Beyond Stress Ischemia: Unveiling the Multifaceted Nature of Coronary Vulnerable Plaques Using Cardiac Computed Tomography
Abstract
:1. Introduction
2. Calcium Score
3. Plaque Morphology
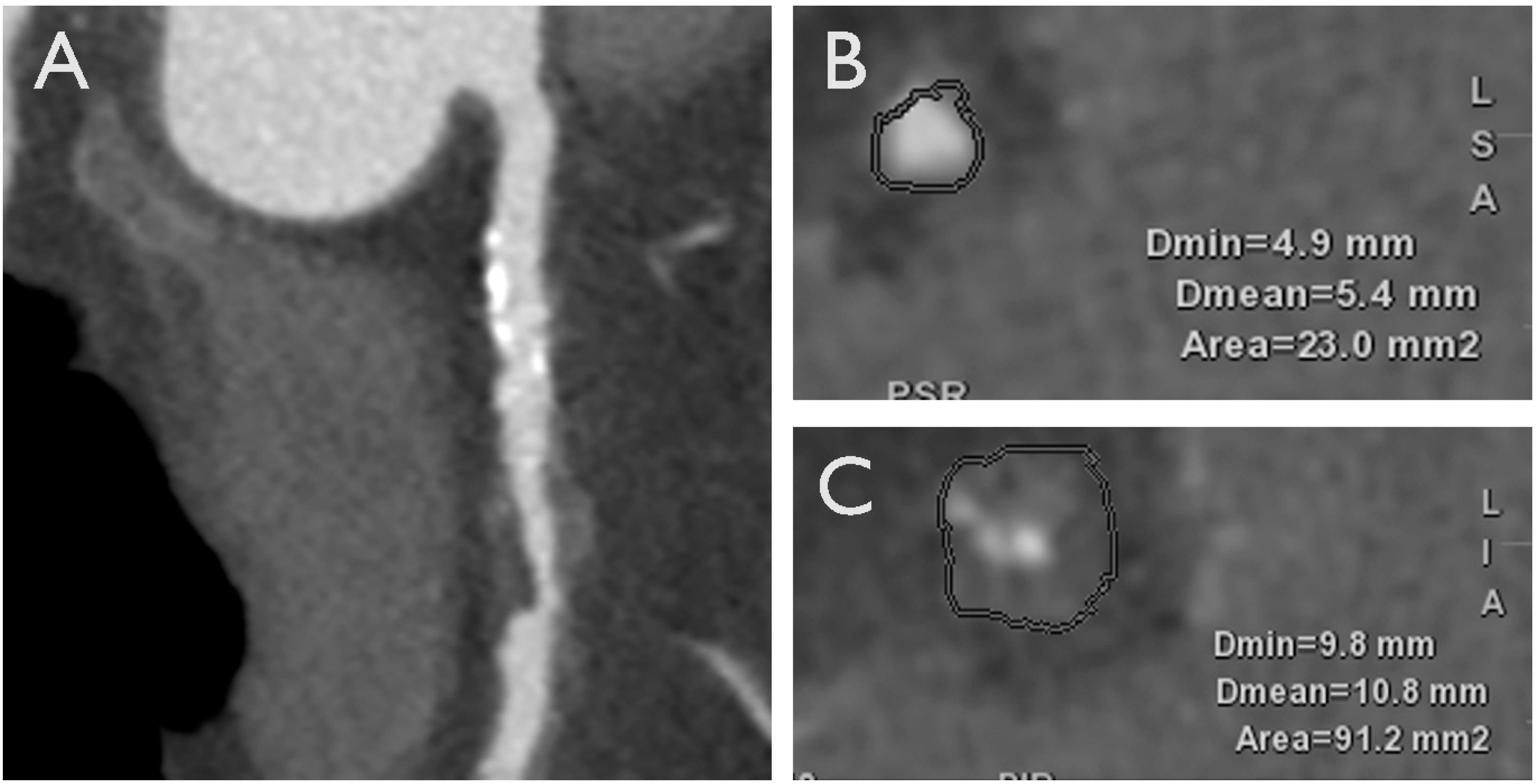
3.1. Adverse Plaque Characteristics
3.2. Adverse Geometric Characteristics
3.3. Adverse Hemodynamic Characteristics
4. Use of CCTA to Facilitate PCI
5. Epicardial and Pericoronary Adipose Tissue
6. New Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Neglia, D.; Liga, R.; Gimelli, A.; Podlesnikar, T.; Cvijić, M.; Pontone, G.; Miglioranza, M.H.; Guaricci, A.I.; Seitun, S.; Clemente, A.; et al. Use of cardiac imaging in chronic coronary syndromes: The EURECA Imaging registry. Eur. Heart J. 2022, 44, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Gaibazzi, N.; Porter, T.; Lorenzoni, V.; Pontone, G.; De Santis, D.; De Rosa, A.; Guaricci, A.I. Effect of Coronary Revascularization on the Prognostic Value of Stress Myocardial Contrast Wall Motion and Perfusion Imaging. J. Am. Heart Assoc. 2017, 6, e006202. [Google Scholar] [CrossRef] [PubMed]
- Hachamovitch, R.; Hayes, S.W.; Friedman, J.D.; Cohen, I.; Berman, D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003, 107, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Berman, D.S.; Maron, D.J.; Mancini, G.B.; Hayes, S.W.; Hartigan, P.M.; Weintraub, W.S.; O’Rourke, R.A.; Dada, M.; Spertus, J.A.; et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008, 117, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahn, J.M.; Kang, D.Y.; Yun, S.C.; Ahn, Y.K.; Kim, W.J.; Nam, C.W.; Jeong, J.O.; Chae, I.H.; Shiomi, H.; et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): A multicentre, open-label, randomised controlled trial. Lancet 2024, 403, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; Lopez-Sendon, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Mintz, G.S.; McPherson, J.A.; De Bruyne, B.; Farhat, N.Z.; Marso, S.P.; Serruys, P.W.; Stone, G.W.; Maehara, A. Predictors of Plaque Rupture Within Nonculprit Fibroatheromas in Patients With Acute Coronary Syndromes: The PROSPECT Study. JACC Cardiovasc. Imaging 2015, 8, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Maffei, E.; Seitun, S.; Martini, C.; Palumbo, A.; Tarantini, G.; Berti, E.; Grilli, R.; Tedeschi, C.; Messalli, G.; Guaricci, A.; et al. CT coronary angiography and exercise ECG in a population with chest pain and low-to-intermediate pre-test likelihood of coronary artery disease. Heart 2010, 96, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Guaricci, A.I.; Maffei, E.; Brunetti, N.D.; Montrone, D.; Di Biase, L.; Tedeschi, C.; Gentile, G.; Macarini, L.; Midiri, M.; Cademartiri, F.; et al. Heart rate control with oral ivabradine in computed tomography coronary angiography: A randomized comparison of 7.5 mg vs 5 mg regimen. Int. J. Cardiol. 2013, 168, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Andreini, D.; Baggiano, A.; Bertella, E.; Mushtaq, S.; Conte, E.; Beltrama, V.; Guaricci, A.I.; Pepi, M. Functional relevance of coronary artery disease by cardiac magnetic resonance and cardiac computed tomography: Myocardial perfusion and fractional flow reserve. Biomed. Res. Int. 2015, 2015, 297696. [Google Scholar] [CrossRef] [PubMed]
- Maffei, E.; Seitun, S.; Martini, C.; Aldrovandi, A.; Cervellin, G.; Tedeschi, C.; Guaricci, A.; Messalli, G.; Catalano, O.; Cademartiri, F. Prognostic value of computed tomography coronary angiography in patients with chest pain of suspected cardiac origin. Radiol. Med. 2011, 116, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Van Assen, M.; Tesche, C.; De Cecco, C.N.; Chiesa, M.; Scafuri, S.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Guaricci, A.I.; et al. Artificial Intelligence in Coronary Computed Tomography Angiography: From Anatomy to Prognosis. Biomed. Res. Int. 2020, 2020, 6649410. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Andreini, D.; Bertella, E.; Baggiano, A.; Mushtaq, S.; Loguercio, M.; Segurini, C.; Conte, E.; Beltrama, V.; Annoni, A.; et al. Impact of an intra-cycle motion correction algorithm on overall evaluability and diagnostic accuracy of computed tomography coronary angiography. Eur. Radiol. 2016, 26, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Baggiano, A.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Del Torto, A.; Mushtaq, S.; et al. Diagnostic accuracy of simultaneous evaluation of coronary arteries and myocardial perfusion with single stress cardiac computed tomography acquisition compared to invasive coronary angiography plus invasive fractional flow reserve. Int. J. Cardiol. 2018, 273, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Muscogiuri, G.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Baggiano, A.; Fazzari, F.; Mushtaq, S.; Conte, E.; Annoni, A.; et al. Impact of a New Adaptive Statistical Iterative Reconstruction (ASIR)-V Algorithm on Image Quality in Coronary Computed Tomography Angiography. Acad. Radiol. 2018, 25, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Chiesa, M.; Baggiano, A.; Spadafora, P.; De Santis, R.; Guglielmo, M.; Scafuri, S.; Fusini, L.; Mushtaq, S.; Conte, E.; et al. Diagnostic performance of deep learning algorithm for analysis of computed tomography myocardial perfusion. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Rossi, A.; Baggiano, A.; Andreini, D.; Conte, E.; Fusini, L.; Gebhard, C.; Rabbat, M.G.; Guaricci, A.; Guglielmo, M.; et al. Progression of non-obstructive coronary plaque: A practical CCTA-based risk score from the PARADIGM registry. Eur. Radiol. 2024, 34, 2665–2676. [Google Scholar] [CrossRef]
- Pontone, G.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Fazzari, F.; Berzovini, C.; et al. Quantitative vs. qualitative evaluation of static stress computed tomography perfusion to detect haemodynamically significant coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Baggiano, A.; Fusini, L.; Del Torto, A.; Vivona, P.; Guglielmo, M.; Muscogiuri, G.; Soldi, M.; Martini, C.; Fraschini, E.; Rabbat, M.G.; et al. Sequential Strategy Including FFR(CT) Plus Stress-CTP Impacts on Management of Patients with Stable Chest Pain: The Stress-CTP RIPCORD Study. J. Clin. Med. 2020, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- Baggiano, A.; Conte, E.; Spiritigliozzi, L.; Mushtaq, S.; Annoni, A.; Carerj, M.L.; Cilia, F.; Fazzari, F.; Formenti, A.; Frappampina, A.; et al. Quantification of extracellular volume with cardiac computed tomography in patients with dilated cardiomyopathy. J. Cardiovasc. Comput. Tomogr. 2023, 17, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lo Iacono, F.; Maragna, R.; Guglielmo, M.; Chiesa, M.; Fusini, L.; Annoni, A.; Babbaro, M.; Baggiano, A.; Carerj, M.L.; Cilia, F.; et al. Identification of subclinical cardiac amyloidosis in aortic stenosis patients undergoing transaortic valve replacement using radiomic analysis of computed tomography myocardial texture. J. Cardiovasc. Comput. Tomogr. 2023, 17, 286–288. [Google Scholar] [CrossRef]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Tintut, Y.; Alfonso, Z.; Saini, T.; Radcliff, K.; Watson, K.; Boström, K.; Demer, L.L. Multilineage potential of cells from the artery wall. Circulation 2003, 108, 2505–2510. [Google Scholar] [CrossRef] [PubMed]
- Obisesan, O.H.; Osei, A.D.; Uddin, S.M.I.; Dzaye, O.; Blaha, M.J. An Update on Coronary Artery Calcium Interpretation at Chest and Cardiac CT. Radiol. Cardiothorac. Imaging 2021, 3, e200484. [Google Scholar] [CrossRef]
- Dzaye, O.; Razavi, A.C.; Dardari, Z.A.; Shaw, L.J.; Berman, D.S.; Budoff, M.J.; Miedema, M.D.; Nasir, K.; Rozanski, A.; Rumberger, J.A.; et al. Modeling the Recommended Age for Initiating Coronary Artery Calcium Testing Among At-Risk Young Adults. J. Am. Coll. Cardiol. 2021, 78, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Rev. Esp. Cardiol. 2022, 75, 429. [Google Scholar] [CrossRef]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; Watson, K.E.; et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Ambale-Venkatesh, B.; Yang, X.; Wu, C.O.; Liu, K.; Hundley, W.G.; McClelland, R.; Gomes, A.S.; Folsom, A.R.; Shea, S.; Guallar, E.; et al. Cardiovascular Event Prediction by Machine Learning: The Multi-Ethnic Study of Atherosclerosis. Circ. Res. 2017, 121, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Kronmal, R.A.; McClelland, R.L.; Detrano, R.; Shea, S.; Lima, J.A.; Cushman, M.; Bild, D.E.; Burke, G.L. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2007, 115, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Lehmann, N.; Churzidse, S.; Rauwolf, M.; Mahabadi, A.A.; Möhlenkamp, S.; Moebus, S.; Bauer, M.; Kälsch, H.; Budde, T.; et al. Progression of coronary artery calcification seems to be inevitable, but predictable—Results of the Heinz Nixdorf Recall (HNR) study. Eur. Heart J. 2014, 35, 2960–2971. [Google Scholar] [CrossRef]
- Lehmann, N.; Erbel, R.; Mahabadi, A.A.; Rauwolf, M.; Möhlenkamp, S.; Moebus, S.; Kälsch, H.; Budde, T.; Schmermund, A.; Stang, A.; et al. Value of Progression of Coronary Artery Calcification for Risk Prediction of Coronary and Cardiovascular Events: Result of the HNR Study (Heinz Nixdorf Recall). Circulation 2018, 137, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Ju, J.; Lin, Q.; Xu, H. Coronary Artery Calcification Under Statin Therapy and Its Effect on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2020, 7, 600497. [Google Scholar] [CrossRef] [PubMed]
- Verghese, D.; Manubolu, S.; Budoff, M.J. Contemporary use of coronary artery calcium for the allocation of aspirin in light of the 2022 USPSTF guideline recommendations. Am. J. Prev. Cardiol. 2022, 12, 100427. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.J.; Mortensen, M.B.; Kianoush, S.; Tota-Maharaj, R.; Cainzos-Achirica, M. Coronary Artery Calcium Scoring: Is It Time for a Change in Methodology? JACC Cardiovasc. Imaging 2017, 10, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.J.; Budoff, M.J.; DeFilippis, A.P.; Blankstein, R.; Rivera, J.J.; Agatston, A.; O’Leary, D.H.; Lima, J.; Blumenthal, R.S.; Nasir, K. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: Implications for the JUPITER population from MESA, a population-based cohort study. Lancet 2011, 378, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Nasir, K.; Bittencourt, M.S.; Blaha, M.J.; Blankstein, R.; Agatson, A.S.; Rivera, J.J.; Miedema, M.D.; Sibley, C.T.; Shaw, L.J.; Blumenthal, R.S.; et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2015, 66, 1657–1668. [Google Scholar] [CrossRef]
- Miedema, M.D.; Duprez, D.A.; Misialek, J.R.; Blaha, M.J.; Nasir, K.; Silverman, M.G.; Blankstein, R.; Budoff, M.J.; Greenland, P.; Folsom, A.R. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: Estimates from the multi-ethnic study of atherosclerosis. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 453–460. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; Martin, S.S.; Dardari, Z.A.; Miedema, M.D.; Sandfort, V.; Yeboah, J.; Budoff, M.J.; Goff, D.C., Jr.; Psaty, B.M.; Post, W.S.; et al. Coronary Artery Calcium to Guide a Personalized Risk-Based Approach to Initiation and Intensification of Antihypertensive Therapy. Circulation 2017, 135, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Puchner, S.B.; Liu, T.; Mayrhofer, T.; Truong, Q.A.; Lee, H.; Fleg, J.L.; Nagurney, J.T.; Udelson, J.E.; Hoffmann, U.; Ferencik, M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: Results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 2014, 64, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D.; Magnoni, M.; Conte, E.; Masson, S.; Mushtaq, S.; Berti, S.; Canestrari, M.; Casolo, G.; Gabrielli, D.; Latini, R.; et al. Coronary Plaque Features on CTA Can Identify Patients at Increased Risk of Cardiovascular Events. JACC Cardiovasc. Imaging 2020, 13, 1704–1717. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Ferencik, M.; Voros, S.; Merkely, B.; Hoffmann, U. Comprehensive plaque assessment by coronary CT angiography. Nat. Rev. Cardiol. 2014, 11, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Denenberg, J.O.; Ix, J.H.; McClelland, R.L.; Wassel, C.L.; Rifkin, D.E.; Carr, J.J.; Budoff, M.J.; Allison, M.A. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014, 311, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, R.; Arsanjani, R.; Achenbach, S.; Gransar, H.; Cheng, V.Y.; Dunning, A.; Lin, F.Y.; Al-Mallah, M.; Budoff, M.J.; Callister, T.Q.; et al. Age-related risk of major adverse cardiac event risk and coronary artery disease extent and severity by coronary CT angiography: Results from 15 187 patients from the International Multisite CONFIRM Study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Min, J.K.; Dunning, A.; Lin, F.Y.; Achenbach, S.; Al-Mallah, M.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; Chang, H.J.; Cheng, V.; et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J. Am. Coll. Cardiol. 2011, 58, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Francone, M.; Andreini, D.; Buffa, V.; Cademartiri, F.; Carbone, I.; Clemente, A.; Guaricci, A.I.; Guglielmo, M.; Indolfi, C.; et al. SIRM-SIC appropriateness criteria for the use of Cardiac Computed Tomography. Part 1: Congenital heart diseases, primary prevention, risk assessment before surgery, suspected CAD in symptomatic patients, plaque and epicardial adipose tissue characterization, and functional assessment of stenosis. Radiol. Med. 2021, 126, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Carrabba, N.; Pontone, G.; Andreini, D.; Buffa, V.; Cademartiri, F.; Carbone, I.; Clemente, A.; Guaricci, A.I.; Guglielmo, M.; Indolfi, C.; et al. Appropriateness criteria for the use of cardiac computed tomography, SIC-SIRM part 2: Acute chest pain evaluation; stent and coronary artery bypass graft patency evaluation; planning of coronary revascularization and transcatheter valve procedures; cardiomyopathies, electrophysiological applications, cardiac masses, cardio-oncology and pericardial diseases evaluation. J. Cardiovasc. Med. 2022, 23, 290–303. [Google Scholar] [CrossRef]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Kondo, T.; Narula, J. Evaluation of plaque morphology by coronary CT angiography. Cardiol. Clin. 2012, 30, 69–75. [Google Scholar] [CrossRef]
- VanderLaan, P.A.; Reardon, C.A.; Getz, G.S. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 12–22. [Google Scholar] [CrossRef]
- Kwak, B.R.; Bäck, M.; Bochaton-Piallat, M.L.; Caligiuri, G.; Daemen, M.J.; Davies, P.F.; Hoefer, I.E.; Holvoet, P.; Jo, H.; Krams, R.; et al. Biomechanical factors in atherosclerosis: Mechanisms and clinical implications. Eur. Heart J. 2014, 35, 3013–3020, 3020a–3020d. [Google Scholar] [CrossRef] [PubMed]
- Jackson, Z.S.; Dajnowiec, D.; Gotlieb, A.I.; Langille, B.L. Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Lin, A.; Kuronuma, K.; Tzolos, E.; Kwan, A.C.; Klein, E.; Andreini, D.; Bax, J.J.; Cademartiri, F.; Chinnaiyan, K.; et al. Association of Plaque Location and Vessel Geometry Determined by Coronary Computed Tomographic Angiography With Future Acute Coronary Syndrome-Causing Culprit Lesions. JAMA Cardiol. 2022, 7, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.C.; Loree, H.M.; Kamm, R.D.; Fishbein, M.C.; Lee, R.T. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation 1993, 87, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.; Loree, H.M.; Cheng, G.C.; Lieberman, E.H.; Jaramillo, N.; Schoen, F.J. Computational structural analysis based on intravascular ultrasound imaging before in vitro angioplasty: Prediction of plaque fracture locations. J. Am. Coll. Cardiol. 1993, 21, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Vengrenyuk, Y.; Carlier, S.; Xanthos, S.; Cardoso, L.; Ganatos, P.; Virmani, R.; Einav, S.; Gilchrist, L.; Weinbaum, S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc. Natl. Acad. Sci. USA 2006, 103, 14678–14683. [Google Scholar] [CrossRef] [PubMed]
- Loree, H.M.; Kamm, R.D.; Stringfellow, R.G.; Lee, R.T. Effects of fibrous cap thickness on peak circumferential stress in model atherosclerotic vessels. Circ. Res. 1992, 71, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Teng, Z.; Calvert, P.A.; Rajani, N.K.; Hennessy, O.; Nerlekar, N.; Obaid, D.R.; Costopoulos, C.; Huang, Y.; Hoole, S.P.; et al. Plaque Structural Stress Estimations Improve Prediction of Future Major Adverse Cardiovascular Events After Intracoronary Imaging. Circ. Cardiovasc. Imaging 2016, 9, e004172. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Choi, G.; Koo, B.K.; Hwang, D.; Park, J.; Zhang, J.; Kim, K.J.; Tong, Y.; Kim, H.J.; Grady, L.; et al. Identification of High-Risk Plaques Destined to Cause Acute Coronary Syndrome Using Coronary Computed Tomographic Angiography and Computational Fluid Dynamics. JACC Cardiovasc. Imaging 2019, 12, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D.; Collet, C.; Leipsic, J.; Nieman, K.; Bittencurt, M.; De Mey, J.; Buls, N.; Onuma, Y.; Mushtaq, S.; Conte, E.; et al. Pre-procedural planning of coronary revascularization by cardiac computed tomography: An expert consensus document of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2022, 16, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Tajima, A.; Bouisset, F.; Ohashi, H.; Sakai, K.; Mizukami, T.; Rizzini, M.L.; Gallo, D.; Chiastra, C.; Morbiducci, U.; Ali, Z.A.; et al. Advanced CT Imaging for the Assessment of Calcific Coronary Artery Disease and PCI Planning. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101299. [Google Scholar] [CrossRef]
- Zuo, W.; Lin, J.; Sun, R.; Su, Y.; Ma, G. Performance of the J-CTO score versus other risk scores for predicting procedural difficulty in coronary chronic total occlusion interventions. Ann. Med. 2022, 54, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, M.; Penso, M.; Carerj, M.L.; Giacari, C.M.; Volpe, A.; Fusini, L.; Baggiano, A.; Mushtaq, S.; Annoni, A.; Cannata, F.; et al. DEep LearnIng-based QuaNtification of epicardial adipose tissue predicts MACE in patients undergoing stress CMR. Atherosclerosis 2024, 117549. [Google Scholar] [CrossRef] [PubMed]
- Napoli, G.; Pergola, V.; Basile, P.; De Feo, D.; Bertrandino, F.; Baggiano, A.; Mushtaq, S.; Fusini, L.; Fazzari, F.; Carrabba, N.; et al. Epicardial and Pericoronary Adipose Tissue, Coronary Inflammation, and Acute Coronary Syndromes. J. Clin. Med. 2023, 12, 7212. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Ferencik, M.; Mayrhofer, T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Lu, M.T.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Patel, M.R.; et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; MacLean, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Chang, H.J.; Sung, J.M.; Park, H.B.; Heo, R.; Rizvi, A.; Lin, F.Y.; Kumar, A.; Hadamitzky, M.; Kim, Y.J.; et al. Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM Study. JACC Cardiovasc. Imaging 2018, 10, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Maroules, C.D.; Rybicki, F.J.; Ghoshhajra, B.B.; Batlle, J.C.; Branch, K.; Chinnaiyan, K.; Hamilton-Craig, C.; Hoffmann, U.; Litt, H.; Meyersohn, N.; et al. 2022 use of coronary computed tomographic angiography for patients presenting with acute chest pain to the emergency department: An expert consensus document of the Society of cardiovascular computed tomography (SCCT): Endorsed by the American College of Radiology (ACR) and North American Society for cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2023, 17, 146–163. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, K.M.; Chen, M.Y.; Dey, A.K.; Virmani, R.; Finn, A.V.; Khamis, R.Y.; Choi, A.D.; Min, J.K.; Williams, M.C.; Buckler, A.J.; et al. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1226–1243. [Google Scholar] [CrossRef] [PubMed]


| IVUS | OCT | NIRS | CCTA | |
|---|---|---|---|---|
| Spatial resolution | 20–100 μm | 10–15 μm | 0.4 mm | |
| Lipid-rich core |    |    |    |    |
| Fibrous cap thickness |  |    |  |  |
| Calcifications |    |    |  |    (*) (*) |
| Positive vessel remodeling |    |  |  |    |
| Coronary Inflammation |  |    |  |  |
| Neovascularization |  |    |  |  |
| PCAT inflammation |  |  |  |    |
 N/A;
N/A; 

 low;
low; 

 medium;
medium; 

 high.
high.| AGATSON Score | Visual Assessment Score | CAC-DRS | |
|---|---|---|---|
| Calculation | Sum of the attenuation (in HU) and area of all CAC lesions in the coronary arteries. | Calcium quantitative assessment for each of the main epicardial coronary arteries. | Sum of the Agatston score or visual assessment score for each coronary artery. |
| Levels |
|
| 0–3 levels as calculated from AGATSON score (A) or visual assessment (V) score plus the number of affected coronary arteries. |
| Interpretation |
| Sum of the score for each of the coronary arteries and can be categorized into three categories of severity: 0, 1–3, and 4–12. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napoli, G.; Mushtaq, S.; Basile, P.; Carella, M.C.; De Feo, D.; Latorre, M.D.; Baggiano, A.; Ciccone, M.M.; Pontone, G.; Guaricci, A.I. Beyond Stress Ischemia: Unveiling the Multifaceted Nature of Coronary Vulnerable Plaques Using Cardiac Computed Tomography. J. Clin. Med. 2024, 13, 4277. https://doi.org/10.3390/jcm13144277
Napoli G, Mushtaq S, Basile P, Carella MC, De Feo D, Latorre MD, Baggiano A, Ciccone MM, Pontone G, Guaricci AI. Beyond Stress Ischemia: Unveiling the Multifaceted Nature of Coronary Vulnerable Plaques Using Cardiac Computed Tomography. Journal of Clinical Medicine. 2024; 13(14):4277. https://doi.org/10.3390/jcm13144277
Chicago/Turabian StyleNapoli, Gianluigi, Saima Mushtaq, Paolo Basile, Maria Cristina Carella, Daniele De Feo, Michele Davide Latorre, Andrea Baggiano, Marco Matteo Ciccone, Gianluca Pontone, and Andrea Igoren Guaricci. 2024. "Beyond Stress Ischemia: Unveiling the Multifaceted Nature of Coronary Vulnerable Plaques Using Cardiac Computed Tomography" Journal of Clinical Medicine 13, no. 14: 4277. https://doi.org/10.3390/jcm13144277







