Real-Life Clinical Outcomes of Benralizumab Treatment in Patients with Uncontrolled Severe Asthma and Coexisting Chronic Rhinosinusitis with Nasal Polyposis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Type of Study
2.2. Study Period
2.3. Population
2.4. Inclusion Criteria
2.5. Efficacy Monitoring
2.6. Statistical Study
2.7. Ethical Considerations
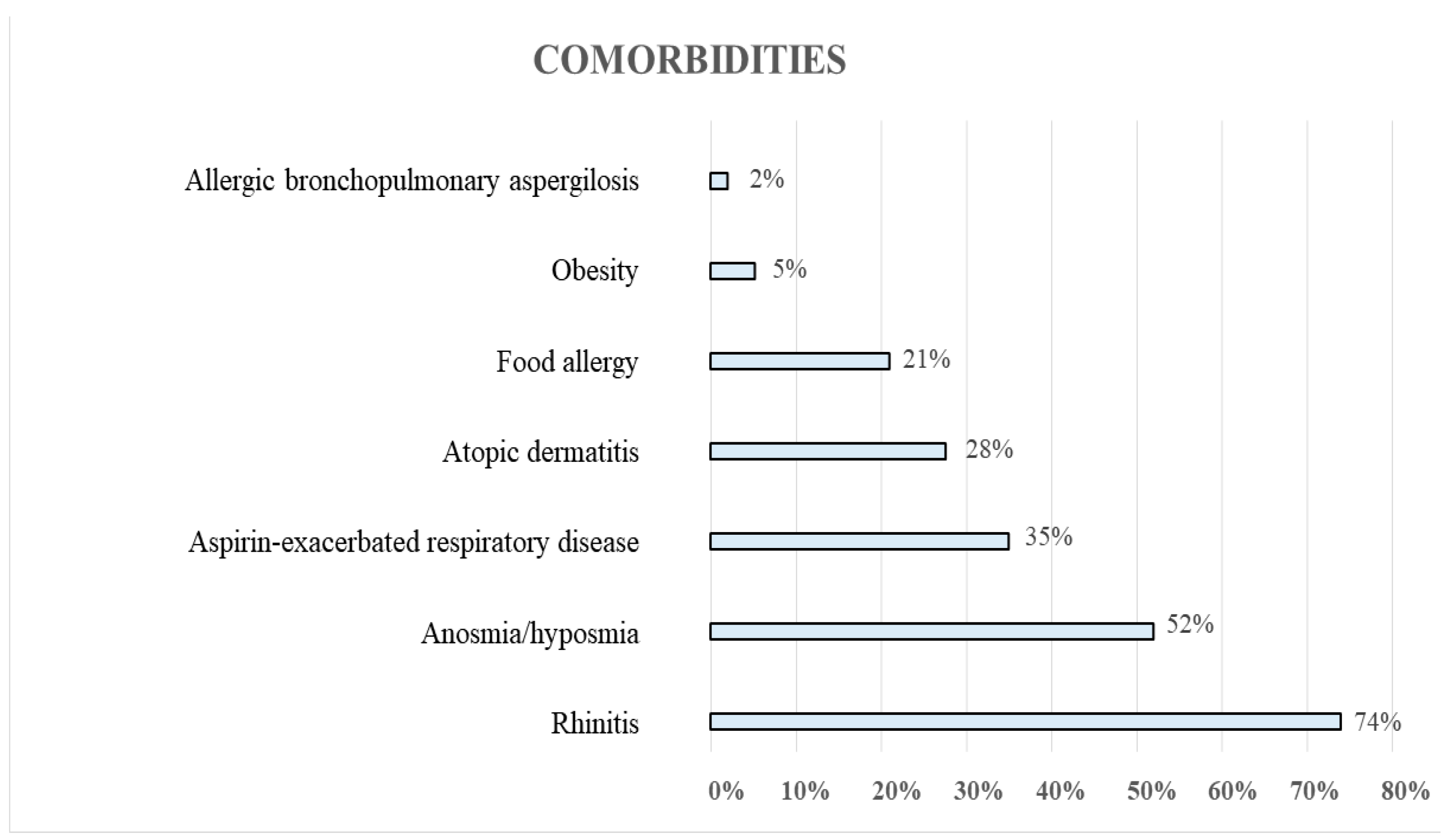
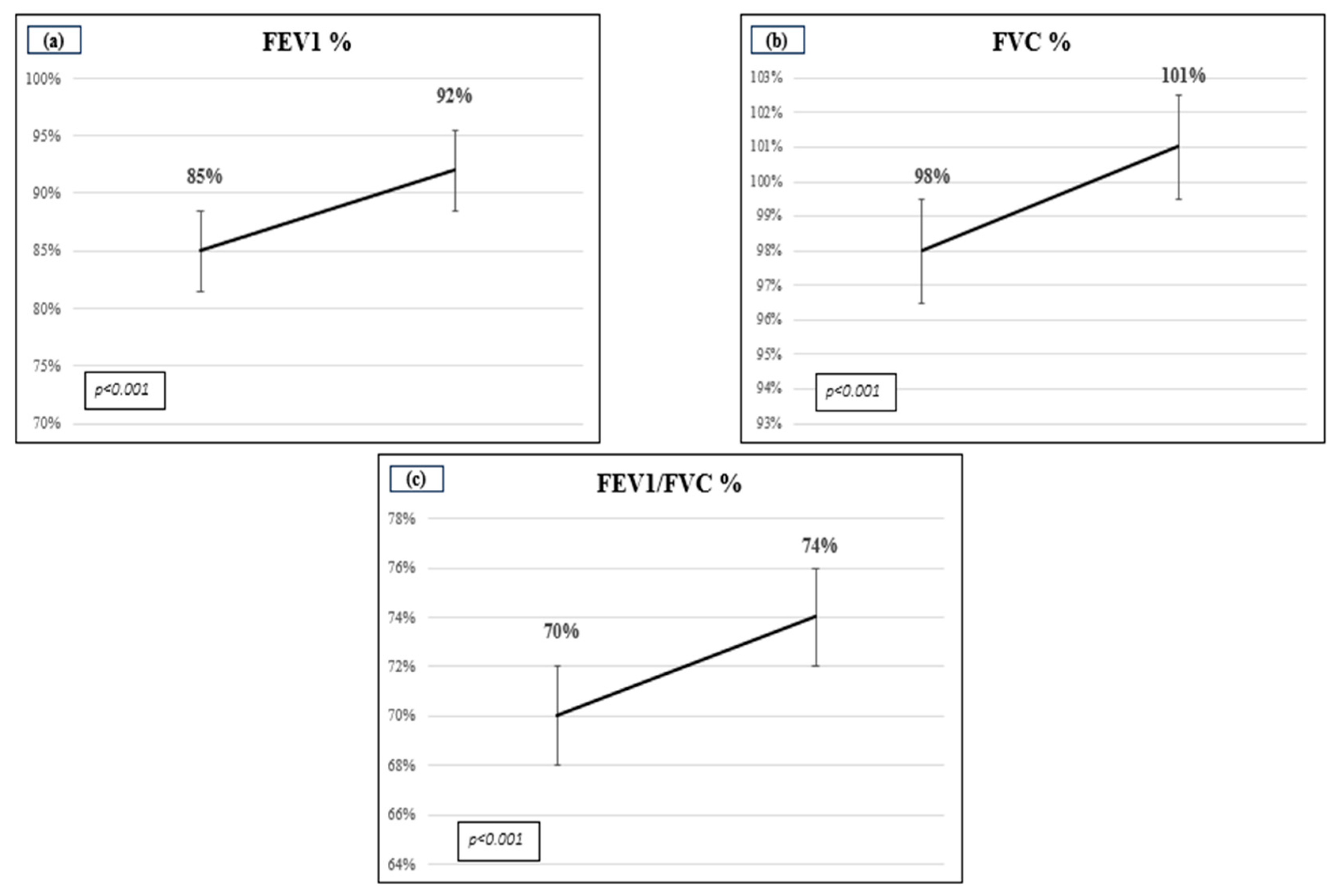
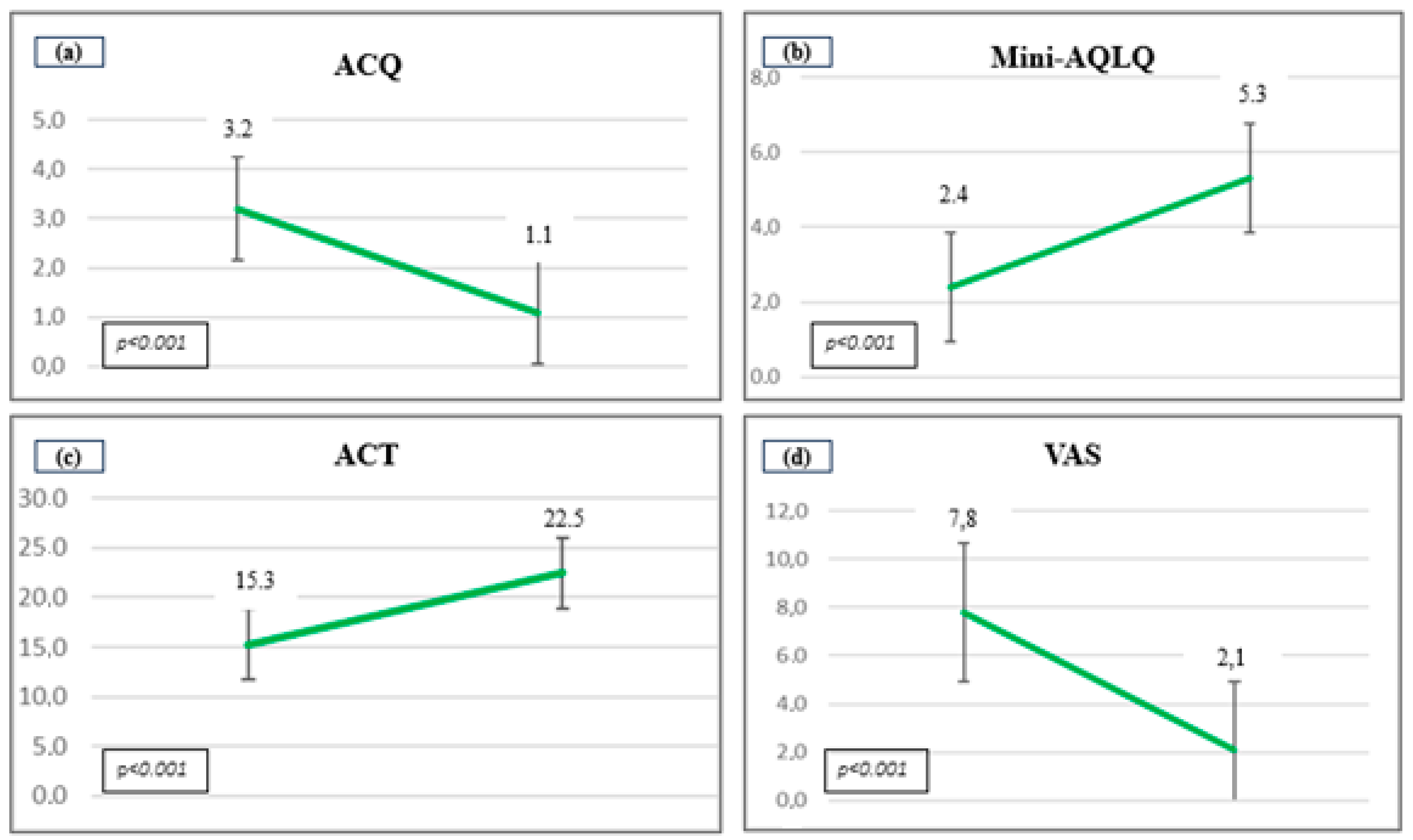
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plaza, V.; Alobid, I.; Álvarez, C.; Blanco, M.; Ferreira, J.; García, G.; Gómez-Outes, A.; Garín Escrivá, N.; Gómez, F.; Hidalgo, A.; et al. GEMA 5.3. Spanish Guideline on the Management of Asthma. Open Respir. Arch. 2023, 5, 100277. [Google Scholar] [CrossRef] [PubMed]
- Canonica, G.W.; Harrison, T.W.; Chanez, P.; Menzella, F.; Louis, R.; Cosio, B.G.; Lugogo, N.L.; Mohan, A.; Burden, A.; Garcia, E. Benralizumab improves symptoms of patients with severe, eosinophilic asthma with a diagnosis of nasal polyposis. Allergy 2022, 77, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, D.; Paggiaro, P.; Latorre, M.; Folli, C.; Testino, E.; Bassi, A.; Milanese, M.; Heffler, E.; Manfredi, A.; Riccio, A.M.; et al. Severe asthma: One disease and multiple definitions. World Allergy Organ. J. 2021, 14, 100606. [Google Scholar] [CrossRef] [PubMed]
- Alobid, I.; Benítez, P.; Bernal-Sprekelsen, M.; Roca, J.; Alonso, J.; Picado, C.; Mullol, J. Nasal polyposis and its impact on quality of life: Comparison between the effects of medical and surgical treatments. Allergy 2005, 60, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Kusano, S.; Kukimoto-Niino, M.; Hino, N.; Ohsawa, N.; Ikutani, M.; Takaki, S.; Sakamoto, K.; Hara-Yokoyama, M.; Shirouzu, M.; Takatsu, K.; et al. Structural basis of interleukin-5 dimer recognition by its α receptor. Protein Sci. 2012, 21, 850–864. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef]
- Agache, I.; Beltran, J.; Akdis, C.; Akdis, M.; Canelo-Aybar, C.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Del Giacco, S.; et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines—Recommendations on the use of biologicals in severe asthma. Allergy 2020, 75, 1023–1042. [Google Scholar] [CrossRef] [PubMed]
- Dávila, G.I.; Moreno, B.F.; Quirce, S. Benralizumab: A New Approach for the Treatment of Severe Eosinophilic Asthma. J. Investig. Allergol. Clin. Immunol. 2019, 29, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Schleich, F.; Moermans, C.; Seidel, L.; Kempeneers, C.; Louis, G.; Rogister, F.; Tombu, S.; Pottier, L.; Poirrier, A.L.; Ziant, S.; et al. Benralizumab in severe eosinophilic asthma in real life: Confirmed effectiveness and contrasted effect on sputum eosinophilia versus exhaled nitric oxide fraction—PROMISE. ERJ Open Res. 2023, 9, 00383–02023. [Google Scholar] [CrossRef]
- Barroso, B.; Valverde-Monge, M.; Betancor, D.; Gómez-López, A.; Villalobos-Vilda, C.; González-Cano, B.; Sastre, J. Improvement in Smell Using Monoclonal Antibodies Among Patients With Chronic Rhinosinusitis With Nasal Polyps: A Systematic Review. J. Investig. Allergol. Clin. Immunol. 2023, 33, 419–430. [Google Scholar] [CrossRef]
- Tversky, J.; Lane, A.P.; Azar, A. Benralizumab effect on severe chronic rhinosinusitis with nasal polyps (CRSwNP): A randomized double-blind placebo-controlled trial. Clin. Exp. Allergy 2021, 51, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, O.; Minamoto, S. Rapid effect of benralizumab for severe asthma with chronic rhinosinusitis with nasal polyps. Pulm. Pharmacol. Ther. 2020, 64, 101965. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.Y.; Gevaert, P.; Heffler, E.; Hopkins, C.; Tversky, J.R.; Barker, P.; Cohen, D.; Emson, C.; et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2022, 149, 1309–1317. [Google Scholar] [CrossRef]
- Harrison, T.W.; Chanez, P.; Menzella, F.; Canonica, G.W.; Louis, R.; Cosio, B.G.; Lugogo, N.L.; Mohan, A.; Burden, A.; McDermott, L.; et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): A randomised, controlled, phase 3b trial. Lancet Respir. Med. 2021, 9, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Alobid, I.; Colás, C.; Castillo, J.A.; Arismendi, E.; Del Cuvillo, A.; Gómez-Outes, A.; Sastre, J.; Mullol, J. Spanish Consensus on the Management of Chronic Rhinosinusitis With Nasal Polyps (POLIposis NAsal/POLINA 2.0). J. Investig. Allergol. Clin. Immunol. 2023, 33, 317–331. [Google Scholar] [CrossRef] [PubMed]
- García-Río, F.; Calle, M.; Burgos, F.; Casan, P.; Del Campo, F.; Galdiz, J.B.; Giner, J.; González-Mangado, N.; Ortega, F.; Puente, L. Spirometry. Arch. Bronconeumol. 2013, 9, 388–401. [Google Scholar] [CrossRef]
- Jia, C.E.; Zhang, H.P.; Lv, Y.; Liang, R.; Jiang, Y.Q.; Powell, H.; Fu, J.J.; Wang, L.; Gibson, P.G.; Wang, G. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2013, 131, 695–703. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Weitzel, E.K.; Chen, P.G. The predictive utility of the 22-item sino-nasal outcome test (SNOT-22): A scoping review. Int. Forum Allergy Rhinol. 2022, 12, 83–102. [Google Scholar] [CrossRef]
- Pérez de Llano, L.; Dávila, I.; Martínez-Moragón, E.; Domínguez-Ortega, J.; Almonacid, C.; Colás, C.; García-Rivero, J.L.; Carmona, L.; García de Yébenes, M.J.; Cosío, B.G. Development of a Tool to Measure the Clinical Response to Biologic Therapy in Uncontrolled Severe Asthma: The FEV1, Exacerbations, Oral Corticosteroids, Symptoms Score. J. Allergy Clin. Immunol. Pract. 2021, 9, 2725–2731. [Google Scholar] [CrossRef]
- Alvarez-Gutiérrez, F.J.; Blanco-Aparicio, M.; Casas-Maldonado, F.; Plaza, V.; González-Barcala, F.J.; Carretero-Gracia, J.Á.; Castilla-Martínez, M.; Cisneros, C.; Diaz-Pérez, D.; Domingo-Ribas, C.; et al. Documento de consenso de asma grave en adultos. Actualización 2022 [Consensus document for severe asthma in adults. 2022 update]. Open Respir Arch. 2022, 4, 100192. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, M.; Menzella, F.; Altieri, E.; Bargagli, E.; Bracciale, P.; Brussino, L.; Caiaffa, M.F.; Canonica, G.W.; Caruso, C.; Centanni, S.; et al. Benralizumab in Patients With Severe Eosinophilic Asthma With and Without Chronic Rhinosinusitis With Nasal Polyps: An ANANKE Study post-hoc Analysis. Front. Allergy. 2022, 3, 881218. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moragon, E.; Chiner, E.; Suliana Mogrovejo, A.; Palop Cervera, M.; Lluch Tortajada, I.; Boira, I.; Sánchez Vera, A.F. Real-world clinical remission of severe asthma with benralizumab in Spanish adults with severe asthma. J. Asthma. 2024, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Monge, M.; Sánchez-Carrasco, P.; Betancor, D.; Barroso, B.; Rodrigo-Muñoz, J.M.; Mahillo-Fernández, I.; Arismendi, E.; Bobolea, I.; Cárdaba, B.; Cruz, M.J.; et al. Comparison of Long-term Response and Remission to Omalizumab and Anti-IL-5/IL-5R Using Different Criteria in a Real-life Cohort of Severe Asthma Patients. Arch. Bronconeumol. 2024, 60, 23–32. [Google Scholar] [CrossRef]
- Padilla-Galo, A.; Moya Carmona, I.; Ausín, P.; Carazo Fernández, L.; García-Moguel, I.; Velasco-Garrido, J.L.; Andújar-Espinosa, R.; Casas-Maldonado, F.; Martínez- Moragón, E.; Martínez Rivera, C.; et al. Achieving clinical outcomes with benralizumab in severe eosinophilic asthma patients in a real-world setting: Orbe II study. Respir. Res. 2023, 24, 235. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral Glucocorticoid- Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef]
- Bagnasco, D.; Brussino, L.; Bonavia, M.; Calzolari, E.; Caminati, M.; Caruso, C.; D’Amato, M.; De Ferrari, L.; Di Marco, F.; Imeri, G.; et al. Efficacy of Benralizumab in severe asthma in real life and focus on nasal polyposis. Respir. Med. 2020, 171, 106080. [Google Scholar] [CrossRef]
- Lombardo, N.; Pelaia, C.; Ciriolo, M.; Della Corte, M.; Piazzetta, G.; Lobello, N.; Viola, P.; Pelaia, G. Real-life effects of benralizumab on allergic chronic rhinosinusitis and nasal polyposis associated with severe asthma. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420950851. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, S.; Crimi, C.; Pelaia, C.; Benfante, A.; Caiaffa, M.F.; Calabrese, C.; Carpagnano, G.E.; Ciotta, D.; D’Amato, M.; Macchia, L.; et al. Benralizumab Effectiveness in Severe Eosinophilic Asthma with and without Chronic Rhinosinusitis with Nasal Polyps: A Real-World Multicenter Study. J. Allergy Clin. Immunol. Pract. 2021, 9, 4371–4380.e4. [Google Scholar] [CrossRef]
- Pini, L.; Bagnasco, D.; Beghè, B.; Braido, F.; Cameli, P.; Caminati, M.; Caruso, C.; Crimi, C.; Guarnieri, G.; Latorre, M.; et al. Unlocking the Long-Term Effectiveness of Benralizumab in Severe Eosinophilic Asthma: A Three-Year Real-Life Study. J. Clin. Med. 2024, 13, 3013. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef] [PubMed]
- Mareque, M.; Climente, M.; Martinez-Moragon, E.; Padilla, A.; Oyagüez, I.; Touron, C.; Torres, C.; Martinez, A. Cost-effectiveness of benralizumab versus mepolizumab and dupilumab in patients with severe uncontrolled eosinophilic asthma in Spain. J. Asthma. 2023, 60, 1210–1220. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristics | Value/Mean ± Standard Deviation |
|---|---|
| Mean age (years) | 55 ± 14 |
| BMI (Kg/m2) | 27 ± 8 |
| Sex | 64% women, 36% men |
| Eosinophils, cel/μL | 786 ± 504 |
| IgE (UI/mL) | 361 ± 807 |
| Atopy/allergy | 17 patients |
| FEV1 (Liters and %) | 2.45 ± 0.8 (84 ± 18%) |
| FEV1/FVC (%) | 69.5 ± 10 (%) |
| FVC (liters and %) | 3.47 ± 1.01 (97 ± 16%) |
| ACT // ACQ// VAS | 15 ± 2 // 3 ± 1 // 7.5 ± 1 |
| SNOT-22 | 55 ± 17 |
| VAS: | |
| Obstruction | 8 ± 1 |
| Runny nose | 7.8 ± 1 |
| Anosmia | 7.6 ± 1 |
| Facial pressure | 7.8 ± 1 |
| Mini-AQLQ | 2.4 ± 0.4 |
| Number emergency visits / year | 2.62 ± 2.7 |
| Number of hospital admissions/year | 0.84 ± 2 |
| Days of hospital admission | 3 ± 8 |
| Global exacerbations | 5.6 ± 5 |
| Oral glucocorticoid cycles | 3.16 ± 3 |
| Time of treatment (months) | 14.4 ± 8 |
| Previous ENT surgery (one or more) | Total: 55% (32) One: 47% (15) Two: 31% (10) Three or more: 22% (7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiner, E.; Murcia, M.; Boira, I.; Bernabeu, M.Á.; Esteban, V.; Martínez-Moragón, E. Real-Life Clinical Outcomes of Benralizumab Treatment in Patients with Uncontrolled Severe Asthma and Coexisting Chronic Rhinosinusitis with Nasal Polyposis. J. Clin. Med. 2024, 13, 4247. https://doi.org/10.3390/jcm13144247
Chiner E, Murcia M, Boira I, Bernabeu MÁ, Esteban V, Martínez-Moragón E. Real-Life Clinical Outcomes of Benralizumab Treatment in Patients with Uncontrolled Severe Asthma and Coexisting Chronic Rhinosinusitis with Nasal Polyposis. Journal of Clinical Medicine. 2024; 13(14):4247. https://doi.org/10.3390/jcm13144247
Chicago/Turabian StyleChiner, Eusebi, María Murcia, Ignacio Boira, María Ángeles Bernabeu, Violeta Esteban, and Eva Martínez-Moragón. 2024. "Real-Life Clinical Outcomes of Benralizumab Treatment in Patients with Uncontrolled Severe Asthma and Coexisting Chronic Rhinosinusitis with Nasal Polyposis" Journal of Clinical Medicine 13, no. 14: 4247. https://doi.org/10.3390/jcm13144247





