Brain Responses Difference between Sexes for Strong Desire to Void: A Functional Magnetic Resonance Imaging Study in Adults Based on Graph Theory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Image Acquisition and Preprocessing
2.3. Establishment of Connection Matrix/Networks
2.4. Graph Analysis—Network Property Analysis
2.5. Statistical Analyses
3. Results
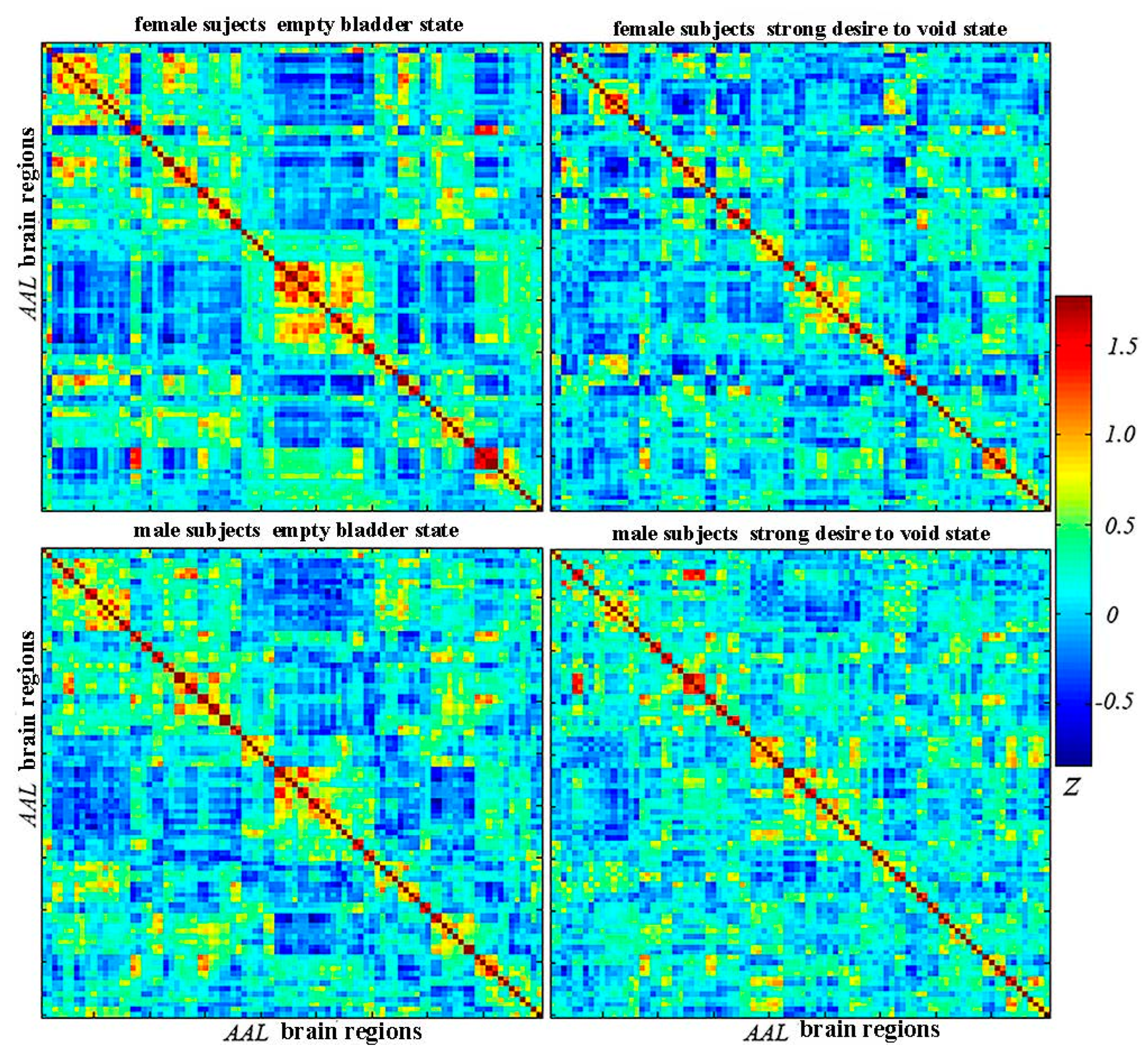
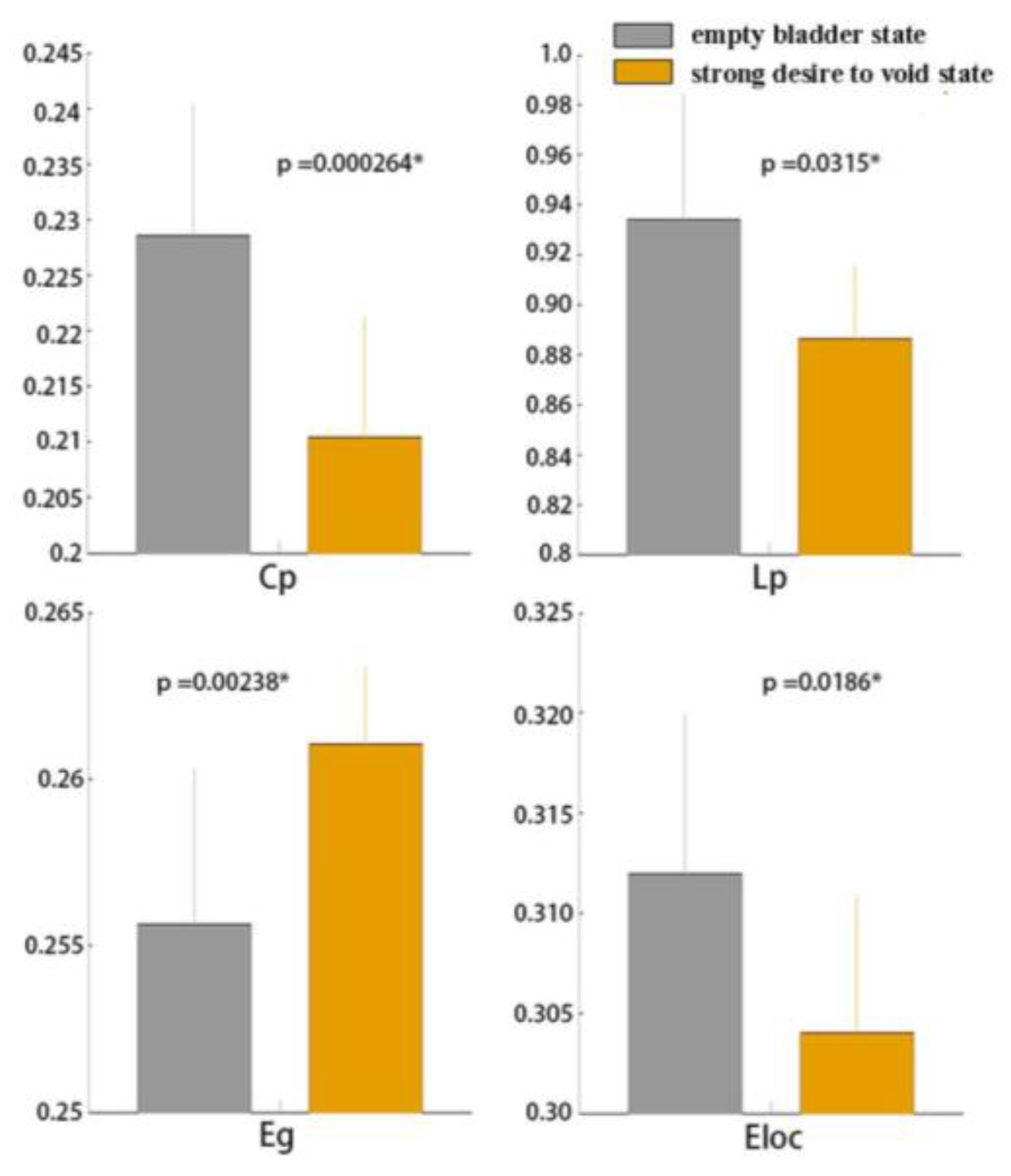
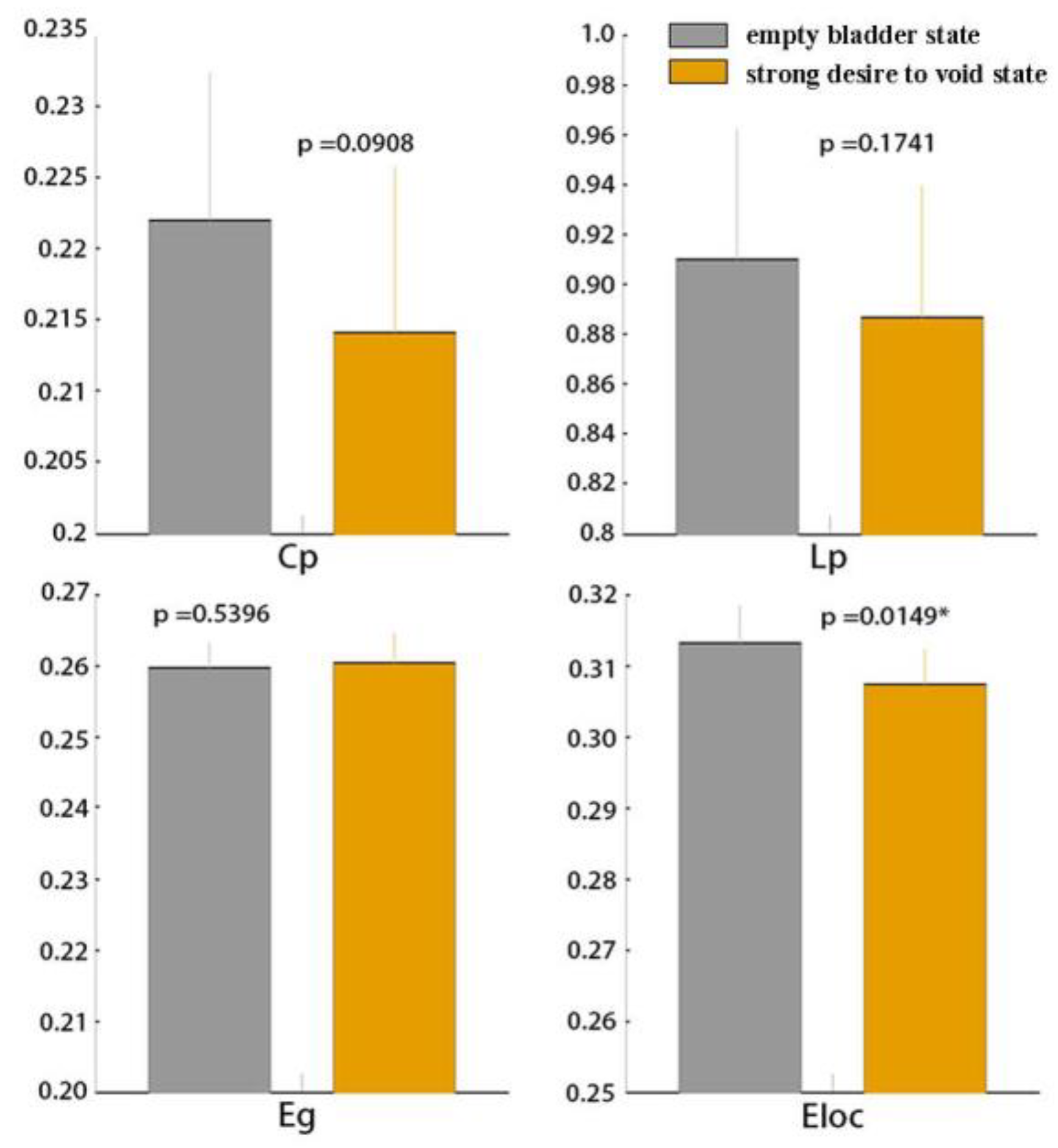
3.1. Global Functional Network Properties
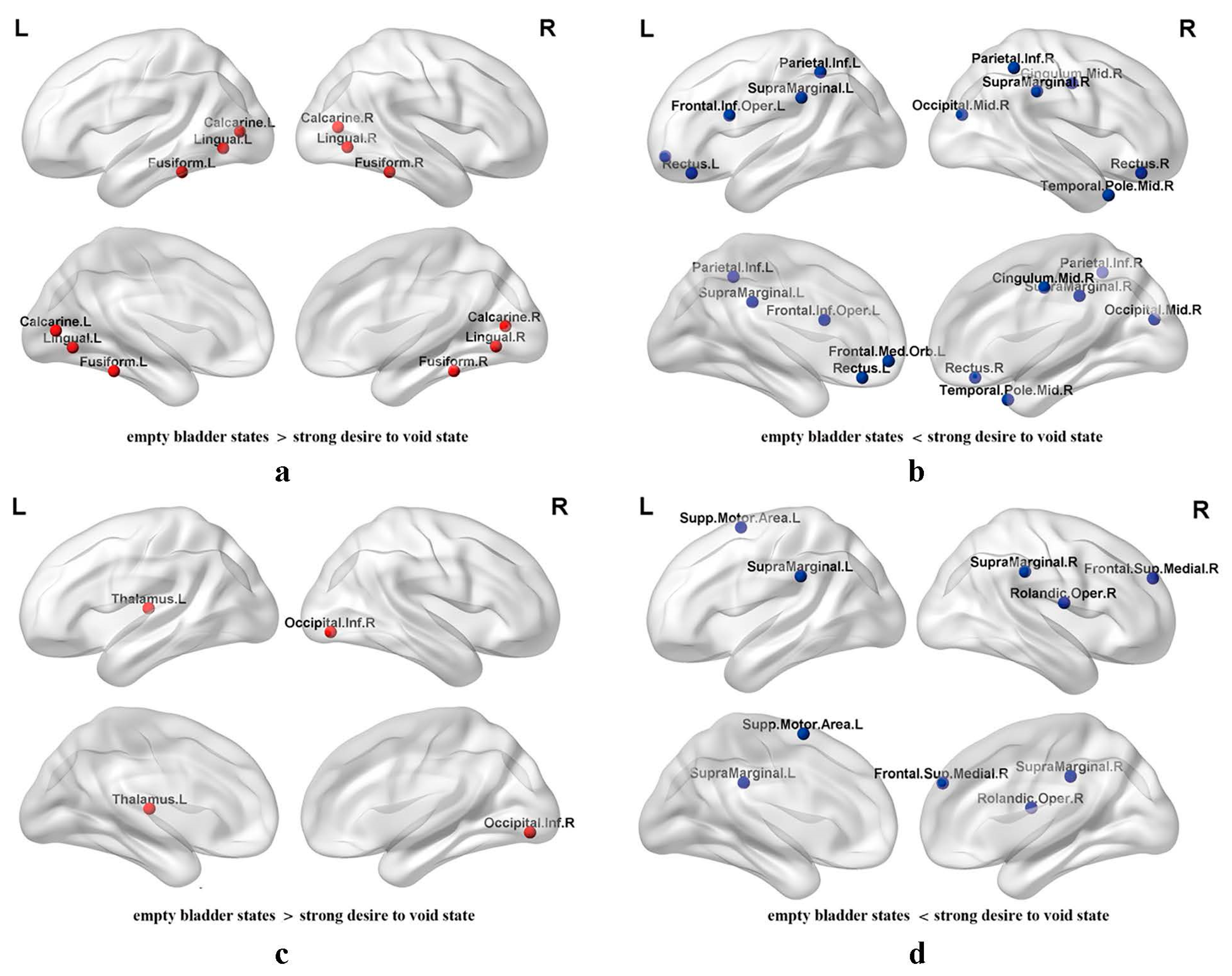
3.2. Regional Nodal Efficiency of Functional Brain Networks
4. Discussion
4.1. Global Graph Metrics
4.2. Regional Nodal Metrics
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.H.; Wang, C.C.; Kuo, H.C. Videourodynamic findings of lower urinary tract dysfunctions in men with persistent storage lower urinary tract symptoms after medical treatment. PLoS ONE 2018, 132, e0190704. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Am. J. Obstet. Gynecol. 2002, 187, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Rosier, P.F.W.M.; Schaefer, W.; Lose, G.; Goldman, H.B.; Guralnick, M.; Eustice, S.; Dickinson, T.; Hashim, H. International Continence Society Good Urodynamic Practices and Terms 2016: Urodynamics, uroflowmetry, cystometry, and pressure-flow study. Neurourol. Urodyn. 2017, 36, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Kaynar, M.; Kucur, M.; Kiliç, O.; Akand, M.; Gul, M.; Goktas, S. The Effect of Bladder Sensation on Uroflowmetry Parameters in Healthy Young Men. Neurourol. Urodyn. 2016, 35, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y. OAB symptoms: Assessment and discriminator for etiopathology. Curr. Opin. Urol. 2014, 244, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Savard, E.; Chesnel, C.; Declemy, A.; Hentzen, C.; Charlanes, A.; Le Breton, F.; Amarenco, G. Effect of need to void on Parkinsonian gait. Progrès En Urologie 2020, 30, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Hentzen, C.; Turmel, N.; Chesnel, C.; Charlanes, A.; Le Breton, F.; Ismaël, S.S.; Amarenco, G. Effect of a strong desire to void on walking speed in individuals with multiple sclerosis and urinary disorders. Ann. Phys. Rehabil. Med. 2020, 63, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Clement, K.D.; Burden, H.; Warren, K.; Lapitan, M.C.; Omar, M.I.; Drake, M.J. Invasive urodynamic studies for the management of lower urinary tract symptoms (LUTS) in men with voiding dysfunction. Cochrane Database Syst. Rev. 2015, 28, CD011179. [Google Scholar] [CrossRef] [PubMed]
- Gammie, A.; Kaper, M.; Dorrepaal, C.; Kos, T.; Abrams, P. Signs and Symptoms of Detrusor Underactivity: An Analysis of Clinical Presentation and Urodynamic Tests from a Large Group of Patients Undergoing Pressure Flow Studies. Eur Urol. 2015, 69, 361–369. [Google Scholar] [CrossRef]
- Panza, J.; Hill, B.; Heft, J.; Biller, D. Influence of the urethral pressure transducer in measuring Valsalva leak point pressure in women undergoing multichannel urodynamic testing. Neurourol. Urodyn. 2019, 39, 682–687. [Google Scholar] [CrossRef]
- Griffiths, D. Functional imaging of structures involved in neural control of the lower urinary tract. In Neurology of Sexual and Bladder Disorders of Handb Clin Neurol; Vodusek, D.B., Boller, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 121–133. [Google Scholar]
- Zuo, L.; Chen, J.; Wang, S.; Zhou, Y.; Wang, B.; Gu, H. Intra- and inter-resting-state networks abnormalities in overactive bladder syndrome patients: An independent component analysis of resting-state fMRI. World J. Urol. 2020, 384, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhou, Y.; Wang, S.; Wang, B.; Gu, H.; Chen, J. Abnormal Brain Functional Connectivity Strength in the Overactive Bladder Syndrome: A Resting-State fMRI Study. Urology 2019, 131, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Khavari, R.; Karmonik, C.; Shy, M.; Fletcher, S.; Boone, T. Functional Magnetic Resonance Imaging with Concurrent Urodynamic Testing Identifies Brain Structures Involved in Micturition Cycle in Patients with Multiple Sclerosis. J. Urol. 2017, 197, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, U.; Michels, L.; Zempleni, M.Z.; Schurch, B.; Kollias, S. The Supraspinal Neural Correlate of Bladder Cold Sensation—An fMRI Study. Hum. Brain Mapp. 2011, 32, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Jarrahi, B.; Mantini, D.; Balsters, J.H.; Michels, L.; Kessler, T.M.; Mehnert, U.; Kollias, S.S. Differential functional brain network connectivity during visceral interoception as revealed by independent component analysis of fMRI TIME-series. Hum. Brain Mapp. 2015, 36, 4438–4468. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Gao, Y.; Liao, L.; Ying, X. Brain functional network alterations caused by a strong desire to void in healthy adults: A graph theory analysis study. Neurourol. Urodyn. 2020, 39, 1966–1976. [Google Scholar] [CrossRef]
- Deruyver, Y.; Hakim, L.; Franken, J.; De Ridder, D. The use of imaging techniques in understanding lower urinary tract (dys)function. Auton. Neurosci. 2016, 200, 11–20. [Google Scholar] [CrossRef]
- Blok, B.F.; De Weerd, H.; Holstege, G. Ultrastructural evidence for apaucity of projections from the lumbosacral cord to the pontine micturition center or M-region in the cat: A new concept for the organization of the micturition reflex with the periaqueductal gray as central relay. J. Comp. Neurol. 1995, 359, 300–309. [Google Scholar] [CrossRef]
- Blok, B.F.; Willemsen, A.T.; Holstege, G. A PET study on brain control of micturition in humans. Brain 1997, 120, 111–121. [Google Scholar] [CrossRef]
- Tai, C.; Wang, J.; Jin, T.; Wang, P.; Kim, S.-G.; Roppolo, J.R.; de Groat, W.C. Brain Switch for Reflex Micturition Control Detected by fMRI in Rats. J. Neurophysiol. 2009, 102, 2719–2730. [Google Scholar] [CrossRef]
- Kershen, R.T.; Kalisvaart, J.; Appell, R.A. Functional brain imaging and the bladder: New insights into cerebral control over micturition. Curr. Urol. Rep. 2003, 4, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.; Derbyshire, S.; Stenger, A.; Resnick, N. Brain control of normal and overactive bladder. J. Urol. 2005, 174, 1862–1867. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Damasio, H.; Damasio, A.R. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000, 10, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Matzel, K.E.; Chartier-Kastler, E.; Knowles, C.H.; Lehur, P.A.; Muñoz-Duyos, A.; Ratto, C.; Rydningen, M.B.; Sørensen, M.; van Kerrebroeck, P.; de Wachter, S. Sacral neuromodulation: Standardized electrode placement technique. Neuromodulation 2017, 20, 816–824. [Google Scholar] [CrossRef]
- Drake, M.J.; Fowler, C.J.; Griffiths, D.; Mayer, E.; Paton, J.F.; Birder, L. Neural control of the lower urinary and gastrointestinal tracts: Supraspinal CNS mechanisms. Neurourol. Urodyn. 2010, 29, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Shuke, N.; Kaneko, S.; Okizaki, A.; Sato, J.; Aburano, T.; Li, Y.; Mizunaga, M.; Yachiku, S. Cerebral control of bladder storage in patients with detrusor overactivity. Nucl. Med. Commun. 2008, 29, 1081–1085. [Google Scholar] [CrossRef]
- Kuhtz-Buschbeck, J.P.; van der Horst, C.; Wolff, S.; Filippow, N.; Nabavi, A.; Jansen, O.; Braun, P.M. Activation of the supplementary motor area (SMA) during voluntary pelvic floor muscle contractions–an fMRI study. Neuroimage 2007, 35, 449–457. [Google Scholar] [CrossRef]
- Raichle, M.E.; Snyder, A.Z. A default mode of brain function: A brief history of an evolving idea. Neuroimage 2007, 37, 1083–1090. [Google Scholar] [CrossRef]
- Davey, C.G.; Harrison, B.J. The brain’s center of gravity: How the default mode network helps us to understand the self. World Psychiatry 2018, 173, 278–279. [Google Scholar] [CrossRef]
- Schwiedrzik, C.M.; Sudmann, S.S.; Thesen, T.; Wang, X.; Groppe, D.M.; Mégevand, P.; Doyle, W.; Mehta, A.D.; Devinsky, O.; Melloni, L. Medial prefrontal cortex supports perceptual memory. Curr. Biol. 2018, 2818, R1094–R1095. [Google Scholar] [CrossRef]
- Griffiths, D.; Tadic, S.D. Bladder control, urgency, and urge incontinence: Evidence from functional brain imaging. Neurourol. Urodyn. 2008, 27, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Tadic, S.D.; Griffiths, D.; Schaefer, W.; Murrin, A.; Clarkson, B.; Resnick, N.M. Brain activity underlying impaired continence control in older women with overactive bladder. Neurourol. Urodyn. 2012, 31, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Schrum, A.; Wolff, S.; van der Horst, C.; Kuhtz-Buschbeck, J.P. Motor cortical representation of the pelvic floor muscles. J. Urol. 2011, 186, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Griffiths, D.J. A decade of functional brain imaging applied to bladder control. Neurourol. Urodyn. 2010, 29, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lou, W.; Zhang, W.; Tong, R.K.; Jin, R.; Peng, W. Gyrus rectus asymmetry predicts trait alexithymia, cognitive empathy, and social function in neurotypical adults. Cereb. Cortex. 2023, 33, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Ballmaier, M.; Toga, A.W.; Blanton, R.E.; Sowell, E.R.; Lavretsky, H.; Peterson, J.; Pham, D.; Kumar, A. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: An MRI-based parcellation of the prefrontal cortex. Am. J. Psychiatry 2004, 161, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Morrell, M.J.; Vogt, B.A. Contributions of anterior cingulate cortex to behaviour. Brain 1995, 118, 279–306. [Google Scholar] [CrossRef]
- Athwal, B.S.; Berkley, K.J.; Hussain, I.; Brennan, A.; Craggs, M.; Sakakibara, R.; Frackowiak, R.S.; Fowler, C.J. Brain responses to changes in bladder volume and urge to void in healthy men. Brain 2001, 124, 369–377. [Google Scholar] [CrossRef]
- Ongür, D.; Price, J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex N. Y. N. 2000, 103, 206–219. [Google Scholar] [CrossRef]
- Critchley, H.D.; Mathias, C.J.; Josephs, O.; John, O.D.; Zanini, S.; Dewar, B.K.; Cipolotti, L.; Shallice, T.; Dolan, R.J. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 2003, 126, 2139–2152. [Google Scholar] [CrossRef]
- Wager, T.D.; Waugh, C.E.; Lindquist, M.; Noll, D.C.; Fredrickson, B.L.; Taylor, S.F. Brain mediators of cardiovascular responses to social threat. Neuroimage 2009, 47, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Botvinick, M.; Carter, C.S. Anterior cingulate and prefrontal cortex: Who’s in control? Nat. Neurosci. 2000, 3, 421–423. [Google Scholar] [CrossRef]
- Gehring, W.J.; Knight, R.T. Prefrontal-cingulate interactions in action monitoring. Nat. Neurosci. 2000, 3, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Karmonik, C.; Soltes, A.; Tran, K.; Lincoln, J.A.; Boone, T.; Khavari, R. Altered bladder-related brain network in multiple sclerosis women with voiding dysfunction. Neurourol. Urodyn. 2022, 41, 1612–1619. [Google Scholar] [CrossRef]
- Khalsa, S.S.; Rudrauf, D.; Feinstein, J.S.; Tranel, D. The pathways of interoceptive awareness. Nat. Neurosci. 2009, 12, 1494–1496. [Google Scholar] [CrossRef]
- Caspers, S.; Geyer, S.; Schleicher, A.; Mohlberg, H.; Amunts, K.; Zilles, K. The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. Neuroimage 2006, 332, 430–448. [Google Scholar] [CrossRef]
- Ben-Shabat, E.; Matyas, T.A.; Pell, G.S.; Brodtmann, A.; Care, L.M. The right supramarginal gyrus is important for Proprioception in healthy and stroke-affected Participants: A Functional MRI study. Front. Neurol. 2015, 6, 248. [Google Scholar] [CrossRef]
- Wada, S.; Honma, M.; Masaoka, Y.; Yoshida, M.; Koiwa, N.; Sugiyama, H.; Iizuka, N.; Kubota, S.; Kokudai, Y.; Yoshikawa, A.; et al. Volume of the right supramarginal gyrus is associated with a maintenance of emotion recognition ability. PLoS ONE 2021, 16, e0254623. [Google Scholar] [CrossRef] [PubMed]
- Fogassi, L.; Luppino, G. Motor functions of the parietal lobe. Curr. Opin. Neurobiol. 2005, 156, 626–631. [Google Scholar] [CrossRef]
- Weinera, K.S.; Zilles, K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia 2016, 83, 48–62. [Google Scholar] [CrossRef]
- Palejwala, A.H.; O’Connor, K.P.; Milton, C.K.; Anderson, C.; Pelargos, P.; Briggs, R.G.; Conner, A.K.; O’Donoghue, D.L.; Glenn, C.A.; Sughrue, M.E. Anatomy and white matter connections of the fusiform gyrus. Sci. Rep. 2020, 10, 13489. [Google Scholar] [CrossRef] [PubMed]
- Ketai, L.H.; Komesu, Y.M.; Dodd, A.B.; Rogers, R.G.; Ling, J.M.; Mayer, A.R. Urgency urinary incontinence and the interoceptive network: A functional magnetic resonance imaging study. Am. J. Obstet. Gynecol. 2016, 215, 449.e1–449.e17. [Google Scholar] [CrossRef] [PubMed]
- Nardos, R.; Karstens, L.; Carpenter, S.; Aykes, K.; Krisky, C.; Stevens, C.; Gregory, W.T.; Fair, D.A. Abnormal functional connectivity in women with urgency urinary incontinence: Can we predict disease presence and severity in individual women using Rs-fcMRI. Neurourol. Urodyn. 2016, 35, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Uono, S.; Sato, W.; Kochiyama, T.; Kubota, Y.; Sawada, R.; Yoshimura, S.; Toichi, M. Time course of gamma-band oscillation associated with face processing in the inferior occipital gyrus and fusiform gyrus: A combined fMRI and MEG study. Hum. Brain Mapp. 2017, 384, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Blok, B.; Sturms, L.M.; Holstege, G. Brain activation during micturition in women. Brain 1998, 121, 2033–2042. [Google Scholar] [CrossRef]
- Arya, N.G.; Weissbart, S.J.; Xu, S.; Rao, H. Brain Activation in Response to Bladder Filling in Healthy Adults: An Activation Likelihood Estimation Meta-Analysis of Neuroimaging Studies. Neurourol. Urodyn. 2017, 36, 960–965. [Google Scholar] [CrossRef]

| Characteristics | Females | Males |
|---|---|---|
| Numbers | 10 | 10 |
| Age (mean ± SD) | 51.4 ± 5.9 | 51.6 ± 5.4 |
| Education (years) | 7.2 ± 1.6 | 10.9 ± 3.7 |
| Mean score of visual analog scale | 6.7 ± 0.9 | 6.9 ± 0.8 |
| Bladder volume under strong desire to void (mL) | 436.0 ± 129.7 | 407.0 ± 139.1 |
| Adverse events after the study | ||
| Frequency/Urgency/Leakage (times) | 0 | 0 |
| Dysuria/Urinary retention/Hematuria | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, X.; Gao, Y.; Liao, L. Brain Responses Difference between Sexes for Strong Desire to Void: A Functional Magnetic Resonance Imaging Study in Adults Based on Graph Theory. J. Clin. Med. 2024, 13, 4284. https://doi.org/10.3390/jcm13154284
Ying X, Gao Y, Liao L. Brain Responses Difference between Sexes for Strong Desire to Void: A Functional Magnetic Resonance Imaging Study in Adults Based on Graph Theory. Journal of Clinical Medicine. 2024; 13(15):4284. https://doi.org/10.3390/jcm13154284
Chicago/Turabian StyleYing, Xiaoqian, Yi Gao, and Limin Liao. 2024. "Brain Responses Difference between Sexes for Strong Desire to Void: A Functional Magnetic Resonance Imaging Study in Adults Based on Graph Theory" Journal of Clinical Medicine 13, no. 15: 4284. https://doi.org/10.3390/jcm13154284







