau in Cognitively Unimpaired Elders: The Modulatory Role of APOE. Front. Aging Neurosci. 2021, 13, 663446. [Google Scholar] [CrossRef] [PubMed]
- Aini, N.; Chu, H.; Banda, K.J.; Chen, R.; Lee, T.-Y.; Pien, L.-C.; Liu, D.; Lai, Y.-J.; Kang, X.L.; Chou, K.-R. Prevalence of Sleep-Related Breathing Disorders and Associated Risk Factors among People with Dementia: A Meta-Analysis. Sleep Med. 2023, 103, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, S.-J.; Ma, M.-Y.; Bao, Y.-P.; Han, Y.; Wang, Y.-M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep Disturbances Increase the Risk of Dementia: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2018, 40, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic Intermittent Hypoxia Induces Oxidative Stress and Inflammation in Brain Regions Associated with Early-Stage Neurodegeneration. Physiol. Rep. 2017, 5, e13258. [Google Scholar] [CrossRef] [PubMed]
- Canevelli, M.; Wallace, L.M.K.; Bruno, G.; Cesari, M.; Rockwood, K.; Ward, D.D. Frailty Is Associated with the Clinical Expression of Neuropsychological Deficits in Older Adults. Eur. J. Neurol. 2024, 31, e16072. [Google Scholar] [CrossRef] [PubMed]
- García-Chanes, R.E.; Avila-Funes, J.A.; Borda, M.G.; Pérez-Zepeda, M.U.; Gutiérrez-Robledo, L.M. Higher Frailty Levels Are Associated with Lower Cognitive Test Scores in a Multi-Country Study: Evidence from the Study on Global Ageing and Adult Health. Front. Med. 2023, 10, 1166365. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Pareja, F.; Gómez De La Cámara, A.; Del Ser, T.; Contador, I.; Llamas-Velasco, S.; López-Arrieta, J.M.; Martín-Arriscado, C.; Hernández-Gallego, J.; Vega, S.; Benito-León, J. The Health Status: The Ignored Risk Factor in Dementia Incidence. NEDICES Cohort. Aging Clin. Exp. Res. 2022, 34, 1275–1283. [Google Scholar] [CrossRef]
- Sargent, L.; Nalls, M.; Amella, E.J.; Slattum, P.W.; Mueller, M.; Bandinelli, S.; Tian, Q.; Swift-Scanlan, T.; Lageman, S.K.; Singleton, A. Shared Mechanisms for Cognitive Impairment and Physical Frailty: A Model for Complex Systems. Alzheimers Dement. Transl. Res. Clin. Interv. 2020, 6, e12027. [Google Scholar] [CrossRef]
- Wallace, L.M.K.; Theou, O.; Godin, J.; Andrew, M.K.; Bennett, D.A.; Rockwood, K. Investigation of Frailty as a Moderator of the Relationship between Neuropathology and Dementia in Alzheimer’s Disease: A Cross-Sectional Analysis of Data from the Rush Memory and Aging Project. Lancet Neurol. 2019, 18, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Langballe, E.M.; Ask, H.; Holmen, J.; Stordal, E.; Saltvedt, I.; Selbæk, G.; Fikseaunet, A.; Bergh, S.; Nafstad, P.; Tambs, K. Alcohol Consumption and Risk of Dementia up to 27 Years Later in a Large, Population-Based Sample: The HUNT Study, Norway. Eur. J. Epidemiol. 2015, 30, 1049–1056. [Google Scholar] [CrossRef]
- Wang, G.; Li, D.Y.; Vance, D.E.; Li, W. Alcohol Use Disorder as a Risk Factor for Cognitive Impairment. J. Alzheimers Dis. 2023, 94, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Hasan, O.S.M.; Black, S.E.; Shield, K.D.; Schwarzinger, M. Alcohol Use and Dementia: A Systematic Scoping Review. Alzheimers Res. Ther. 2019, 11, 1. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.-T. Alcohol Consumption and Dementia Risk: A Dose–Response Meta-Analysis of Prospective Studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef]
- Ganguli, M.; Bilt, J.V.; Saxton, J.A.; Shen, C.; Dodge, H.H. Alcohol Consumption and Cognitive Function in Late Life: A Longitudinal Community Study. Neurology 2005, 65, 1210–1217. [Google Scholar] [CrossRef]
- Ruitenberg, A.; Van Swieten, J.C.; Witteman, J.C.; Mehta, K.M.; Van Duijn, C.M.; Hofman, A.; Breteler, M.M. Alcohol Consumption and Risk of Dementia: The Rotterdam Study. Lancet 2002, 359, 281–286. [Google Scholar] [CrossRef]
- Xie, C.; Feng, Y. Alcohol Consumption and Risk of Alzheimer’s Disease: A Dose–Response Meta-analysis. Geriatr. Gerontol. Int. 2022, 22, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Overview—Dementia, Disability and Frailty in Later Life—Mid-Life Approaches to Delay or Prevent Onset—Guidance—NICE. Available online: https://www.nice.org.uk/guidance/ng16 (accessed on 28 September 2023).
- Niu, H.; Qu, Y.; Li, Z.; Wang, R.; Li, L.; Li, M.; Lv, X.; Gao, C.; Song, Y.; Li, B. Smoking and Risk for Alzheimer Disease: A Meta-Analysis Based on Both Case-Control and Cohort Study. J. Nerv. Ment. Dis. 2018, 206, 680–685. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, Y.; Zhang, Y.; Guo, J.J.; Zhao, Y. Smoking Is Associated with an Increased Risk of Dementia: A Meta-Analysis of Prospective Cohort Studies with Investigation of Potential Effect Modifiers. PLoS ONE 2015, 10, e0118333. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Wang, J.; Xue, Q.; Yang, X.; Kang, Y.; Li, M.; Xu, J.; Li, G.; Li, C.; et al. Association of Cigarette Smoking with Cerebrospinal Fluid Biomarkers of Neurodegeneration, Neuroinflammation, and Oxidation. JAMA Netw. Open 2020, 3, e2018777. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Tang, J.; Guo, X.; Li, K.; Li, D. Dietary Fat Intake and Risk of Alzheimer’s Disease and Dementia: A Meta-Analysis of Cohort Studies. Curr. Alzheimer Res. 2018, 15, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Vicens, P.; Torrente, M. Modifiable Risk Factors for Dementia: The Role of Gut Microbiota. Curr. Alzheimer Res. 2021, 18, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Aridi, Y.; Walker, J.; Wright, O. The Association between the Mediterranean Dietary Pattern and Cognitive Health: A Systematic Review. Nutrients 2017, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Moradi, S.; Moradinazar, M.; Mostafai, R.; Pasdar, Y. Dietary Pattern in Relation to the Risk of Alzheimer’s Disease: A Systematic Review. Neurol. Sci. 2019, 40, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Asoudeh, F.; Naeini, F.; Sadeghi, E.; Travica, N.; Mohammadi, H. Association between Animal Protein Sources and Risk of Neurodegenerative Diseases: A Systematic Review and Dose-Response Meta-Analysis. Nutr. Rev. 2023, 81, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J. Alzheimers Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef] [PubMed]
- Fotuhi, M.; Mohassel, P.; Yaffe, K. Fish Consumption, Long-Chain Omega-3 Fatty Acids and Risk of Cognitive Decline or Alzheimer Disease: A Complex Association. Nat. Rev. Neurol. 2009, 5, 140–152. [Google Scholar] [CrossRef]
- Li, F.-J.; Shen, L.; Ji, H.-F. Dietary Intakes of Vitamin E, Vitamin C, and β-Carotene and Risk of Alzheimer’s Disease: A Meta-Analysis. J. Alzheimers Dis. 2012, 31, 253–258. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.M.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M.; et al. Vitamin D and the Risk of Dementia and Alzheimer Disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef]
- Kalra, A.; Teixeira, A.L.; Diniz, B.S. Association of Vitamin D Levels with Incident All Cause Dementia in Longitudinal Observational Studies: A Systematic Review and Meta-Analysis. J. Prev. Alzheimers Dis. 2019, 7, 14–20. [Google Scholar] [CrossRef]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D Deficiency as a Risk Factor for Dementia and Alzheimer’s Disease: An Updated Meta-Analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Shab-Bidar, S. Vitamin D Status and Risk of Dementia and Alzheimer’s Disease: A Meta-Analysis of Dose-Response. Nutr. Neurosci. 2019, 22, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.-F. Vitamin D Deficiency Is Associated with Increased Risk of Alzheimer’s Disease and Dementia: Evidence from Meta-Analysis. Nutr. J. 2015, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Hersi, M.; Irvine, B.; Gupta, P.; Gomes, J.; Birkett, N.; Krewski, D. Risk Factors Associated with the Onset and Progression of Alzheimer’s Disease: A Systematic Review of the Evidence. NeuroToxicology 2017, 61, 143–187. [Google Scholar] [CrossRef]
- Hörder, H.; Johansson, L.; Guo, X.; Grimby, G.; Kern, S.; Östling, S.; Skoog, I. Midlife Cardiovascular Fitness and Dementia: A 44-Year Longitudinal Population Study in Women. Neurology 2018, 90, e1298–e1305. [Google Scholar] [CrossRef]
- Spartano, N.L.; Ngandu, T. Fitness and Dementia Risk: Further Evidence of the Heart-Brain Connection. Neurology 2018, 90, 675–676. [Google Scholar] [CrossRef]
- Collins, A.M.; Molina-Hidalgo, C.; Aghjayan, S.L.; Fanning, J.; Erlenbach, E.D.; Gothe, N.P.; Velazquez-Diaz, D.; Erickson, K.I. Differentiating the Influence of Sedentary Behavior and Physical Activity on Brain Health in Late Adulthood. Exp. Gerontol. 2023, 180, 112246. [Google Scholar] [CrossRef] [PubMed]
- Dorsman, K.A.; Weiner-Light, S.; Staffaroni, A.M.; Brown, J.A.; Wolf, A.; Cobigo, Y.; Walters, S.; Kramer, J.H.; Casaletto, K.B. Get Moving! Increases in Physical Activity Are Associated with Increasing Functional Connectivity Trajectories in Typically Aging Adults. Front. Aging Neurosci. 2020, 12, 104. [Google Scholar] [CrossRef]
- Erickson, K.I.; Donofry, S.D.; Sewell, K.R.; Brown, B.M.; Stillman, C.M. Cognitive Aging and the Promise of Physical Activity. Annu. Rev. Clin. Psychol. 2022, 18, 417–442. [Google Scholar] [CrossRef]
- Huang, A.R.; Roth, D.L.; Cidav, T.; Chung, S.; Amjad, H.; Thorpe, R.J.; Boyd, C.M.; Cudjoe, T.K.M. Social Isolation and 9-year Dementia Risk in community-dwelling Medicare Beneficiaries in the United States. J. Am. Geriatr. Soc. 2023, 71, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Evans, I.E.M.; Martyr, A.; Collins, R.; Brayne, C.; Clare, L. Social Isolation and Cognitive Function in Later Life: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2019, 70 (Suppl. 1), S119–S144. [Google Scholar] [CrossRef] [PubMed]
- Najar, J.; Aakre, J.A.; Vassilaki, M.; Wetterberg, H.; Rydén, L.; Zettergren, A.; Skoog, I.; Jack, C.R.; Knopman, D.S.; Petersen, R.C.; et al. Sex Difference in the Relation Between Marital Status and Dementia Risk in Two Population-Based Cohorts. J. Alzheimers Dis. 2021, 83, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Biddle, K.D.; Jacobs, H.I.L.; D’Oleire Uquillas, F.; Zide, B.S.; Kirn, D.R.; Properzi, M.R.; Rentz, D.M.; Johnson, K.A.; Sperling, R.A.; Donovan, N.J. Associations of Widowhood and β-Amyloid with Cognitive Decline in Cognitively Unimpaired Older Adults. JAMA Netw. Open 2020, 3, e200121. [Google Scholar] [CrossRef] [PubMed]
- Sundström, A.; Westerlund, O.; Mousavi-Nasab, H.; Adolfsson, R.; Nilsson, L.G. The Relationship between Marital and Parental Status and the Risk of Dementia. Int. Psychogeriatr. 2014, 26, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Guarnera, J.; Yuen, E.; Macpherson, H. The Impact of Loneliness and Social Isolation on Cognitive Aging: A Narrative Review. J. Alzheimers Dis. Rep. 2023, 7, 699–714. [Google Scholar] [CrossRef]
- Lam, J.A.; Murray, E.R.; Yu, K.E.; Ramsey, M.; Nguyen, T.T.; Mishra, J.; Martis, B.; Thomas, M.L.; Lee, E.E. Neurobiology of Loneliness: A Systematic Review. Neuropsychopharmacology 2021, 46, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Kulesza, R.J.; González-González, L.O.; Reynoso-Robles, R.; Mukherjee, P.S.; Torres-Jardón, R. Air Pollution, Combustion and Friction Derived Nanoparticles, and Alzheimer’s Disease in Urban Children and Young Adults. J. Alzheimers Dis. 2019, 70, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Carey, I.M.; Anderson, H.R.; Atkinson, R.W.; Beevers, S.D.; Cook, D.G.; Strachan, D.P.; Dajnak, D.; Gulliver, J.; Kelly, F.J. Are Noise and Air Pollution Related to the Incidence of Dementia? A Cohort Study in London, England. BMJ Open 2018, 8, e022404. [Google Scholar] [CrossRef]
- Jung, C.-R.; Lin, Y.-T.; Hwang, B.-F. Ozone, Particulate Matter, and Newly Diagnosed Alzheimer’s Disease: A Population-Based Cohort Study in Taiwan. J. Alzheimers Dis. 2015, 44, 573–584. [Google Scholar] [CrossRef]
- Yuan, S.; Huang, X.; Zhang, L.; Ling, Y.; Tan, S.; Peng, M.; Xu, A.; Lyu, J. Associations of Air Pollution with All-Cause Dementia, Alzheimer’s Disease, and Vascular Dementia: A Prospective Cohort Study Based on 437,932 Participants from the UK Biobank. Front. Neurosci. 2023, 17, 1216686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Weuve, J.; Langa, K.M.; D’Souza, J.; Szpiro, A.; Faul, J.; Mendes De Leon, C.; Gao, J.; Kaufman, J.D.; Sheppard, L.; et al. Comparison of Particulate Air Pollution from Different Emission Sources and Incident Dementia in the US. JAMA Intern. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Ee, N.; Peters, J.; Booth, A.; Mudway, I.; Anstey, K.J. Air Pollution and Dementia: A Systematic Review. J. Alzheimers Dis. 2019, 70 (Suppl. 1), S145–S163. [Google Scholar] [CrossRef]
- Hussenoeder, F.S.; Riedel-Heller, S.G. Primary Prevention of Dementia: From Modifiable Risk Factors to a Public Brain Health Agenda? Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.; Ngandu, T.; Rusanen, M.; Antikainen, R.; Bäckman, L.; Havulinna, S.; Hänninen, T.; Laatikainen, T.; Lehtisalo, J.; Levälahti, E.; et al. Multidomain Lifestyle Intervention Benefits a Large Elderly Population at Risk for Cognitive Decline and Dementia Regardless of Baseline Characteristics: The FINGER Trial. Alzheimers Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.-N.; Dantoine, T.; Dartigues, J.-F.; et al. Effect of Long-Term Omega 3 Polyunsaturated Fatty Acid Supplementation with or without Multidomain Intervention on Cognitive Function in Elderly Adults with Memory Complaints (MAPT): A Randomised, Placebo-Controlled Trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Van Charante, E.P.M.; Richard, E.; Eurelings, L.S.; Van Dalen, J.-W.; Ligthart, S.A.; Van Bussel, E.F.; Hoevenaar-Blom, M.P.; Vermeulen, M.; Van Gool, W.A. Effectiveness of a 6-Year Multidomain Vascular Care Intervention to Prevent Dementia (preDIVA): A Cluster-Randomised Controlled Trial. Lancet 2016, 388, 797–805. [Google Scholar] [CrossRef]
- Takada, L.T.; Aláez-Verson, C.; Burgute, B.D.; Nitrini, R.; Sosa, A.L.; Castilhos, R.M.; Chaves, M.F.; Longoria, E.-M.; Carrillo-Sánchez, K.; Brucki, S.M.D.; et al. Discovery and Validation of Dominantly Inherited Alzheimer’s Disease Mutations in Populations from Latin America. Alzheimers Res. Ther. 2022, 14, 108. [Google Scholar] [CrossRef]
- Lai, D.; Zhang, M.; Li, R.; Zhang, C.; Zhang, P.; Liu, Y.; Gao, S.; Foroud, T. Identifying Genes Associated with Alzheimer’s Disease Using Gene-Based Polygenic Risk Score. J. Alzheimers Dis. 2023, 96, 1639–1649. [Google Scholar] [CrossRef]
- Maloney, B.; Lahiri, D.K. Epigenetics of Dementia: Understanding the Disease as a Transformation Rather than a State. Lancet Neurol. 2016, 15, 760–774. [Google Scholar] [CrossRef]
- Bennett, D.A.; Buchman, A.S.; Boyle, P.A.; Barnes, L.L.; Wilson, R.S.; Schneider, J.A. Religious Orders Study and Rush Memory and Aging Project. J. Alzheimers Dis. 2018, 64 (Suppl. 1), S161–S189. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer Disease Genetics Consortium (ADGC); The European Alzheimer’s Disease Initiative (EADI); Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (CHARGE); Genetic and Environmental Risk in AD/Defining Genetic, Polygenic and Environmental Risk for Alzheimer’s Disease Consortium (GERAD/PERADES); Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; et al. Genetic Meta-Analysis of Diagnosed Alzheimer’s Disease Identifies New Risk Loci and Implicates Aβ, Tau, Immunity and Lipid Processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef]
- Knopman, D.S.; Roberts, R. Vascular Risk Factors: Imaging and Neuropathologic Correlates. J. Alzheimers Dis. 2010, 20, 699–709. [Google Scholar] [CrossRef]
- Azarpazhooh, M.R.; Avan, A.; Cipriano, L.E.; Munoz, D.G.; Erfanian, M.; Amiri, A.; Stranges, S.; Hachinski, V. A Third of Community-Dwelling Elderly with Intermediate and High Level of Alzheimer’s Neuropathologic Changes Are Not Demented: A Meta-Analysis. Ageing Res. Rev. 2020, 58, 101002. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L. Centenarians as Models of Resistance and Resilience to Alzheimer’s Disease and Related Dementias. Adv. Geriatr. Med. Res. 2020, 2, e200018. [Google Scholar] [CrossRef]
- Perls, T.T. Cognitive Trajectories and Resilience in Centenarians—Findings from the 100-Plus Study. JAMA Netw. Open 2021, 4, e2032538. [Google Scholar] [CrossRef]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. The Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup. Whitepaper: Defining and Investigating Cognitive Reserve, Brain Reserve, and Brain Maintenance. Alzheimers Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Arenaza-Urquijo, E.M.; Vemuri, P. Resistance vs Resilience to Alzheimer Disease: Clarifying Terminology for Preclinical Studies. Neurology 2018, 90, 695–703. [Google Scholar] [CrossRef]
- Stern, Y.; Albert, M.; Barnes, C.A.; Cabeza, R.; Pascual-Leone, A.; Rapp, P.R. A framework for concepts of reserve and resilience in aging. Neurobiol. Aging 2023, 124, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.A.; Borenstein, A.R.; Gosche, K.M.; Snowdon, D.A. Very Early Detection of Alzheimer Neuropathology and the Role of Brain Reserve in Modifying Its Clinical Expression. J. Geriatr. Psychiatry Neurol. 2005, 18, 218–223. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive Reserve in Ageing and Alzheimer’s Disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Arenaza-Urquijo, E.M.; Vemuri, P. Improving the resistance and resilience framework for aging and dementia studies. Alzheimers Res. Ther. 2020, 12, 41. [Google Scholar] [CrossRef] [PubMed]
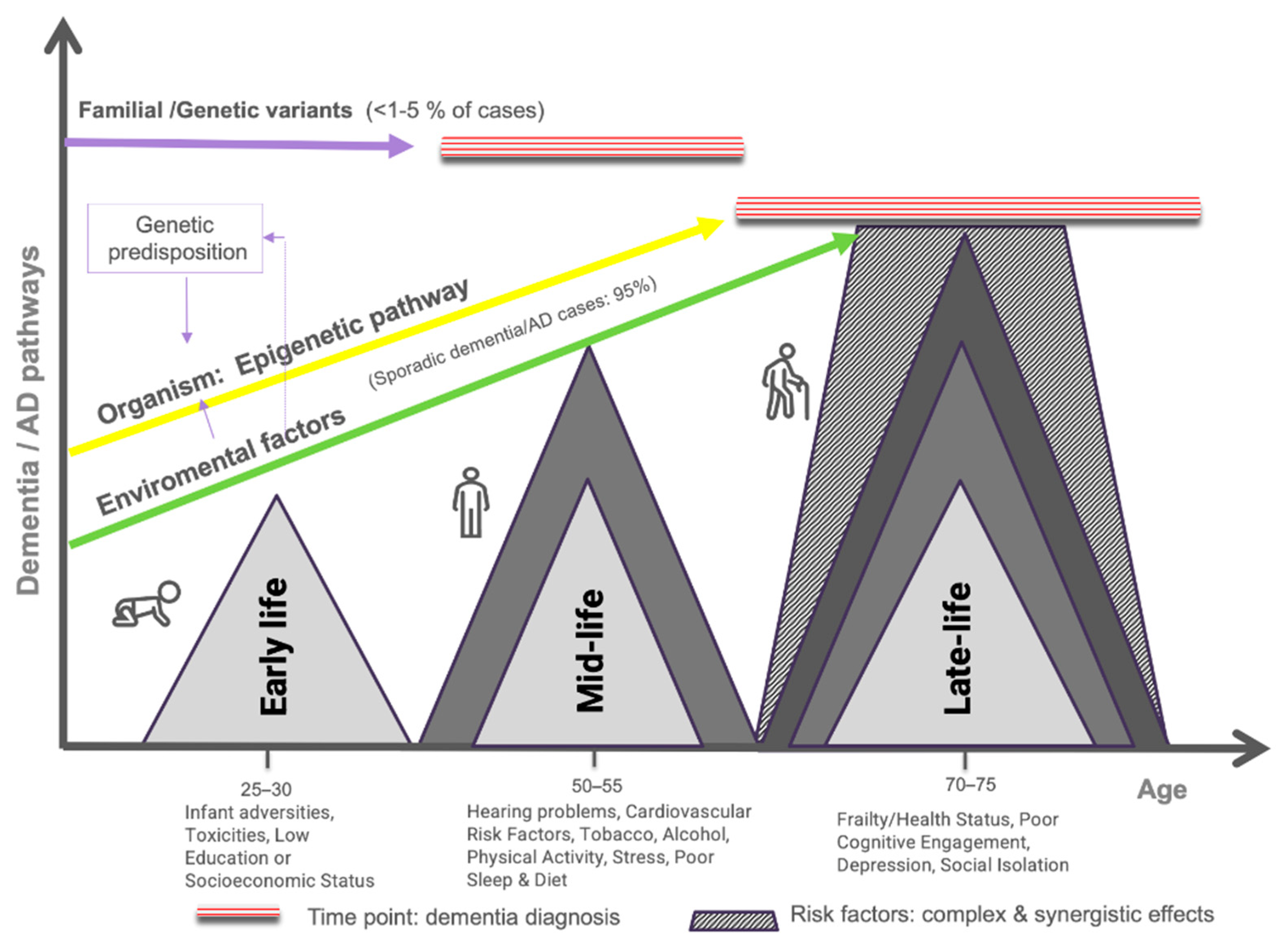

| Author/Year/Country | Follow-Up (Years) | Dementia Subtype | Preval | Incidence | Study Name |
|---|---|---|---|---|---|
| Western countries | |||||
| Manton/2005/US [25] ** | 18 | Mixed | ↓ | — | LNLTCS |
| Langa/2008/US [33] * | 9 | Dementia | ↓ | — | HRS |
| Hall/2009/US [34] * | 9 | Dementia | # | — | Indianapolis cohort. (African Americans) |
| Hebert/2010/US [35] * | 10 | AD | — | # | Chicago neighborhoods |
| Lobo/2011/Spain [36] * | >10 | Dementia | ↓ | — α | ZARADEMP |
| Rocca/2011/US [26] ** | 10–20 | Dem/AD | — | ↓ # | Several |
| Schrijvers/2012/Holland [37] * | 10 | Dem | — | ↓ & | Rotterdam |
| Wiberg/2013/Sweden [38] * | 30 | Dementia | # | — | Gothenburg cohorts |
| Abdulrahman/2014/UK [39] ** | 12 | AD | ↑ | ↑ | PEDW (Wales) |
| Grasset/2016/France [40] * | 10 | Dementia | — | ↓ ω | PAQUID-Three City |
| Matthews/2016/UK [41] * | 7–12 | Dementia | — | ↓ | CFAS |
| Satizabal/2016//US [3] * | 30 | Dementia | — | ↓ | Framingham Heart |
| Kosteniuk/2016/Canada [42] ** | 8 | Dementia | ↑ | ↓ | Saskatchewan heath data |
| Wimo/2016/Sweden [43] * | 6 | Dementia | ↓ | — | Sweden, rural area |
| Ahmadi/2017/UK [44] ** | 10 | Dementia | — | ↓ | ELSA |
| Cerasuolo/2017/Canada [45] | 12 | Dementia | — | ↓ ~ | Several |
| Derby/2017/US [34] * | 22 | Dementia | — | ↓ | Einstein Aging Study |
| Noble/2017/US [46] * | 7 | Dementia | — | ↓ | WH-I-Aging Study |
| Peres/2017/France [47] * | 20 | Dementia | ↓ | — | PAQUID&AMI |
| Chen/2018/US [48] ** | 12 | Dementia | ↓ | — | HR Study |
| Hendrie/2018/US [49] * | 9 | Dem/AD | — | ↓ | WHICAP |
| Seblova/2018/Sweden [50] ** | 30 | Dementia | — | ↓ | National Swedish Registry |
| Rajan/2018/US [51] | 18 | AD | # | # | CHAP Study |
| Sullivan/2019/US [52] * | 40 | Dementia | — | ↓ | Monongahela Valley |
| Ding/2020/Sweden [53] * | 25 | Dementia | — | ↓ | Stockholm (2 Cohorts) |
| Wolters/2020/US-Europe [12] * | 27 | Dem/AD | — | ↓ | Several |
| Farina/2022/US [54] ** | 16 | Dem/AD | ↓ | ↓ | HRS |
| Van Bussell/2022/Netherland [55] | 12 | Dementia | — | # | Dutch Primary Care |
| Chen-Y-/2023/UK [56] | 17 | Dementia | — | ↓γ, ↑γ | ELS of Aging |
| Non-Western countries | |||||
| Gao/2016/Nigeria [57] | 9 | Dem/AD | — | # | IIDP |
| Ohara/2017/Japan [58] | 27 | Dem/AD | ↑ | ↑ | Hisayama |
| Ding/2020/China [59] | 29 | Dementia | ↑ | ↑ | SESD&SAS (Shanghai) |
| Shimizu/2022/Japan [60] * | 19 | Dem/AD | ↑ | — | Nayakama town |
| Huang/2024/Taiwan [61] | 13 | Dementia | — | ↑ | National Taiwan cohort |
| Author/Publication Year | Risk Factors | Dementia Outcome | Preventable %, Study Type |
|---|---|---|---|
| Henderson, (1988) [115] | CRF, SERF, environmental RF | AD | - |
| EURODEM group [116] | Clinical and environmental RF | AD | |
| EURODEM group [125] | Lifestyle RF | AD | |
| Haan and Wallace (2004) [126] | VRF, genetics, and exposure RF | AD/VaD | |
| Jansson (2005) [127] | Clinical and biological RF | AD | 50% |
| Middleton and Yaffe (2009) [128] | CRF and lifestyle | Dementia | |
| Ritchie et al. (2010) [129] | CRF | Dementia | Specific % by RFs |
| Barnes and Jaffe (2011) [130] | 7 CRF | EA | up to 50.7% ¥ |
| Song et al. (2011) [131] | Frailty index | Dementia/AD | - |
| Mangialasche et al. (2012) [23] | Clinical and lifestyle RF | Dementia/AD/VaD | |
| Anstey et al. (2013) [132] | 11 RF and 4 PF | AD | |
| Di Marco et al. (2014) [133] | Modifiable lifestyle RF | Dementia | Systematic Review of Cohorts |
| Anstey et al. (2015) [134] | CRF and lifestyle RF | Dementia | - |
| Baumgart et al. (2015) [135] | CRF and lifestyle RF | Dementia | |
| Deckers et al. (2015) [136] | CRF (midlife) | Dementia/MCI | Expert Delphi Panel |
| Xu et al. (2015) [114] | 93 clinical, lifestyle, exposure RF | AD | 66% |
| Hazar et al. (2016) [137] | 5 CRF | AD | 33% |
| Killin et al. (2016) [138] | Environmental RF | Dementia | |
| Wu et al. (2016) [139] | 11 RF | Dementia | |
| Bellou et al. (2017) [140] | Environmental RF and CRF | Dem/AD/VaD | |
| Livingston et al. (2017) [112] | 9 CRF | Dementia | |
| Rakesh et al. (2017) [141] | Multiples CRF and lifestyle RF | Dementia | |
| Larsson and Markus, (2018) [142] | VRF | Dementia/AD | Systematic Review & MA |
| Anstey et al. (2019) [143] | RF: AD 34; Dem 69, VaD 8 | AD/Dem/VaD | |
| Armstrong (2019) [144] | 51 RF | AD | |
| Edwards III GA et al. (2019) [145] | CRF and lifestyle RF | AD | |
| Peters et al. (2019) [146] | Co-ocurring RF | Dementia | Systematic Review & MA |
| Rochoy et al. (2019) [147] | Multiples RF | AD | |
| Yu et al. (2020) [148] | 104 modifiable RF, 11 interventions | AD | |
| Liang et al. (2020) [149] | Modifiable RF | Dementia | Bayesian Analysis & MA |
| Livingston et al. (2020) [15] | 12 CRF | Dementia | 40% |
| Kuo et al. (2020) [150] | Modifiable/Non-modifiable RF | Dementia/AD | Review |
| Rolandi et al. (2020) [151] | Modifiable FR cohort | Dementia | 40% |
| Weiss et al. (2020) [16] | 65 RF | Dementia | |
| Zhang et al. (2023) [17] | 210 CRF, lifestyle, SERF | Dementia | 47.0–72.6% |
| Jones et al. (2024) [152] | 14 Modifiable RF | AD/VaD | Umbrella-Review & MA |
| Stephan et al. (2024) [153] | Modifiable RF | Dementia | Systematic Review & MA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contador, I.; Buch-Vicente, B.; del Ser, T.; Llamas-Velasco, S.; Villarejo-Galende, A.; Benito-León, J.; Bermejo-Pareja, F. Charting Alzheimer’s Disease and Dementia: Epidemiological Insights, Risk Factors and Prevention Pathways. J. Clin. Med. 2024, 13, 4100. https://doi.org/10.3390/jcm13144100
Contador I, Buch-Vicente B, del Ser T, Llamas-Velasco S, Villarejo-Galende A, Benito-León J, Bermejo-Pareja F. Charting Alzheimer’s Disease and Dementia: Epidemiological Insights, Risk Factors and Prevention Pathways. Journal of Clinical Medicine. 2024; 13(14):4100. https://doi.org/10.3390/jcm13144100
Chicago/Turabian StyleContador, Israel, Bárbara Buch-Vicente, Teodoro del Ser, Sara Llamas-Velasco, Alberto Villarejo-Galende, Julián Benito-León, and Félix Bermejo-Pareja. 2024. "Charting Alzheimer’s Disease and Dementia: Epidemiological Insights, Risk Factors and Prevention Pathways" Journal of Clinical Medicine 13, no. 14: 4100. https://doi.org/10.3390/jcm13144100
APA StyleContador, I., Buch-Vicente, B., del Ser, T., Llamas-Velasco, S., Villarejo-Galende, A., Benito-León, J., & Bermejo-Pareja, F. (2024). Charting Alzheimer’s Disease and Dementia: Epidemiological Insights, Risk Factors and Prevention Pathways. Journal of Clinical Medicine, 13(14), 4100. https://doi.org/10.3390/jcm13144100






