Revisiting Latex-Fruit Syndrome after 30 Years of Research: A Comprehensive Literature Review and Description of Two Cases
Abstract
:1. Introduction
2. Natural Rubber Latex (NRL) Allergy
3. Latex and Its Allergens
4. Definition of Latex-Fruit Syndrome
5. Diagnosis of LFS
- -
- The symptoms of an allergy to fruit;
- -
- The symptoms appearing in an occupational setting;
- -
- The role of potential cofactors.
6. Materials and Methods
7. Results
- What is the prevalence of latex-fruit syndrome?
- What are the most prevalent cross-reactions with fruit allergens in subjects with latex-fruit syndrome?
- What is the clinical manifestation of latex-fruit syndrome?
8. Epidemiology of LFS
| Autor | Number of Participants | Country | Tested Allergens | The Most Prevalent Allergenic Fruit | Prevalence of LFS (Based on…) | |
|---|---|---|---|---|---|---|
| 1 | Mäkinen-Kiljunen 1994 [53] | n = 47 | Finland | Latex, Banana | Banana | 52% (History) 35% (SPT) |
| 2 | Lavaud et al. 1995 [55] | n = 17 | France | Latex, Banana, Avocado | Avocado | 59% (History + SPT) |
| 3 | Delbourg et al. 1996 [56] | n = 19 | France | Latex, Banana | Banana | 50% (History) 36% (SPT) |
| 4 | Alenius et al. 1996 [57] | n = 22 | Finland | Latex, Banana | Banana | 45% (Immunobloting) 78% (SPT) |
| 5 | Beezhold et al. 1996 [58] | n = 47 | Canada | Avocado, Potato, Banana, Tomato, Chestnut, Kiwi, Pineapple, Milk | Avocado | 27% (History + SPT) 70% (SPT) |
| 6 | Brehler et al. 1997 [50] | n = 136 | Germany | Latex, Papaya, Papain, Avocado, Chestnut, Banana, Ficus Spp., Passion Fruit, Melon, Mango, Kiwi, Peach, Pineapple, Tomato, Guava | Banana and kiwi | 42.6% (History) 69.1% (sIgE) 32.1% (History + sIgE) |
| 7 | Kim and Hussain 1999 [59] | n = 137 | USA | Latex, Banana, Avocado, Kiwi, Tomato, Watermelon, Peach, Carrot, Apple, Chestnut, Cherry, Coconut, Apricot, Strawberry, Loquat | Banana | 21% (History) |
| 8 | Levy et al. 2000 [60] | n = 24 | France | Avocado, Banana, Apple, Peach Celery, Kiwi, Mango, Tomato, Chestnut, Cantaloupe, Pineapple, Papaya | Banana | 46% (SPT) |
| n = 20 | Papaya | 24% (SPT) | ||||
| 9 | Chen and Lan 2002 [2] | n = 26 | Taiwan | Latex, Avocado, Apple, Pear, Kiwi, Papaya, Pineapple, Peach, Cherry, Plum, Apricot, Banana, Melon, Nectarine, Grape, Fig, Passion Fruit, Tomatoes, Celery, Carrot, Hazelnut, Chestnut, Potatoes | Unknown | 26.9% (History + IgE) |
| 10 | Ebo et al. 2003 [49] | n = 42 | Belgium | Avocado, Banana, Chestnut, Fig, Kiwi, Papaya, Peanut, Pineapple, Tomato, Ficus Benjamina | Papaya (sIgE) Banana (history) | 88% (sIgE) 58% (History) |
| 11 | Isola et al. 2003 [61] | n = 82 | Italy | Kiwi, Banana, Avocado, Papaya | Kiwi | 47.5% (Skin test) |
| 12 | Radauer et al. 2011 [62] | n = 34 | Austria | Banana, Avocado | Banana | 44% (History) |
| 13 | Ricci et al. 2013 [54] | n = 22 | Italy | Latex, Kiwi, Chestnut, Peach, Cherry, Apple, Melon | Kiwi | 36% (History + sIgE) |
| 14 | Santos et al. 2018 [52] | n = 150 | Brazil | Banana, Latex | Banana | 12.7% (SPT) 4% (History) |

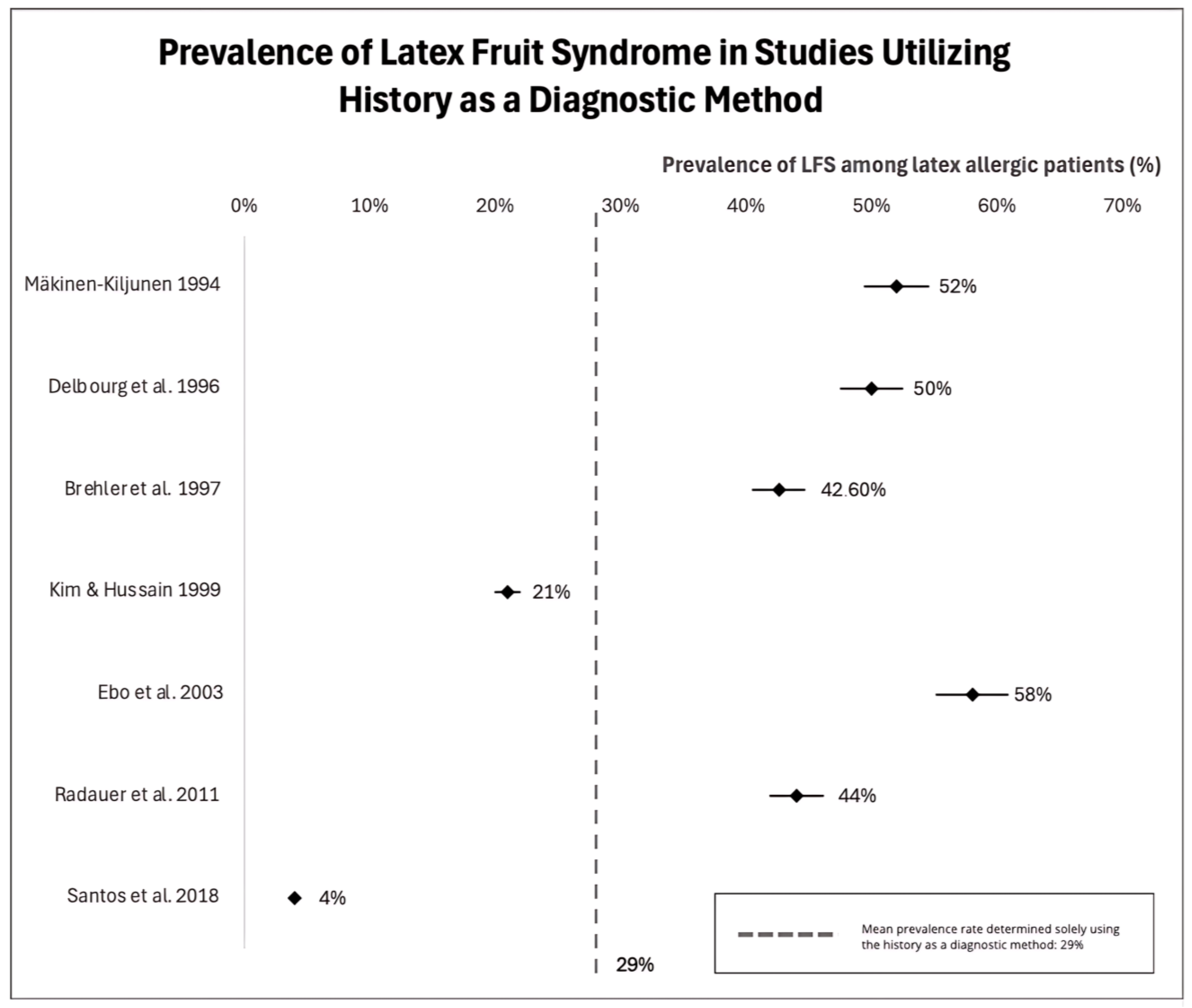
9. The Most Prevalent Allergenic Fruit in Individuals with LFS
10. Clinical Picture of LFS
11. Case Reports
- Patient #1
- Patient #2
12. Differences between Grading Systems
13. Limitations
14. Future Perspectives
- I.
- After three decades of thorough investigation into LFS, this syndrome presents a potential for additional investigation and comprehension. The continuous study and analysis of LFS have paved the way for plenty of opportunities for deeper exploration and insight into this field.
- →
- Given the heterogeneity in the prevalence rates documented in previous studies, it has become imperative to advocate for the implementation of epidemiological examinations that comprise a diverse range of populations. Future investigations should establish consistent and comparable diagnostic criteria while evaluating hypersensitivity to fruits among latex-allergic patients. These investigations ought to encompass examinations for a spectrum of allergens that can potentially induce hypersensitivity reactions. The wide range of available fruit allergens allows for the selection of individual allergens for testing by SPT and serum sIgE measurement. This allows for a more efficient diagnostic management of LFS.
- →
- Expanding the diagnosis with relevant allergenic components of fruits in the diagnostic process can play a crucial role in pinpointing the specific molecules responsible for LFS and exposing potential cross-reactive patterns. Such an approach can not only reinforce the diagnostic accuracy, but also help with creating therapeutic strategies tailored to an individual patient’s needs.
- →
- Raising awareness among healthcare professionals about the connection between a latex allergy and fruit hypersensitivity is vital for proper diagnosis and subsequent treatment. It is essential to increase the awareness of the healthcare professionals about the allergen components involved in LFS, facilitating an accurate diagnosis and appropriate treatment strategies.
- →
- In addition, future research that examines the specific characteristics of allergen molecules and, in the future, on epitopes involved in LFS should provide a more profound understanding of this syndrome and support the development of better diagnostic tools and treatment options.
- →
- Improving the warnings and labeling of latex items may aid in averting inadvertent contact and reducing the likelihood of allergic responses in individuals with LFS. Consequently, the implementation of a singular, globally recognized warning icon on products that include latex is worth considering.
- →
- Enhancing the accessibility of latex alternatives such as polyurethane and polyisoprene plays a pivotal role in diminishing the likelihood of allergic responses within the demographic of both healthcare practitioners and individuals who frequently encounter latex-based items. By implementing these substitutes, the healthcare industry could effectively mitigate the risks posed by latex allergies, thereby ensuring a conducive and risk-free environment for both medical professionals and patients alike.
- II.
- Furthermore, addressing significant gaps in systemic allergy grading systems is imperative for standardizing clinical assessment and management practices.
- →
- Implementing a standardized tool for assessing generalized allergic reactions in hospital Emergency Departments (EDs) and allergology units is crucial for enhancing communication and ensuring prompt interventions. Moreover, developing a globally adaptable anaphylaxis reporting form based on established grading systems should enable consistent documentation and robust data collection for epidemiological analysis and quality improvement efforts in Hospital EDs.
- →
- The primacy of epinephrine in anaphylaxis management should be kept in mind by medical personnel, regardless of the grading scale. To achieve the best patient outcomes in LFS and related allergies, it is vital to ensure familiarity with epinephrine administration techniques and prioritize ongoing education and training initiatives.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanco, C.; Carrillo, T.; Castillo, R.; Quiralte, J.; Cuevas, M. Latex allergy: Clinical features and cross-reactivity with fruits. Ann. Allergy 1994, 73, 4. [Google Scholar]
- Chen, Y.H.; Lan, J.L. Latex allergy and latex-fruit syndrome among medical workers in Taiwan. J. Formos. Med. Assoc. 2002, 101, 9. [Google Scholar]
- Parisi, C.A.S.; Kelly, K.J.; Ansotegui, I.J.; Gonzalez-Díaz, S.N.; Bilò, M.B.; Cardona, V.; Park, H.S.; Braschi, M.C.; Macias-Weinmann, A.; Piga, M.A.; et al. Update on latex allergy: New insights into an old problem. World Allergy Organ. J. 2021, 14, 100569. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; McIntosh, J.; Liu, J. Current prevalence rate of latex allergy: Why it remains a problem? J. Occup. Health 2016, 58, 2. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Kohli, A. Latex Allergy. StatPearls. July 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545164/ (accessed on 13 July 2023).
- Nucera, E.; Aruanno, A.; Rizzi, A.; Centrone, M. Latex allergy: Current status and future perspectives. J. Asthma Allergy 2020, 13, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Sommergruber, K.; Hilger, C.; Santos, A.; De Las Vecillas, L.; Dramburg, S. Molecular Allergology User’s Guide 2.0; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Mühl-Benninghaus, R. Spina bifida [Spina bifida]. Radiologe 2018, 58, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Šutovský, J. Surgical Treatment of Neural Tube Defects. In Spina Bifida and Craniosynostosis—New Perspectives and Clinical Applications; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Ebo, D.G.; Bridts, C.H.; Rihs, H.P. Hevea latex-associated allergies: Piecing together the puzzle of the latex IgE reactivity profile. Expert Rev. Mol. Diagn. 2020, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Gracz-Bernaciak, J.; Mazur, O.; Nawrot, R. Functional Studies of Plant Latex as a Rich Source of Bioactive Compounds: Focus on Proteins and Alkaloids. Int. J. Mol. Sci. 2021, 22, 12427. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. S23), 1–250. [Google Scholar] [CrossRef]
- Allergen Search Results. Available online: https://allergen.org/search.php?allergenname=&allergensource=latex&TaxSource=&TaxOrder=&foodallerg=all&bioname= (accessed on 28 May 2024).
- Yeang, H.Y.; Cheong, K.F.; Sunderasan, E.; Hamzah, S.; Chew, N.P.; Hamid, S.; Hamilton, R.G.; Cardosa, M.J. The 14.6 kd rubber elongation factor (Hev b 1) and 24 kd (Hev b 3) rubber particle proteins are recognized by IgE from patients with spina bifida and latex allergy. J. Allergy Clin. Immunol. 1996, 98, 628–639. [Google Scholar] [CrossRef]
- Hev b 1 Allergen Details. Available online: https://allergen.org/viewallergen.php?aid=355 (accessed on 26 May 2024).
- Barre, A.; Culerrier, R.; Granier, C.; Selman, L.; Peumans, W.J.; Van Damme, E.J.; Bienvenu, F.; Bienvenu, J.; Rougé, P. Mapping of IgE-binding epitopes on the major latex allergen Hev b 2 and the cross-reacting 1,3beta-glucanase fruit allergens as a molecular basis for the latex-fruit syndrome. Mol. Immunol. 2009, 46, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Hev b 3 Allergen Details. Available online: https://allergen.org/viewallergen.php?aid=361 (accessed on 26 May 2024).
- Malik, A.; Arif, S.A.M.; Ahmad, S.; Sunderasan, E. A molecular and in silico characterization of Hev b 4, a glycosylated latex allergen. Int. J. Biol. Macromol. 2008, 42, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Hev b 4 Allergen Details. Available online: https://allergen.org/viewallergen.php?aid=362 (accessed on 26 May 2024).
- Lehto, M.; Kotovuori, A.; Palosuo, K.; Varjonen, E.; Lehtimäki, S.; Kalkkinen, N.; Palosuo, T.; Reunala, T.; Alenius, H. Hev b 6.01 and Hev b 5 induce pro-inflammatory cytokines and chemokines from peripheral blood mononuclear cells in latex allergy. Clin. Exp. Allergy 2007, 37, 133–140. [Google Scholar] [CrossRef]
- Yeang, H.Y.; Arif, S.A.; Raulf-Heimsoth, M.; Loke, Y.H.; Sander, I.; Sulong, S.H.; Lau, C.H.; Hamilton, R.G. Hev b 5 and Hev b 13 as allergen markers to estimate the allergenic potency of latex gloves. J. Allergy Clin. Immunol. 2004, 114, 593–598. [Google Scholar] [CrossRef]
- Chen, Z.; Posch, A.; Cremer, R.; Raulf-Heimsoth, M.; Baur, X. Identification of hevein (Hev b 6.02) in Hevea latex as a major cross-reacting allergen with avocado fruit in patients with latex allergy. J. Allergy Clin. Immunol. 1998, 102, 3. [Google Scholar] [CrossRef]
- Yagami, A.; Suzuki, K.; Saito, H.; Matsunaga, K. Hev b 6.02 Is the Most Important Allergen in Health Care Workers Sensitized Occupationally by Natural Rubber Latex Gloves. Allergol. Int. 2009, 58, 347–355. [Google Scholar] [CrossRef]
- Raulf-Heimsoth, M.; Rozynek, P.; Brüning, T.; Rihs, H.P. Characterization of B- and T-cell responses and HLA-DR4 binding motifs of the latex allergen Hev b 6.01 (prohevein) and its post-transcriptionally formed proteins Hev b 6.02 and Hev b 6.03. Allergy 2004, 59, 724–733. [Google Scholar] [CrossRef]
- Wagner, B.; Buck, D.; Hafner, C.; Sowka, S.; Niggemann, B.; Scheiner, O.; Breiteneder, H. Hev b 7 is a Hevea brasiliensis protein associated with latex allergy in children with spina bifida. J. Allergy Clin. Immunol. 2001, 108, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Hev b 7 Allergen Details. Available online: https://allergen.org/viewallergen.php?aid=365 (accessed on 26 May 2024).
- Ganglberger, E.; Radauer, C.; Wagner, S.; Ríordáin, G.; Beezhold, D.H.; Brehler, R.; Niggemann, B.; Scheiner, O.; Jensen-Jarolim, E.; Breiteneder, H. Hev b 8, the Hevea brasiliensis latex profilin, is a cross-reactive allergen of latex, plant foods and pollen. Int. Arch. Allergy Immunol. 2001, 125, 216–227. [Google Scholar] [CrossRef]
- Allergome—Hev b 8. Available online: http://allergome.com/script/dettaglio_native.php?id_molecule=397&id_native=279 (accessed on 4 June 2024).
- Wagner, S.; Breiteneder, H.; Simon-Nobbe, B.; Susani, M.; Krebitz, M.; Niggemann, B.; Brehler, R.; Scheiner, O.; Hoffmann-Sommergruber, K. Hev b 9, an enolase and a new cross-reactive allergen from hevea latex and molds. Purification, characterization, cloning and expression. Eur. J. Biochem. 2000, 267, 7006–7014. [Google Scholar] [CrossRef]
- Hev b 9 Allergen Details. Available online: https://allergen.org/viewallergen.php?aid=367 (accessed on 26 May 2024).
- Hev b 10 Allergen Details. Available online: https://allergen.org/viewallergen.php?aid=356 (accessed on 26 May 2024).
- Wagner, S.; Sowka, S.; Mayer, C.; Crameri, R.; Focke, M.; Kurup, V.P.; Scheiner, O.; Breiteneder, H. Identification of a Hevea brasiliensis latex manganese superoxide dismutase (Hev b 10) as a cross-reactive allergen. Int. Arch. Allergy Immunol. 2001, 125, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hev b 11 Allergen Details. Available online: https://allergen.org/viewallergen.php?aid=357 (accessed on 26 May 2024).
- O’Riordain, G.; Radauer, C.; Hoffmann-Sommergruber, K.; Adhami, F.; Peterbauer, C.K.; Blanco, C.; Godnic-Cvar, J.; Scheiner, O.; Ebner, C.; Breiteneder, H. Cloning and molecular characterization of the Hevea brasiliensis allergen Hev b 11, a class I chitinase. Clin. Exp. Allergy 2002, 32, 455–462. [Google Scholar] [CrossRef]
- Barre, A.; Van Damme, E.J.M.; Simplicien, M.; Benoist, H.; Rougé, P. Are Dietary Lectins Relevant Allergens in Plant Food Allergy? Foods 2020, 9, 1724. [Google Scholar] [CrossRef]
- Beezhold, D.H.; Hickey, V.L.; Kostyal, D.A.; Puhl, H.; Zuidmeer, L.; van Ree, R.; Sussman, G.L. Lipid transfer protein from Hevea brasiliensis (Hev b 12), a cross-reactive latex protein. Ann. Allergy Asthma Immunol. 2003, 90, 439–445. [Google Scholar] [CrossRef]
- Hev b 12 Allergen Details. Available online: https://allergen.org/viewallergen.php?aid=358 (accessed on 26 May 2024).
- Teixeira, L.D.B.; Epifânio, V.L.A.A.; Lachat, J.J.; Foss, N.T.; Coutinho-Netto, J. Oral treatment with Hev b 13 prevents experimental arthritis in mice. Clin. Exp. Immunol. 2012, 168, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Hev b 13 Allergen Details. Available online: https://allergen.org/viewallergen.php?aid=359 (accessed on 26 May 2024).
- Hev b 14 Allergen Details. Available online: https://allergen.org/viewallergen.php?aid=691 (accessed on 26 May 2024).
- Rihs, H.P.; Sander, I.; Heimann, H.; Meurer, U.; Brüning, T.; Raulf, M. The new latex allergen Hev b 15: IgE-binding properties of a recombinant serine protease inhibitor. J. Investig. Allergol. Clin. Immunol. 2015, 25, 160–162. [Google Scholar]
- Blanco, C. Latex-fruit syndrome. Curr. Allergy Asthma Rep. 2003, 3, 1. [Google Scholar] [CrossRef]
- Posch, A.; Wheeler, C.H.; Chen, Z.; Flagge, A.; Dunn, M.J.; Papenfuss, F.; Raulf-Heimsoth, M.; Baur, X. Class I endochitinase containing a hevein domain is the causative allergen in latex-associated avocado allergy. Clin. Exp. Allergy 1999, 29, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.H.H.; Raulf-Heimsoth, M.; Posch, A. Evaluation of patatin as a major cross-reactive allergen in latex-induced potato allergy. Ann. Allergy Asthma Immunol. 2002, 89, 6. [Google Scholar] [CrossRef]
- Raulf-Heimsoth, M.; Kespohl, S.; Crespo, J.F.; Rodriguez, J.; Feliu, A.; Brüning, T.; Rihs, H.P. Natural rubber latex and chestnut allergy: Cross-reactivity or co-sensitization? Allergy 2007, 62, 1277–1281. [Google Scholar] [CrossRef]
- Rihs, H.P.; Ruëff, F.; Lundberg, M.; Rozynek, P.; Barber, D.; Scheurer, S.; Cisteró-Bahima, A.; Brüning, T.; Raulf-Heimsoth, M. Relevance of the recombinant lipid transfer protein of Hevea brasiliensis: IgE-binding reactivity in fruit-allergic adults. Ann. Allergy Asthma Immunol. 2006, 97, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; Sánchez-García, F.; Torres-Galván, M.J.; Dumpierrez, A.G.; Almeida, L.; Figueroa, J.; Ortega, N.; Castillo, R.; Gallego, M.D.; Carrillo, T. Genetic basis of the latex-fruit syndrome: Association with HLA class II alleles in a Spanish population. J. Allergy Clin. Immunol. 2004, 114, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Bridts, C.; Hagendorens, M.M.; De Clerck, L.S.; Stevens, W.J. The prevalence and diagnostic value of specific IgE antibodies to inhalant, animal and plant food, and ficus allergens in patients with natural rubber latex allergy. Acta Clin. Belg. 2003, 58, 3. [Google Scholar] [CrossRef] [PubMed]
- Brehler, R.; Theissen, U.; Mohr, C.; Luger, T. Latex-fruit syndrome: Frequency of cross-reacting IgE antibodies. Allergy 1997, 52, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, J.H.; Alenius, H.; Kalkkinen, N.; Turjanmaa, K.; Palosuo, T.; Reunala, T. Hevein-like protein domains as a possible cause for allergen cross-reactivity between latex and banana. J. Allergy Clin. Immunol. 1998, 102, 1005–1012. [Google Scholar] [CrossRef]
- Dos Santos, A.N.F.; De Jesus Santos, J.M.; Oliveira, F.M. Skin hypersensitivity to banana and risk for latex-fruit syndrome in healthcare professionals. Mundo da Saude 2018, 42, 1. [Google Scholar] [CrossRef]
- Mäkinen-Kiljunen, S. Banana allergy in patients with immediate-type hypersensitivity to natural rubber latex: Characterization of cross-reacting antibodies and allergens. J. Allergy Clin. Immunol. 1994, 93, 990–996. [Google Scholar] [CrossRef]
- Ricci, G.; Piccinno, V.; Calamelli, E.; Giannetti, A.; Pession, A. Latex-fruit syndrome in Italian children and adolescents with natural rubber latex allergy. Int. J. Immunopathol. Pharmacol. 2013, 26, 263–268. [Google Scholar] [CrossRef]
- Lavaud, F.; Prevost, A.; Cossart, C.; Guerin, L.; Bernard, J.; Kochman, S. Allergy to latex, avocado pear, and banana: Evidence for a 30 kD antigen in immunoblotting. J. Allergy Clin. Immunol. 1995, 95, 2. [Google Scholar] [CrossRef]
- Delbourg, M.F.; Guilloux, L.; Moneret-Vautrin, D.A.; Ville, G. Hypersensitivity to banana in latex-allergic patients. Identification of two major banana allergens of 33 and 37 kD. Ann. Allergy Asthma Immunol. 1996, 76, 4. [Google Scholar] [CrossRef]
- Alenius, H.; Mäkinen-Kiljunen, S.; Ahlroth, M.; Turjanmaa, K.; Reunala, T.; Palosuo, T. Crossreactivity between allergens in natural rubber latex and banana studied by immunoblot inhibition. Clin. Exp. Allergy 1996, 26, 3. [Google Scholar] [CrossRef]
- Beezhold, D.H.; Sussman, G.L.; Liss, G.M.; Chang, N.S. Latex allergy can induce clinical reactions to specific foods. Clin. Exp. Allergy 1996, 26, 4. [Google Scholar] [CrossRef]
- KT, K.; HH, H. Prevalence of food allergy in 137 latex-allergic patients. Allergy Asthma Proc. 1999, 20, 95–97. [Google Scholar] [CrossRef]
- Levy, D.A.; Mounedji, N.; Noirot, C.; Leynadier, F. Allergic sensitization and clinical reactions to latex, food and pollen in adult patients. Clin. Exp. Allergy 2000, 30, 2. [Google Scholar] [CrossRef] [PubMed]
- Isola, S.; Ricciardi, L.; Saitta, S.; Fedele, R.; Mazzeo, L.; Fogliani, O.; Gangemi, S.; Purello-D’Ambrosio, F. Latex allergy and fruit cross-reaction in subjects who are nonatopic. Allergy Asthma Proc. 2003, 24, 193–197. [Google Scholar] [PubMed]
- Radauer, C.; Adhami, F.; Fürtler, I.; Wagner, S.; Allwardt, D.; Scala, E.; Ebner, C.; Hafner, C.; Hemmer, W.; Mari, A.; et al. Latex-allergic patients sensitized to the major allergen hevein and hevein-like domains of class I chitinases show no increased frequency of latex-associated plant food allergy. Mol. Immunol. 2011, 48, 600–609. [Google Scholar] [CrossRef]
- García-Menaya, J.M.; Cordobés-Durán, C.; Bobadilla-González, P.; Ledesma, A.; Pérez-Rangel, I.; Sánchez-Vega, S.; Zambonino, M.A.; Corrales-Vargas, S. Anaphylactic reaction to bell pepper (Capsicum annuum) in a patient with a latex-fruit syndrome. Allergol. Immunopathol. 2014, 42, 263–265. [Google Scholar] [CrossRef]
- Pereira, C.; Tavares, B.; Loureiro, G.; Lundberg, M.; Chieira, C. Turnip and zucchini: New foods in the latex-fruit syndrome. Allergy 2007, 62, 452–453. [Google Scholar] [CrossRef]
- Jalil, M.; Hostoffer, R.; Wu, S.S. Jackfruit Anaphylaxis in a Latex Allergic Non-Healthcare Worker. Allergy Rhinol. 2021, 12, 21526567211009195. [Google Scholar] [CrossRef]
- Sanchez, Z.A.; Santana, G.H.; Plata, E.R.; Tadeo, J.M.; Robaina, J.G.; Colino, C.G. Syndrome latex-fruit: Report of a case with cassava anaphylaxis. Clin. Transl. Allergy 2013, 3 (Suppl. S3), P155. [Google Scholar] [CrossRef]
- Leoni, C.; Volpicella, M.; Dileo, M.C.G.; Gattulli, B.A.R.; Ceci, L.R. Chitinases as food allergens. Molecules 2019, 24, 2087. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Ansotegui, I.J.; Campbell, D.E.; Cardona, V.; Carr, S.; Custovic, A.; Durham, S.; Ebisawa, M.; Geller, M.; Gonzalez-Estrada, A.; et al. Updated grading system for systemic allergic reactions: Joint Statement of the World Allergy Organization Anaphylaxis Committee and Allergen Immunotherapy Committee. World Allergy Organ. J. 2024, 17, 100876. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Behrendt, H. Anaphylaxis and anaphylactoid reactions: Classification and pathophysiology. Clin. Rev. Allergy Immunol. 1999, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Blazowski, L.; Majak, P.; Kurzawa, R.; Kuna, P.; Jerzynska, J. A severity grading system of food-induced acute allergic reactions to avoid the delay of epinephrine administration. Ann. Allergy Asthma Immunol. 2019, 127, 4. [Google Scholar] [CrossRef] [PubMed]
- Mueller, H.L. Diagnosis and treatment of insect sensitivity. J. Asthma 1966, 3, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A. Anaphylaxis and emergency treatment. Pediatrics 2003, 111, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.S.; Sanchez-Borges, M.; Lockey, R.F. World Allergy Organization Systemic Allergic Reaction Grading System: Is a Modification Needed? J. Allergy Clin. Immunol. Pract. 2017, 5, 1. [Google Scholar] [CrossRef]
- Dribin, T.E.; Schnadower, D.; Spergel, J.M.; Campbell, R.L.; Shaker, M.; Neuman, M.I.; Michelson, K.A.; Capucilli, P.S.; Camargo, C.A., Jr.; Brousseau, D.C.; et al. Severity grading system for acute allergic reactions: A multidisciplinary Delphi study. J. Allergy Clin. Immunol. 2021, 148, 173–181. [Google Scholar] [CrossRef]
| Molecule | Biochemical Name | Clinical Relevance | Cross-Reactivity | Route of Exposure | Reference |
|---|---|---|---|---|---|
| Hev b 1 | Rubber Elongation Factor (REF) | Major in children with spina bifida | Papain | Contact | [3,14,15] |
| Hev b 2 | Beta 1-3-glucanase | Minor | - | Contact | [16] |
| Hev b 3 | Small rubber particle protein (SRPP) | Major in children with spina bifida | - | Contact | [3,17] |
| Hev b 4 | Lecithinase homologue | Minor | - | Contact | [18,19] |
| Hev b 5 | Acidic structural protein | Major in healthcare professionals | Kiwi | Contact | [20,21] |
| Hev b 6.01 | Prohevein | Major healthcare workers and spina bifida | Banana, avocado | Contact | [3,20] |
| Hev b 6.02 | Hevein | - | Banana, avocado, chestnut | Contact | [22,23] |
| Hev b 6.03 | Hevein C | - | Contact | [24] | |
| Hev b 7 | Patatin-like protein | Minor | Solanaceae (tomato and potato) | Contact | [3,25,26] |
| Hev b 8 | Profilin | Minor | Birch pollen, Ambrosia artemisiifolia, Capsicum annuum, Chenopodium album, and other allergenic sources containing profilins | Contact | [27,28] |
| Hev b 9 | Enolase | Minor | Cladosporium herbarum and Alternaria alternata | Contact | [29,30] |
| Hev b 10 | Manganese superoxide dismutase (MnSOD) | Minor | Aspergillus fumigatus | Contact | [31,32] |
| Hev b 11 | Chitinase Class I | Minor | Ficus benjamina, banana, avocado, chestnut, wheat, kiwi, and other allergenic sources containing chitinases | Contact | [10,33,34,35] |
| Hev b 12 | Non-specific lipid transfer protein type 1 (nsLTP1) | Minor | Apple, peach, bell pepper, banana, potato, avocado, and other allergenic sources containing nsLTPs | Contact | [7,36,37] |
| Hev b 13 | Esterase | Major | Contact | [21,38,39] | |
| Hev b 14 | Hevamine | Minor | Contact | [40] | |
| Hev b 15 | Serine protease inhibitor | Minor | Contact | [41] |
| Position in Ranking | Fruit | Number of Studies | Number of Countries | Reference |
|---|---|---|---|---|
| 1 | Banana | 9 | 7 | [49,50,52,53,56,57,59,60,62] |
| 2 | Kiwi | 3 | 2 | [50,54,61] |
| 3 | Avocado | 2 | 2 | [55,58] |
| Papaya | 2 | 2 | [49,60] |
| Organ | Symptoms | Number |
|---|---|---|
| Mucocutaneus | 109 | |
| Urticaria | 55 | |
| Angioedema | 33 | |
| Eczema | 7 | |
| Facial edema | 6 | |
| Atopic dermatitis | 3 | |
| Generalized urticaria | 2 | |
| Edema | 1 | |
| Pruritus | 1 | |
| Erythema | 1 | |
| Respiratory | 57 | |
| Asthma | 38 | |
| Rhinoconjunctivitis | 12 | |
| Rhinitis | 7 | |
| Gastrointestinal | 14 | |
| Gastrointestinal | 14 | |
| Cardiovascular | 14 | |
| Anaphylaxis | 14 | |
| Other | 3 | |
| Eye syndrome | 3 |
| Symptoms | Grade Assigned in Accordance with a Given Severity Scoring System | ||||||
|---|---|---|---|---|---|---|---|
| Mueller [71] | Ring [69] | Sampson [72] | Cox [73] | Błażowski [70] | Dribin [74] $ | WAO [68] | |
| Case 1 | |||||||
| Generalized pruritus | Grade 1 | Grade 1 | Grade 2 | Grade 2 * | Grade 1 | Grade 2 ‡ | Grade 2 # |
| Generalized urticaria | Grade 1 | Grade 1 | Grade 2 | Grade 2 * | Grade 1 | Grade 2 ‡ | Grade 2 # |
| Localized ocular angioedema | Not included | Grade 1 | Grade 1 | Grade 2 * | Grade 1 | Grade 2 ‡ | Grade 1 |
| Tongue stiffness and swelling | Not included | Grade 1 | Grade 1 | Grade 2 * | Grade 1 † | Grade 2 ‡ | Grade 1 |
| Vomiting | Grade 2 | Grade 2 | Grade 2 | Grade 3 | Grade 2 | Grade 2 ‡ | Grade 2 |
| Dyspnea | Grade 3 | Grade 3 | Grade 4 | Grade 3 | Grade 2 | Grade 3 | Grade 3 |
| Case 2 | |||||||
| Blood pressure fall | Grade 4 | Grade 3 ** | Grade 5 | Grade 5 | Grade 3 | Grade 5 | Grade 4 |
| Loss of consciousness | Grade 4 | Grade 3 ** | Grade 5 | Grade 5 | Grade 4 | Grade 5 | Grade 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gromek, W.; Kołdej, N.; Świtała, S.; Majsiak, E.; Kurowski, M. Revisiting Latex-Fruit Syndrome after 30 Years of Research: A Comprehensive Literature Review and Description of Two Cases. J. Clin. Med. 2024, 13, 4222. https://doi.org/10.3390/jcm13144222
Gromek W, Kołdej N, Świtała S, Majsiak E, Kurowski M. Revisiting Latex-Fruit Syndrome after 30 Years of Research: A Comprehensive Literature Review and Description of Two Cases. Journal of Clinical Medicine. 2024; 13(14):4222. https://doi.org/10.3390/jcm13144222
Chicago/Turabian StyleGromek, Weronika, Natalia Kołdej, Szymon Świtała, Emilia Majsiak, and Marcin Kurowski. 2024. "Revisiting Latex-Fruit Syndrome after 30 Years of Research: A Comprehensive Literature Review and Description of Two Cases" Journal of Clinical Medicine 13, no. 14: 4222. https://doi.org/10.3390/jcm13144222







