Anti-Inflammatory and Anti-Oxidant Properties of N-Acetylcysteine: A Fresh Perspective
Abstract
:1. Introduction
2. Methods
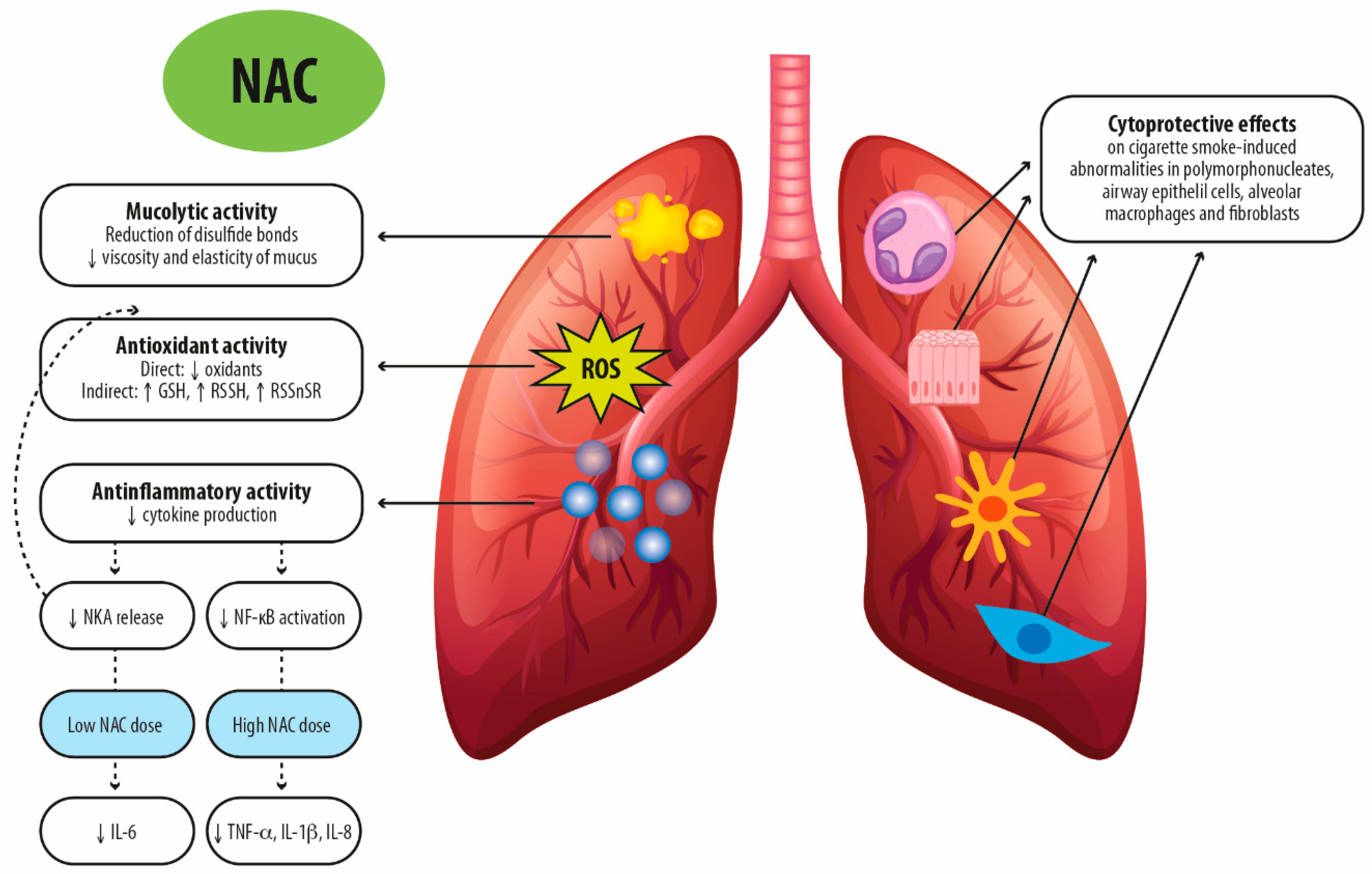
3. NAC Exhibits Pharmacological Actions beyond Mucolytic Activity
3.1. NAC as Anti-Oxidant
3.2. NAC as an Anti-Inflammatory Drug
4. Clinical Effectiveness of NAC in Pulmonary Medicine
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheffner, A.L. The reduction in vitro in viscosity of mucoprotein solutions by a new mucolytic agent, N-acetyl-L-cysteine. Ann. N. Y. Acad. Sci. 1963, 106, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Prescott, L.F.; Park, J.; Ballantyne, A.; Adriaenssens, P.; Proudfoot, A.T. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet 1977, 2, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/medicines/publications/essentialmedicines/en/ (accessed on 6 May 2024).
- Rogers, D.F.; Barnes, P.J. Treatment of airway mucus hypersecretion. Ann. Med. 2006, 38, 116–125. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; Silva, C.P.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants 2023, 12, 548. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Hauber, H.P.; Foley, S.C.; Hamid, Q. Mucin overproduction in chronic inflammatory lung disease. Can. Respir. J. 2006, 13, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Curran, D.R.; Cohn, L. Advances in mucous cell metaplasia: A plug for mucus as a therapeutic focus in chronic airway disease. Am. J. Respir. Cell Mol. Biol. 2010, 42, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.A.; Chen, A.C.; Radicioni, G.; Lourie, R.; Martin, M.; Broomfield, A.; Sheng, Y.H.; Hasnain, S.Z.; Radford-Smith, G.; Simms, L.A.; et al. Airway Mucus Hyperconcentration in Non–Cystic Fibrosis Bronchiectasis. Am. J. Respir. Crit. Care Med. 2020, 201, 661–670. [Google Scholar] [CrossRef]
- Tilley, A.E.; Walters, M.S.; Shaykhiev, R.; Crystal, R.G. Cilia Dysfunction in Lung Disease. Annu. Rev. Physiol. 2015, 77, 379–406. [Google Scholar] [CrossRef]
- MacNee, W. Pathology, pathogenesis, and pathophysiology. BMJ 2006, 332, 1202–1204. [Google Scholar] [CrossRef]
- Calzetta, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. Multifaceted activity of N-acetyl-l-cysteine in chronic obstructive pulmonary disease. Expert. Rev. Respir. Med. 2018, 12, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Randell, S.H.; Boucher, R.C.; University of North Carolina Virtual Lung Group. Effective mucus clearance is essential for respiratory health. Am. J. Respir. Cell Mol. Biol. 2006, 35, 20–28. [Google Scholar] [CrossRef]
- Matsui, H.; Verghese, M.W.; Kesimer, M.; Schwab, U.E.; Randell, S.H.; Sheehan, J.K.; Grubb, B.R.; Boucher, R.C. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J. Immunol. 2005, 175, 1090–1099. [Google Scholar] [CrossRef]
- Papi, A.; Luppi, F.; Franco, F.; Fabbri, L.M. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006, 3, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, A.; Choate, R.; Addrizzo-Harris, D.; Aksamit, T.R.; Barker, A.; Daley, C.L.; Daniels, M.L.; Eden, E.; DiMango, A.; Fennelly, K.; et al. Airway clearance techniques in bronchiectasis: Analysis from the United States Bronchiectasis and Non-TB Mycobacteria Research Registry. Chest 2020, 158, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Borekci, S.; Halis, A.N.; Aygun, G.; Musellim, B. Bacterial colonization and associated factors in patients with bronchiectasis. Ann. Thorac. Med. 2016, 11, 55–59. [Google Scholar] [CrossRef]
- Korfhagen, T.R.; Kitzmiller, J.; Chen, G.; Sridharan, A.; Haitchi, H.M.; Hegde, R.S.; Divanovic, S.; Karp, C.L.; Whitsett, J.A. SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc. Natl. Acad. Sci. USA 2012, 109, 16630–16635. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, M.J.; Byers, D.E.; Alexander-Brett, J.; Wang, X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat. Rev. Immunol. 2014, 14, 686–698. [Google Scholar] [CrossRef]
- Tyner, J.W.; Kim, E.Y.; Ide, K.; Pelletier, M.R.; Roswit, W.T.; Morton, J.D.; Battaile, J.T.; Patel, A.C.; Patterson, G.A.; Castro, M.; et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J. Clin. Investig. 2006, 116, 309–321. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.M.; Verbraecken, J.; Darquennes, K.; De Backer, W.A. Role of N-acetylcysteine in the management of COPD. Int. J. Chron. Obstruct Pulmon Dis. 2006, 1, 425–434. [Google Scholar] [CrossRef] [PubMed]
- De Backer, W.; Van Overveld, F. Sputum ECP levels in COPD patients decrease after treatment with N-acetylcysteine (NAC). Eur. Respir. J. 1997, 12, 225s. [Google Scholar]
- Van Overveld, F.J.; Demkow, U.; Górecka, D.; De Backer, W.A.; Zielinski, J. New developments in the treatment of COPD: Comparing the effects of inhaled corticosteroids and N-acetylcysteine. J. Physiol. Pharmacol. 2005, 56 (Suppl. S4), 135–142. [Google Scholar]
- Sadowska, A.M.; Van Overveld, F.J.; Gorecka, D.; Zdral, A.; Filewska, M.; Demkow, U.A.; Luyten, C.; Saenen, E.; Zielinski, J.; De Backer, W.A. The interrelationship between markers of inflammation and oxidative stress in chronic obstructive pulmonary disease: Modulation by inhaled steroids and antioxidant. Respir. Med. 2005, 99, 241–249. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Facciolo, F.; Rogliani, P.; Matera, M.G. Pharmacological investigation on the anti-oxidant and anti-inflammatory activity of N-acetylcysteine in an ex vivo model of COPD exacerbation. Respir. Res. 2017, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Rogliani, P.; Facciolo, F.; Rinaldi, B.; Cazzola, M.; Matera, M.G. N-Acetylcysteine protects human bronchi by modulating the release of neurokinin A in an ex vivo model of COPD exacerbation. Biomed. Pharmacother. 2018, 103, 1–8. [Google Scholar] [CrossRef]
- Montero, P.; Roger, I.; Estornut, C.; Milara, J.; Cortijo, J. Influence of dose and exposition time in the effectiveness of N-Acetyl-l-cysteine treatment in A549 human epithelial cells. Heliyon 2023, 9, e15613. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S: A Novel Gasotransmitter that Signals by Sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef]
- Tieu, S.; Charchoglyan, A.; Paulsen, L.; Wagter-Lesperance, L.C.; Shandilya, U.K.; Bridle, B.W.; Mallard, B.A.; Karrow, N.A. N-Acetylcysteine and Its Immunomodulatory Properties in Humans and Domesticated Animals. Antioxidants 2023, 12, 1867. [Google Scholar] [CrossRef]
- Santus, P.; Corsico, A.; Solidoro, P.; Braido, F.; Di Marco, F.; Scichilone, N. Oxidative stress and respiratory system: Pharmacological and clinical reappraisal of N-acetylcysteine. COPD J. Chronic Obstr. Pulm. Dis. 2014, 11, 705–717. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease—GOLD. 2024 GOLD Report. Available online: https://goldcopd.org/2024-gold-report/ (accessed on 22 January 2024).
- Miravitlles, M.; Calle, M.; Molina, J.; Almagro, P.; Gómez, J.T.; Trigueros, J.A.; Cosío, B.G.; Casanova, C.; López-Camposb, J.L.; Riescob, J.A.; et al. Spanish COPD Guidelines (GesEPOC) 2021: Updated Pharmacological treatment of stable COPD. Arch. Bronconeumol. 2022, 58, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Criner, G.J.; Bourbeau, J.; Diekemper, R.L.; Ouellette, D.R.; Goodridge, D.; Hernandez, P.; Curren, K.; Balter, M.S.; Bhutani, M.; Camp, P.G.; et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015, 147, 894–942. [Google Scholar] [CrossRef] [PubMed]
- Bourbeau, J.; Bhutani, M.; Hernandez, P.; Marciniuk, D.D.; Aaron, S.D.; Balter, M.; Beauchesne, M.F.; D’Urzo, A.; Goldstein, R.; Kaplan, A.; et al. Full article: CTS position statement: Pharmacotherapy in patients with COPD—An update. Can. J. Respir. Crit. Care Sleep Med. 2017, 1, 222. [Google Scholar]
- Wedzicha, J.A.; Calverley, P.M.; Albert, R.K.; Anzueto, A.; Criner, G.J.; Hurst, J.R.; Miravitlles, M.; Papi, A.; Rabe, K.F.; Rigau, D.; et al. Prevention of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2017, 50, 1602265. [Google Scholar] [CrossRef]
- Gould, N.S.; Day, B.J. Targeting maladaptive glutathione responses in lung disease. Biochem. Pharmacol. 2011, 81, 187. [Google Scholar] [CrossRef] [PubMed]
- Olveira, G.; Olveira, C.; Dorado, A.; García-Fuentes, E.; Rubio, E.; Tinahones, F.; Soriguer, F.; Murri, M. Cellular and plasma oxidative stress biomarkers are raised in adults with bronchiectasis. Clin. Nutr. 2013, 32, 112–117. [Google Scholar] [CrossRef]
- Moldéus, P.; Cotgreave, I.A.; Berggren, M. Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration 1986, 50 (Suppl. S1), 31–42. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Drost, E.M.; Skwarski, K.M.; Sauleda, J.; Soler, N.; Roca, J.; Agusti, A.; MacNee, W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005, 60, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Matera, M.G.; Calzetta, L.; Cazzola, M. Oxidation pathway and exacerbations in COPD: The role of NAC. Expert Rev. Respir. Med. 2016, 10, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.M.; Marsden, M.; Selby, C.; Morrison, D.; MacNee, W. Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissue. Thorax 1994, 49, 670–675. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W.; Bridgeman, M.M.; Marsden, M.; Drost, E.; Lannan, S.; Selby, C.; Donaldson, K. The effects of N-acetylcysteine and glutathione on smoke-induced changes in lung phagocytes and epithelial cells. Am. J. Med. 1991, 91, 60S–66S. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.M.; Marsden, M.; MacNee, W.; Flenley, D.C.; Ryle, A.P. Cysteine and glutathione concentrations in plasma and bronchoalveolar lavage fluid after treatment with N-acetylcysteine. Thorax 1991, 46, 39–42. [Google Scholar] [CrossRef]
- Linden, M.; Wieslander, E.; Eklund, A.; Larsson, K.; Brattsand, R. Effects of oral N-acetylcysteine on cell content and macrophage function in bronchoalveolar lavage from healthy smokers. Eur. Respir. J. 1988, 1, 645–650. [Google Scholar] [CrossRef]
- Kasielski, M.; Nowak, D. Long-term administration of N-acetylcysteine decreases hydrogen peroxide exhalation in subjects with chronic obstructive pulmonary disease. Respir. Med. 2001, 95, 448–456. [Google Scholar] [CrossRef]
- De Benedetto, F.; Aceto, A.; Dragani, B.; Spacone, A.; Formisano, S.; Pela, R.; Donner, C.F.; Sanguinetti, C.M. Long-term oral n-acetylcysteine reduces exhaled hydrogen peroxide in stable COPD. Pulm. Pharmacol. Ther. 2005, 18, 41–47. [Google Scholar] [CrossRef]
- Bridges, R.B. Protective action of thiols on neutrophil function. Eur. J. Respir. Dis. Suppl. 1985, 139, 40–48. [Google Scholar]
- Voisin, C.; Aerts, C.; Wallaert, B. Prevention of in vitro oxidant-mediated alveolar macrophage injury by cellular glutathione and precursors. Bull. Eur. Physiopathol. Respir. 1987, 23, 309–313. [Google Scholar] [PubMed]
- Bergstrand, H.; Björnson, A.; Eklund, A.; Hernbrand, R.; Larsson, K.; Linden, M.; Nilsson, A. Stimuli-induced superoxide radical generation in vitro by human alveolar macrophages from smokers: Modulation by N-acetylcysteine treatment in vivo. J. Free Radic. Biol. Med. 1986, 2, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Moldéus, P.; Berggren, M.; Grafström, R. N-acetylcysteine protection against the toxicity of cigarette smoke and cigarette smoke condensates in various tissues and cells in vitro. Eur. J. Respir. Dis. Suppl. 1985, 139, 123–129. [Google Scholar]
- Cotgreave, I.A.; Moldéus, P. Lung protection by thiol-containing antioxidants. Bull. Eur. Physiopathol. Respir. 1987, 23, 275–277. [Google Scholar] [PubMed]
- Eklund, A.; Eriksson, O.; Hakansson, L.; Larsson, K.; Ohlsson, K.; Venge, P.; Bergstrand, H.; Bjornson, A.; Brattsand, R.; Glennow, C. Oral N-acetylcysteine reduces selected humoral markers of inflammatory cell activity in BAL fluid from healthy smokers: Correlation to effects on cellular variables. Eur. Respir. J. 1988, 1, 832–838. [Google Scholar] [CrossRef]
- Schreck, R.; Meier, B.; Männel, D.N.; Dröge, W.; Baeuerle, P.A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 1992, 175, 1181–1194. [Google Scholar] [CrossRef]
- Jankowska, R.; Passowicz-Muszyńska, E.; Medrala, W.; Banaś, T.; Marcinkowska, A. The influence of n-acetylcysteine on chemiluminescence of granulocytes in peripheral blood of patients with chronic bronchitis. Pneumonol. Alergol. Pol. 1993, 61, 586–591. [Google Scholar] [PubMed]
- Blackwell, T.S.; Blackwell, T.R.; Holden, E.P.; Christman, B.W.; Christman, J.W. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J. Immunol. 1996, 157, 1630–1637. [Google Scholar] [CrossRef]
- Desaki, M.; Takizawa, H.; Kasama, T.; Kobayashi, K.; Morita, Y.; Yamamoto, K. Nuclear factor-kappa b activation in silica-induced interleukin 8 production by human bronchial epithelial cells. Cytokine 2000, 12, 1257–1260. [Google Scholar] [CrossRef]
- Hsu, B.G.; Lee, R.P.; Yang, F.L.; Harn, H.J.; Chen, H.I. Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci. 2006, 79, 2010–2016. [Google Scholar] [CrossRef]
- Palacio, J.R.; Markert, U.R.; Martínez, P. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm. Res. 2011, 60, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Behera, P.; Parida, D.; Mohapatra, A.P.; Das, S.K.; Kumari, S.; Avula, K.; Mohapatra, A.; Syed, G.H.; Senapati, S. Therapeutic role of N-acetyl cysteine (NAC) for the treatment and/or management of SARS-CoV-2-induced lung damage in hamster model. Eur. J. Pharmacol. 2023, 938, 175392. [Google Scholar] [CrossRef]
- Rincon, M.; Irvin, C.G. Role of IL-6 in Asthma and Other Inflammatory Pulmonary Diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Grubek-Jaworska, H.; Paplińska, M.; Hermanowicz-Salamon, J.; Białek-Gosk, K.; Dąbrowska, M.; Grabczak, E.; Domagała-Kulawik, J.; Stępień, J.; Chazan, R. IL-6 and IL-13 in induced sputum of COPD and asthma patients: Correlation with respiratory tests. Respiration 2012, 84, 101–107. [Google Scholar] [CrossRef]
- Bucchioni, E.; Kharitonov, S.A.; Allegra, L.; Barnes, P.J. High levels of interleukin-6 in the exhaled breath condensate of patients with COPD. Respir. Med. 2003, 97, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Seemungal, T.A.; Sapsford, R.J.; Wedzicha, J.A. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000, 55, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Hussein, F.G.M.; Mohammed, R.S.; Khattab, R.A.; Al-Sharawy, L.A. Serum interleukin-6 in chronic obstructive pulmonary disease patients and its relation to severity and acute exacerbation. Egypt. J. Bronchol. 2022, 16, 10. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Zeng, K.; Deng, F.; Lin, C.; Huang, W. Interleukin-6 is a Strong Predictor of the Frequency of COPD Exacerbation within 1 Year. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2945–2951. [Google Scholar] [CrossRef]
- Wilkinson, T.M.A.; Hurst, J.R.; Perera, W.R.; Wilks, M.; Donaldson, G.C.; Wedzicha, J.A. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 2006, 129, 317–324. [Google Scholar] [CrossRef]
- Sadowska, A.M.; Manuel-Y-Keenoy, B.; De Backer, W.A. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose-effects: A review. Pulm. Pharmacol. Ther. 2007, 20, 9–22. [Google Scholar] [CrossRef]
- Calzetta, L.; Luongo, L.; Cazzola, M.; Page, C.; Rogliani, P.; Facciolo, F.; Maione, S.; Capuano, A.; Rinaldi, B.; Matera, M.G. Contribution of sensory nerves to LPS-induced hyperresponsiveness of human isolated bronchi. Life Sci. 2015, 131, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Soggiu, A.; Roncada, P.; Bonizzi, L.; Pistocchini, E.; Urbani, A.; Rinaldi, B.; Matera, M.G. Propofol protects against opioid-induced hyperresponsiveness of airway smooth muscle in a horse model of target-controlled infusion anaesthesia. Eur. J. Pharmacol. 2015, 765, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Page, C.P.; Rinaldi, B.; Capuano, A.; Matera, M.G. Protein prenylation contributes to the effects of LPS on EFS-induced responses in human isolated bronchi. Am. J. Respir. Cell Mol. Biol. 2011, 45, 704–710. [Google Scholar] [CrossRef] [PubMed]
- De Laurentiis, A.; Candolfi, M.; Pisera, D.; Seilicovich, A. Effects of lipopolysaccharide on neurokinin A content and release in the hypothalamic-pituitary axis. Regul. Pept. 2003, 111, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Faryabi, R.; Mozaffari, H.; Darooghegi Mofrad, M. The effects of N-Acetylcysteine on serum level of inflammatory biomarkers in adults. Findings from a systematic review and meta-analysis of randomized clinical trials. Cytokine 2020, 135, 155239. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.S.; Seemungal, T.A.; Wilks, M.; Lloyd-Owen, S.J.; Donaldson, G.C.; Wedzicha, J.A. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002, 57, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Page, C.; Rossolini, G.M.; Pallecchi, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016, 117, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Hassett, D.J.; Borchers, M.T.; Panos, R.J. Chronic obstructive pulmonary disease (COPD): Evaluation from clinical, immunological and bacterial pathogenesis perspectives. J. Microbiol. 2014, 52, 211–226. [Google Scholar] [CrossRef]
- Eldika, N.; Sethi, S. Role of nontypeable Haemophilus influenzae in exacerbations and progression of chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2006, 12, 118–124. [Google Scholar] [CrossRef]
- Martínez-Solano, L.; Macia, M.D.; Fajardo, A.; Oliver, A.; Martinez, J.L. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin. Infect. Dis. 2008, 47, 1526–1533. [Google Scholar] [CrossRef]
- Amaral, E.P.; Conceição, E.L.; Costa, D.L.; Rocha, M.S.; Marinho, J.M.; Cordeiro-Santos, M.; D’Império-Lima, M.R.; Barbosa, T.; Sher, A.; Andrade, B.B. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 2016, 16, 251. [Google Scholar] [CrossRef]
- Lea, J.; Conlin, A.E.; Sekirov, I.; Restelli, V.; Ayakar, K.G.; Turnbull, L.; Doyle, P.; Noble, M.; Rennie, R.; Schreiber, W.E.; et al. In vitro efficacy of N-acetylcysteine on bacteria associated with chronic suppurative otitis media. J. Otolaryngol.-Head Neck Surg. 2014, 43, 20. [Google Scholar] [CrossRef]
- del Prado, G.; Ruiz, V.; Naves, P.; Rodríguez-Cerrato, V.; Soriano, F.; del Carmen Ponte, M. Biofilm formation by Streptococcus pneumoniae strains and effects of human serum albumin, ibuprofen, N-acetyl-l-cysteine, amoxicillin, erythromycin, and levofloxacin. Diagn. Microbiol. Infect. Dis. 2010, 67, 311–318. [Google Scholar] [CrossRef]
- Aslam, S.; Darouiche, R.O. Role of antibiofilm-antimicrobial agents in controlling device-related infections. Int. J. Artif. Organs 2011, 34, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Riise, G.C.; Qvarfordt, I.; Larsson, S.; Eliasson, V.; Andersson, B.A. Inhibitory effect of N-acetylcysteine on adherence of Streptococcus pneumoniae and Haemophilus influenzae to human oropharyngeal epithelial cells in vitro. Respiration 2000, 67, 552–558. [Google Scholar] [CrossRef]
- Zheng, J.P.; Wen, F.Q.; Bai, C.X.; Wan, H.Y.; Kang, J.; Chen, P.; Yao, W.Z.; Ma, L.J.; Li, X.; Raiteri, L.; et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): A randomised, double-blind placebo-controlled trial. Lancet Respir. Med. 2014, 2, 187–194. [Google Scholar] [CrossRef]
- Aylward, M.; Maddock, J.; Dewland, P. Clinical evaluation of acetylcysteine in the treatment of patients with chronic obstructive bronchitis: A balanced double-blind trial with placebo control. Eur. J. Respir. Dis. Suppl. 1980, 111, 81–89. [Google Scholar] [PubMed]
- Pela, R.; Calcagni, A.M.; Subiaco, S.; Isidori, P.; Tubaldi, A.; Sanguinetti, C.M. N-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD. Respiration 1999, 66, 495–500. [Google Scholar] [CrossRef]
- Decramer, M.; Rutten-van Mölken, M.; Dekhuijzen, P.R.; Troosters, T.; van Herwaarden, C.; Pellegrino, R.; van Schayck, C.O.; Olivieri, D.; Del Donno, M.; De Backer, W.; et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet 2005, 365, 1552–1560. [Google Scholar] [CrossRef]
- Fowdar, K.; Chen, H.; He, Z.; Zhang, J.; Zhong, X.; Zhang, J.; Li, M.; Bai, J. The effect of N-acetylcysteine on exacerbations of chronic obstructive pulmonary disease: A meta-analysis and systematic review. Heart Lung 2017, 46, 120–128. [Google Scholar] [CrossRef]
- Schermer, T.; Chavannes, N.; Dekhuijzen, R.; Wouters, E.; Muris, J.; Akkermans, R.; van Schayck, O.; van Weel, C. Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respir. Med. 2009, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.N.; Raiteri, L.; Wong, K.Y.; Yee, K.S.; Ng, L.Y.; Wai, K.Y.; Loo, C.K.; Chan, M.H. High-dose N-acetylcysteine in stable COPD: The 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest 2013, 144, 106–118. [Google Scholar] [CrossRef]
- Shen, Y.; Cai, W.; Lei, S.; Zhang, Z. Effect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: A systematic review and meta-analysis. COPD J. Chronic Obstr. Pulm. Dis. 2014, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Page, C.; Jardim, J.; Chuchalin, A.G.; Rogliani, P.; Matera, M.G. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: A meta-analysis. Eur. Respir. Rev. 2015, 24, 451–461. [Google Scholar] [CrossRef]
- Cazzola, M.; Rogliani, P.; Calzetta, L.; Hanania, N.A.; Matera, M.G. Impact of Mucolytic Agents on COPD Exacerbations: A Pair-wise and Network Meta-analysis. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Stey, C.; Steurer, J.; Bachmann, S.; Medici, T.C.; Tramèr, M.R. The effect of oral N-acetylcysteine in chronic bronchitis: A quantitative systematic review. Eur. Respir. J. 2000, 16, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, E.M.; Berthet, P.; Ruffmann, R.; Leuenberger, P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: A meta-analysis of published double-blind, placebo-controlled clinical trials. Clin. Ther. 2000, 22, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Alfano, F.; Bigoni, T.; Mancini, L.; Mawass, A.; Baraldi, F.; Aljama, C.; Contoli, M.; Miravitlles, M. N-acetylcysteine Treatment in Chronic Obstructive Pulmonary Disease (COPD) and Chronic Bronchitis/Pre-COPD: Distinct Meta-analyses. Arch. Bronconeumol. 2024, 60, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Poole, P.; Chong, J.; Cates, C.J. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 7, CD001287. [Google Scholar] [CrossRef]
- Poole, P.J.; Black, P.N. Oral mucolytic drugs for exacerbations of chronic obstructive pulmonary disease: Systematic review. BMJ 2001, 322, 1271–1274. [Google Scholar] [CrossRef]
- Mokra, D.; Mokry, J.; Barosova, R.; Hanusrichterova, J. Advances in the Use of N-Acetylcysteine in Chronic Respiratory Diseases. Antioxidants 2023, 12, 1713. [Google Scholar] [CrossRef] [PubMed]
- Maghsadi, Z.; Azadmehr, A.; Moghadamnia, A.A.; Feizi, F.; Hamidi, N. N-Acetylcysteine attenuated pulmonary fibrosis induced by bleomycin via immunomodulation responses. Res. Pharm. Sci. 2023, 18, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zhang, J.; Wang, Z.; Wu, Q.; Zhou, X. Efficacy and safety of N-acetylcysteine therapy for idiopathic pulmonary fibrosis: An updated systematic review and meta-analysis. Exp. Ther. Med. 2019, 18, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Idiopathic Pulmonary Fibrosis Clinical Research Network; Martinez, F.J.; de Andrade, J.A.; Anstrom, K.J.; King, T.E.; Raghu, G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Calzetta, L.; Cavalli, F.; Matera, M.G.; Cazzola, M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm. Pharmacol. Ther. 2016, 40, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Podolanczuk, A.J.; Noth, I.; Raghu, G. Idiopathic pulmonary fibrosis: Prime time for a precision-based approach to treatment with N-acetylcysteine. Eur. Respir. J. 2021, 57, 2003551. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Z.; Kotuk, M.; Iraz, M.; Kuku, I.; Ulu, R.; Armutcu, F.; Ozen, S. Attenuation of bleomycin-induced lung fibrosis by oral sulfhydryl containing antioxidants in rats: Erdosteine and N-acetylcysteine. Pulm. Pharmacol. Ther. 2005, 18, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Oldham, J.M.; Ma, S.F.; Martinez, F.J.; Anstrom, K.J.; Raghu, G.; Schwartz, D.A.; Valenzi, E.; Witt, L.; Lee, C.; Vij, R.; et al. TOLLIP, MUC5B, and the Response to N-Acetylcysteine among Individuals with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2015, 192, 1475–1482. [Google Scholar] [CrossRef]
- Qi, Q.; Ailiyaer, Y.; Liu, R.; Zhang, Y.; Li, C.; Liu, M.; Wang, X.; Jing, L.; Li, Y. Effect of N-acetylcysteine on exacerbations of bronchiectasis (BENE): A randomized controlled trial. Respir. Res. 2019, 20, 73. [Google Scholar] [CrossRef]
- Jayaram, L.; King, P.T.; Hunt, J.; Lim, M.; Park, C.; Hu, E.; Dousha, L.; Ha, P.; Bartlett, J.B.; Southcott, A.M.; et al. Evaluation of high dose N-Acetylcysteine on airway inflammation and quality of life outcomes in adults with bronchiectasis: A randomised placebo-controlled pilot study. Pulm. Pharmacol. Ther. 2024, 84, 102283. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, Y.; Zi, K.; Shen, Y.; Wang, T.; Qin, J.; Chen, L.; Chen, M.; Liu, L.; Li, W.; et al. The effect of N-acetylcysteine in patients with non-cystic fibrosis bronchiectasis (NINCFB): Study protocol for a multicentre, double-blind, randomised, placebo-controlled trial. BMC Pulm. Med. 2022, 22, 401. [Google Scholar] [CrossRef] [PubMed]
- Guerini, M.; Condrò, G.; Friuli, V.; Maggi, L.; Perugini, P. N-acetylcysteine (NAC) and Its Role in Clinical Practice Management of Cystic Fibrosis (CF): A Review. Pharmaceuticals 2022, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Skov, M.; Pressler, T.; Lykkesfeldt, J.; Poulsen, H.E.; Jensen, P.Ø.; Johansen, H.K.; Qvist, T.; Kræmer, D.; Høiby, N.; Ciofu, O. The effect of short-term, high-dose oral N-acetylcysteine treatment on oxidative stress markers in cystic fibrosis patients with chronic P. aeruginosa infection—A pilot study. J. Cyst. Fibros. 2015, 14, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Lymp, J.; Thompson, V.; Dunn, C.; Davies, Z.; Chatfield, B.; Nichols, D.; Clancy, J.; Vender, R.; Egan, M.E.; et al. Long-term treatment with oral N-acetylcysteine: Affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J. Cyst. Fibros. 2015, 14, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Tirouvanziam, R.; Conrad, C.K.; Bottiglieri, T.; Herzenberg, L.A.; Moss, R.B.; Herzenberg, L.A. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4628–4633. [Google Scholar] [CrossRef]
- Santus, P.; Danzo, F.; Zuffi, A.; Pini, S.; Saad, M.; Visconti, A.; Radovanovic, D. Oxidative stress and viral Infections: Rationale, experiences, and perspectives on N-acetylcysteine. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8582–8590. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santus, P.; Signorello, J.C.; Danzo, F.; Lazzaroni, G.; Saad, M.; Radovanovic, D. Anti-Inflammatory and Anti-Oxidant Properties of N-Acetylcysteine: A Fresh Perspective. J. Clin. Med. 2024, 13, 4127. https://doi.org/10.3390/jcm13144127
Santus P, Signorello JC, Danzo F, Lazzaroni G, Saad M, Radovanovic D. Anti-Inflammatory and Anti-Oxidant Properties of N-Acetylcysteine: A Fresh Perspective. Journal of Clinical Medicine. 2024; 13(14):4127. https://doi.org/10.3390/jcm13144127
Chicago/Turabian StyleSantus, Pierachille, Juan Camilo Signorello, Fiammetta Danzo, Giada Lazzaroni, Marina Saad, and Dejan Radovanovic. 2024. "Anti-Inflammatory and Anti-Oxidant Properties of N-Acetylcysteine: A Fresh Perspective" Journal of Clinical Medicine 13, no. 14: 4127. https://doi.org/10.3390/jcm13144127




