Aural Manifestations of Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis—Diagnosis, Symptoms, Treatment
Abstract
:1. Characteristics of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis
2. Aural Manifestations of ANCA Vasculitis
OMAAV Diagnostic Criteria
- Intractable otitis media with effusion or granulation, which is resistant to antibiotics and the insertion of a tympanostomy tube.
- The progressive deterioration of bone conduction hearing levels.
- Already diagnosed as AAV (GPA, MPA, or EGPA) based on the involvement of other organs.
- Positivity for serum MPO- or PR3-ANCA.
- Histopathology consistent with AAV, i.e., necrotizing vasculitis predominantly affecting small vessels with or without granulomatous extravascular inflammation.
- At least one of the following accompanying signs/symptoms of AAV-related involvement is witnessed:
- Involvement with upper airway tracts other than ears, scleritis, lungs, and/or kidneys;
- Facial palsy;
- Hypertrophic pachymeningitis;
- Multiple mononeuropathy;
- Transient alleviation of symptoms/signs with administration of 0.5–1.0 mg/kg prednisolone and relapse with discontinuation of treatment.
- Cholesteatoma;
- Cholesterol granuloma;
- Eosinophilic otitis media;
- Tuberculosis;
- Malignant otitis externa, skull-base osteomyelitis;
- Neoplasms (malignancy, inflammatory myofibroblastic tumor, etc.);
- Otitis media or inner ear inflammation caused by autoimmune diseases and vasculitis other than AAV.
3. Diagnosis
Temporal Bone Radiologic Findings
- Hearing loss/ear fullness with the opacification of temporal air cells, without bone destruction and an intact ossicular chain, implies OME (either an acute or chronic phase).
- Pain of the mastoid, purulent and/or bloody discharge, acute hearing loss, fever, and elevated inflammation markers, with the opacification of temporal air cells, without bone destruction, and an intact ossicular chain suggest acute otitis media.
- Purulent and/or bloody discharge, typically of an unpleasant smell, usually without or with moderate pain, chronic hearing loss without fever, and signs of acute inflammation with the opacification of temporal air cells, with bone and ossicular chain destruction, indicate chronic otitis media with or without cholesteatoma.
- Chronic vertigo and sometimes concomitant hearing loss, usually without spontaneous nystagmus and sympathetic symptoms, suggest chronic labyrinthitis in the final stage of the ossification of the inner ear.
- Hearing loss/ear fullness with opacification in computer tomography and fluid signal in the middle ear cavity and mastoid antrum in magnetic resonance suggest OME or acute otitis media when there are additional symptoms like the pain of the mastoid, purulent and/or bloody discharge, acute hearing loss, fever, and elevated inflammation markers or intra-/extratemporal complications of otitis media. Although magnetic resonance is not required for the diagnosis of OME and acute otitis media, it should be performed when complications are suspected.
- In patients with chronic otitis media, magnetic resonance is not usually needed. It could be helpful in cholesteatoma/neoplasm differentiation or if intra-/extratemporal complications are suspected.
- In cholesteatoma, non-echoplanar diffusion-weighted imaging (non-EP DWI, with a b parameter of 800–1000) with Apparent Diffusion Coefficient (ADC) map modalities are helpful. Lesions that are hyperintensive in T2 and non-EP DWI but hypointensive in ADC are most likely cholesteatomas.
- Acute labyrinthitis with sudden symptoms of vertigo with nausea/vomiting, hearing loss, and nystagmus is seen as an enhancement of the inflamed inner ear in MRI with contrast enhancement.
- Chronic labyrinthitis with the symptoms listed before. Whereas the bony obliteration of the inner ear is readily identified using CT, fibrous obliteration is recognizable only using MR imaging. In the T2 images, the high signal seen within the normal inner ear structures is absent, therefore making the involved structures no longer recognizable.
- In peripheral facial nerve paresis, magnetic resonance is the modality of choice in the case of facial nerve paresis. CT can only indirectly assess the intratemporal part of the facial nerve; magnetic resonance can assess all segments of the VII cranial nerve. Contrast enhancement and/or a specific type of MR imaging are used depending on the localization of the facial nerve segment. Three-dimensional heavily T2-weighted MR sequences are usually used to describe the facial nerve in its cisternal and intracanalicular segments. When it is healthy, it is usually depicted as a dark linear structure against bright cerebrospinal fluid. The thickening of the nerve into the internal auditory canal and the inner ear on the affected side should be treated as pathology. On post-contrast T1-weighted images, the facial nerve is the only cranial nerve that may show normal post-contrast enhancement, sometimes asymmetric, due to arteriovenous plexus around certain parts of the nerve. Typical sites of enhancement in healthy patients are the fundal canalicular, anterior genu, and posterior genu with the proximal mastoid segment. Meatal, intralabyrinthic, and extracranial parts should show no enhancement in healthy individuals. In cases of idiopathic paresis or viral neuritis, the postgadolinum enhancement is seen from the distal internal auditory canal (intralabyrinthine segment) to the distal tympanic segment and it is called “fundal tuft sign”. The asymmetric enhancement of the geniculate ganglion can also be observed on the affected site. Although there is a pathological enhancement in contrast-enhanced 3D MRI with gradient-echo sequences (CE-3D-GRE), in ANCA patients with OMAAV syndrome, particularly in tympanic and mastoid segments, which corresponds to the intensity of middle ear inflammation, it is not connected with facial nerve paresis [39]. On the other hand, Kato et al. used 3D-FLAIR MRI to assess patients with facial nerve paresis and the conclusion was that the post-contrast enhancement was connected with facial nerve inflammation and paresis [53]. More studies in this field should be conducted to establish clear guidelines. Moreover, facial nerve paresis can be one of the symptoms of hypertrophic pachymeningitis—see below.
- Hypertrophic pachymeningitis—asymptomatic or associated with headaches ophthalmological symptoms, cranial nerve involvement (including the VII and VIII cranial nerves), and fever. As it was written before, the thickening and enhancement of the dura mater on T1-weighted gadolinium-enhanced MRI suggests HP [54,55].
- Other modalities
4. GPA vs. EGPA vs. MPA—Do They Differ in Aural Manifestations?
5. Other OMAAV
5.1. Anti-Thyroid Drug-Associated OMAAV
5.2. OMAAV COVID
6. Differential Diagnosis
- Cholesterol granuloma;
- Middle ear tumor;
- Bacterial otitis media (diabetes mellitus, old aged, P. aeruginosa: skull base osteomyelitis);
- Tuberculosis otitis media;
- Eosinophilic otitis media (EOM);
- IgG4-RD otitis media.
6.1. EOM vs. OMAAV
6.1.1. Eosinophilic Otitis Media Diagnostic Criteria [66]
- Major: otitis media with effusion or chronic otitis media with eosinophil—dominant effusion.
- Minor:
- Highly viscous middle ear effusion;
- Resistance to conventional treatment for otitis media;
- Association with bronchial asthma;
- Association with nasal polyposis.
6.2. IgG4-RD vs. OMAAV
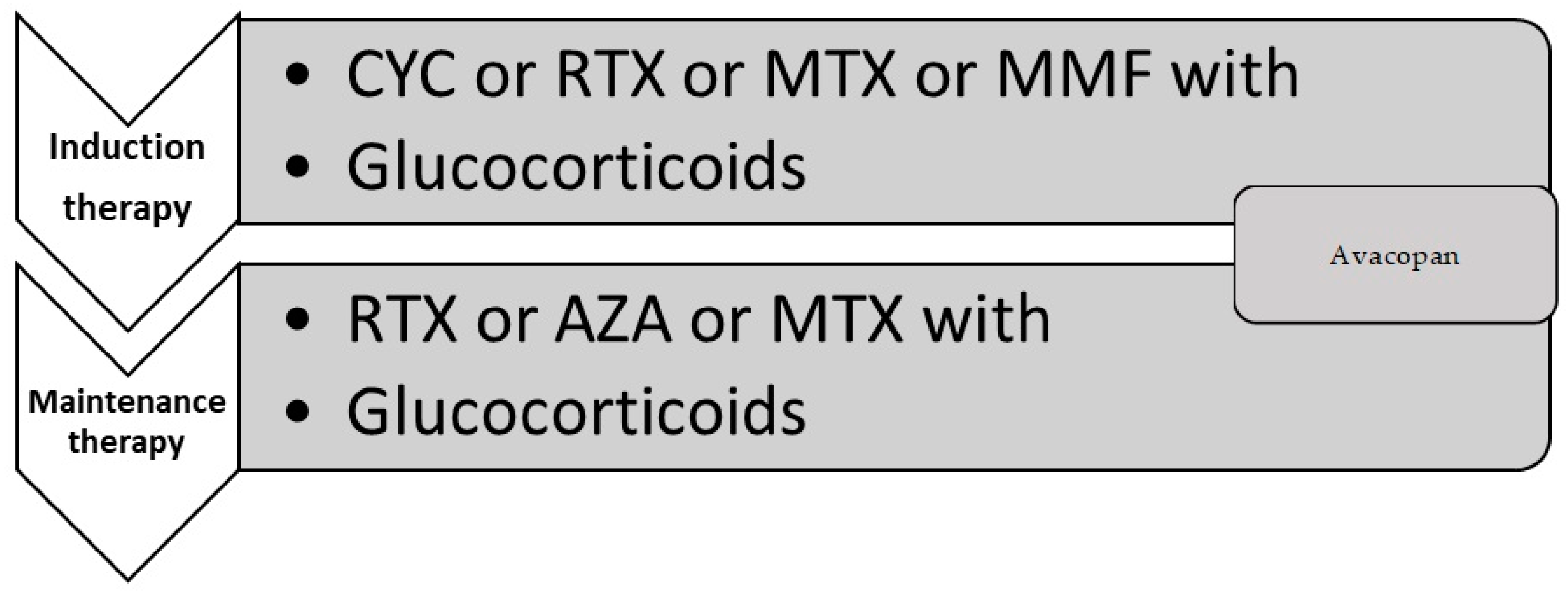
7. Treatment
7.1. Controlling the Treatment
- Facial palsy;
- Hypertrophic pachymeningitis;
- Negativity for both MPO-ANCA and PR3-ANCA;
- Disease relapse;
- Treatment with GCs alone.
7.2. OMAAV Antibacterial Treatment
7.3. Pharmacology
7.4. Surgery
7.5. Cochlear Implantation
7.5.1. Current Adult Selection Criteria for Cochlear Implantation [89]
- Severe or profound hearing loss with a pure-tone average (PTA) of 70 dB or greater hearing level (HL);
- Use of appropriately fitted hearing aids or a trial with amplification;
- Aided scores on open-set sentence tests of less than 50% in the ear to be implanted and 60% in the contralateral ear;
- No evidence of central auditory lesions or lack of an auditory nerve;
- No evidence of contraindications for surgery in general or CI surgery in particular.
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Robson, J.C.; Grayson, P.C.; Ponte, C.; Suppiah, R.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Watts, R.A.; Merkel, P.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann. Rheum. Dis. 2022, 81, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, R.; Robson, J.C.; Grayson, P.C.; Ponte, C.; Craven, A.; Khalid, S.; Judge, A.; Hutchings, A.; Merkel, P.A.; Luqmani, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann. Rheum. Dis. 2022, 81, 321–326. [Google Scholar] [CrossRef]
- Felicetti, M.; Cazzador, D.; Padoan, R.; Pendolino, A.L.; Faccioli, C.; Nardello, E.; Berti, A.; Silvestrini, M.; Paolazzi, G.; Brunori, G.; et al. Ear, nose and throat involvement in granulomatosis with polyangiitis: How it presents and how it determines disease severity and long-term outcomes. Clin. Rheumatol. 2018, 37, 1075–1083. [Google Scholar] [CrossRef]
- Jennings, C.R.; Jones, N.S.; Dugar, J.; Powell, R.J.; Lowe, J. Wegener’s granulomatosis—A review of diagnosis and treatment in 53 subjects. Rhinology 1998, 36, 188–191. [Google Scholar]
- Martinez Del Pero, M.; Rasmussen, N.; Chaudhry, A.; Jani, P.; Jayne, D. Structured clinical assessment of the ear, nose and throat in patients with granulomatosis with polyangiitis (Wegener’s). Eur. Arch. Otorhinolaryngol. 2013, 270, 345–354. [Google Scholar] [CrossRef]
- Wojciechowska, J.; Krajewski, W.; Krajewski, P.; Kręcicki, T. Granulomatosis with Polyangiitis in Otolaryngologist Practice: A Review of Current Knowledge. Clin. Exp. Otorhinolaryngol. 2016, 9, 8–13. [Google Scholar] [CrossRef]
- Srouji, I.A.; Andrews, P.; Edwards, C.; Lund, V.J. Patterns of presentation and diagnosis of patients with Wegener’s granulomatosis: ENT aspects. J. Laryngol. Otol. 2006, 121, 653–658. [Google Scholar] [CrossRef]
- Morales-Angulo, C.; García-Zornoza, R.; Obeso-Agüera, S.; Calvo-Alén, J.; González-Gay, M.A. Manifestaciones otorrinolaringológicas en pacientes con granulomatosis de Wegener (granulomatosis con poliangeitis). Acta Otorrinolaringológica Española 2012, 63, 206–211. [Google Scholar] [CrossRef]
- Puéchal, X. Antineutrophil cytoplasmic antibody-associated vasculitides. Jt. Bone Spine 2007, 74, 427–435. [Google Scholar] [CrossRef]
- Wojciechowska, J.; KręCicki, T. Clinical characteristics of patients with granulomatosis with polyangiitis and microscopic polyangiitis in ENT practice: A comparative analysis. Acta Otorhinolaryngol. Ital. 2018, 38, 517–527. [Google Scholar] [CrossRef]
- Trimarchi, M.; Sinico, R.A.; Teggi, R.; Bussi, M.; Specks, U.; Meroni, P.L. Otorhinolaryngological manifestations in granulomatosis with polyangiitis (Wegener’s). Autoimmun. Rev. 2013, 12, 501–505, Erratum in: Autoimmun Rev. 2015, 14, 80. [Google Scholar] [CrossRef]
- Nishino, H.; Rubino, F.A.; DeRemee, R.A.; Swanson, J.W.; Parisi, J.E. Neurological involvement in Wegener’s granulomatosis: An analysis of 324 consecutive patients at the Mayo Clinic. Ann. Neurol. 1993, 33, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Comarmond, C.; Pagnoux, C.; Khellaf, M.; Cordier, J.F.; Hamidou, M.; Viallard, J.F.; Maurier, F.; Jouneau, S.; Bienvenu, B.; Puéchal, X.; et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013, 65, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Durel, C.A.; Berthiller, J.; Caboni, S.; Jayne, D.; Ninet, J.; Hot, A. Long-Term Followup of a Multicenter Cohort of 101 Patients with Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss). Arthritis Care Res. 2016, 68, 374–387. [Google Scholar] [CrossRef]
- Petersen, H.; Götz, P.; Both, M.; Hey, M.; Ambrosch, P.; Bremer, J.P.; Holle, J.; Moosig, F.; Laudien, M. Manifestation of eosinophilic granulomatosis with polyangiitis in head and neck. Rhinology 2015, 53, 277–285. [Google Scholar] [CrossRef]
- Seo, P.; Stone, J.H. The antineutrophil cytoplasmic antibody-associated vasculitides. Am. J. Med. 2004, 117, 39–50. [Google Scholar] [CrossRef]
- Sinico, R.A.; Di Toma, L.; Maggiore, U.; Bottero, P.; Radice, A.; Tosoni, C.; Grasselli, C.; Pavone, L.; Gregorini, G.; Monti, S.; et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 2005, 52, 2926–2935. [Google Scholar] [CrossRef]
- Moosig, F.; Bremer, J.P.; Hellmich, B.; Holle, J.U.; Holl-Ulrich, K.; Laudien, M.; Matthis, C.; Metzler, C.; Nölle, B.; Richardt, G.; et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): Monocentric experiences in 150 patients. Ann. Rheum. Dis. 2013, 72, 1011–1017. [Google Scholar] [CrossRef]
- Millet, A.; Pederzoli-Ribeil, M.; Guillevin, L.; Witko-Sarsat, V.; Mouthon, L. Antineutrophil cytoplasmic antibody-associated vasculitides: Is it time to split up the group? Ann. Rheum. Dis. 2013, 72, 1273–1279. [Google Scholar] [CrossRef]
- Greco, A.; De Virgilio, A.; Rizzo, M.I.; Gallo, A.; Magliulo, G.; Fusconi, M.; Ruoppolo, G.; Tombolini, M.; Turchetta, R.; de Vincentiis, M. Microscopic polyangiitis: Advances in diagnostic and therapeutic approaches. Autoimmun. Rev. 2015, 14, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.Y.; Lee, L.E.; Park, Y.B.; Lee, S.W. Comparison of the 2022 ACR/EULAR Classification Criteria for Antineutrophil Cytoplasmic Antibody-Associated Vasculitis with Previous Criteria. Yonsei Med. J. 2023, 64, 11–17. [Google Scholar] [CrossRef]
- Luqmani, R.A.; Bacon, P.A.; Moots, R.J.; Janssen, B.A.; Pall, A.; Emery, P.; Savage, C.; Adu, D. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 1994, 87, 671–678. [Google Scholar] [PubMed]
- Prieto, L.; Santed, R.; Cobo, E.; Alonso, J. A new measure for assessing the health-related quality of life of patients with vertigo, dizziness or imbalance: The VDI questionnaire. Qual. Life Res. 1999, 8, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Iino, Y. Pathogenesis and Diagnosis of Otitis Media with ANCA-Associated Vasculitis. Allergol. Int. 2014, 63, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Badia, R.R.; Hendricks, A.R.; Perez, C.L.; Sertich, A.; Ripley, L. Unique Presentation of Microscopic Polyangiitis: Hearing and Vision Loss, Dysphagia, and Renal Dysfunction. Cureus 2021, 13, e14069. [Google Scholar] [CrossRef]
- Ashman, P.E.; Chen, T.; Barinsky, G.L.; Benson, B.; Babu, S.; Bojrab, D.I., 2nd; Svider, P.F. Otologic Manifestations of Eosinophilic Granulomatosis with Polyangiitis: A Systematic Review. Otol. Neurotol. 2021, 42, e380–e387. [Google Scholar] [CrossRef]
- Kawashima, Y.; Noguchi, Y.; Ito, T.; Mizushima, K.; Takahashi, M.; Kitamura, K.; Tsutsumi, T. Otologic Manifestations in Patients with ANCA Associated Vasculitis-Comparative Analysis among Microscopic Polyangiitis, Granulomatosis with Polyangiitis and Eosinophilic Granulomatosis with Polyangiitis. Nippon Jibiinkoka Gakkai Kaiho 2016, 119, 110–117. [Google Scholar] [CrossRef]
- Harabuchi, Y.; Kishibe, K.; Tateyama, K.; Morita, Y.; Yoshida, N.; Okada, M.; Kunimoto, Y.; Watanabe, T.; Inagaki, A.; Yoshida, T.; et al. Clinical characteristics, the diagnostic criteria and management recommendation of otitis media with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (OMAAV) proposed by Japan Otological Society. Auris Nasus Larynx. 2021, 48, 2–14. [Google Scholar] [CrossRef]
- Harabuchi, Y.; Kishibe, K.; Tateyama, K.; Morita, Y.; Yoshida, N.; Kunimoto, Y.; Matsui, T.; Sakaguchi, H.; Okada, M.; Watanabe, T.; et al. Clinical features and treatment outcomes of otitis media with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (OMAAV): A retrospective analysis of 235 patients from a nationwide survey in Japan. Mod. Rheumatol. 2017, 27, 87–94. [Google Scholar] [CrossRef]
- Illum, P.; Thorling, K. Otological Manifestations of Wegeners Granulomatosis. Laryngoscope 1982, 92, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kitazawa, M.; Yagi, C.; Takahashi, K.; Ohshima, S.; Yamagishi, T.; Izumi, S.; Horii, A. Locations and Predictive Factors of Hypertrophic Pachymeningitis in Otitis Media with Antineutrophil Cytoplasmic Antigen-Associated Vasculitis. Otol. Neurotol. 2022, 43, e835–e840. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Shin, J.J.; Schwartz, S.R.; Coggins, R.; Gagnon, L.; Hackell, J.M.; Hoelting, D.; Hunter, L.L.; Kummer, A.W.; Payne, S.C.; et al. Clinical Practice Guideline: Otitis Media with Effusion (Update). Otolaryngol. Head. Neck Surg. 2016, 154 (Suppl. S1), S1–S41. [Google Scholar] [CrossRef]
- Morita, Y.; Kitazawa, M.; Yagi, C.; Nonomura, Y.; Takahashi, K.; Yamagishi, T.; Ohshima, S.; Izumi, S.; Horii, A. Tympanic membrane findings of otitis media with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (OMAAV). Auris Nasus Larynx. 2020, 47, 740–746. [Google Scholar] [CrossRef]
- Morita, S.; Nakamaru, Y.; Nakazawa, D.; Suzuki, M.; Hoshino, K.; Fukuda, A.; Hattanda, F.; Kusunoki, K.; Tomaru, U.; Ishizu, A.; et al. The Diagnostic and Clinical Utility of the Myeloperoxidase-DNA Complex as a Biomarker in Otitis Media with Antineutrophil Cytoplasmic Antibody-associated Vasculitis. Otol. Neurotol. 2019, 40, e99–e106. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Salviz, M.; Domond, H.; Nadol, J.B. Otopathology of Vasculitis in Granulomatosis with Polyangitis. Otol. Neurotol. 2015, 36, 1657–1662. [Google Scholar] [CrossRef]
- Okada, M.; Suemori, K.; Takagi, D.; Teraoka, M.; Yamada, H.; Ishizaki, J.; Matsumoto, T.; Hasegawa, H.; Hato, N. The treatment outcomes of rituximab for intractable otitis media with ANCA-associated vasculitis. Auris Nasus Larynx. 2019, 46, 38–42. [Google Scholar] [CrossRef]
- Holle, J.U.; Gross, W.L. Neurological involvement in Wegener’s granulomatosis. Curr. Opin. Rheumatol. 2011, 23, 7–11. [Google Scholar] [CrossRef]
- Fujikawa, T.; Honda, K.; Ito, T.; Kishino, M.; Kimura, N.; Umezawa, N.; Hirano, M.; Aoki, N.; Kawashima, Y.; Tsutsumi, T. Enhanced fallopian canal as a potential marker for temporal bone vasculitis. Laryngoscope Investig. Otolaryngol. 2020, 5, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yoshida, H.; Kishibe, K.; Morita, Y.; Yoshida, N.; Takahashi, H.; Harabuchi, Y. Cochlear implantation in patients with bilateral deafness caused by otitis media with ANCA-associated vasculitis (OMAAV): A report of four cases. Auris Nasus Larynx. 2018, 45, 922–928. [Google Scholar] [CrossRef]
- Murao, Y.; Yoshida, Y.; Oka, N.; Yorishima, A.; Masuda, S.; Sugimoto, T.; Ono, R.; Hirokawa, Y.; Hirata, S. Fluorodeoxyglucose-positron emission tomography/computed tomography-positive ear lesions responsive to immunosuppressive therapy in a patient with otitis media with antineutrophil cytoplasmic antibody-associated vasculitis. Mod. Rheumatol. Case Rep. 2023, 7, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Hahn, L.D.; Fulbright, R.; Baehring, J.M. Hypertrophic pachymeningitis. J. Neurol. Sci. 2016, 367, 278–283. [Google Scholar] [CrossRef]
- Roszkowska, A.; Morawska-Kochman, M.; Temporale, H.; Sikorska-Żuk, M.; Kręcicki, T. Bilateral Facial Palsy in Rapidly Progressive Course of Wegener’s Granulomatosis: A Case Report. Case Rep. Otolaryngol. 2013, 2013, 875108. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Morita, S.; Fukuda, A.; Yanagi, H.; Hoshino, K.; Nakamaru, Y.; Furuta, Y.; Homma, A. Characteristics of and Prognosis for Facial Palsy in Patients with Otitis Media With ANCA-Associated Vasculitis (OMAAV). Otol. Neurotol. 2021, 42, e1577–e1582. [Google Scholar] [CrossRef] [PubMed]
- Marszał, J.; Bartochowska, A.; Yu, R.; Wierzbicka, M. Facial nerve paresis in the course of masked mastoiditis as a revelator of GPA. Eur. Arch. Otorhinolaryngol. 2022, 279, 4271–4278. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Takahashi, K.; Izumi, S.; Kubota, Y.; Ohshima, S.; Horii, A. Vestibular Involvement in Patients with Otitis Media with Antineutrophil Cytoplasmic Antibody-associated Vasculitis. Otol. Neurotol. 2017, 38, 97–101. [Google Scholar] [CrossRef]
- Takagi, D.; Nakamaru, Y.; Maguchi, S.; Furuta, Y.; Fukuda, S. Otologic manifestations of Wegener’s granulomatosis. Laryngoscope 2002, 112, 1684–1690. [Google Scholar] [CrossRef]
- Fujiwara, K.; Morita, S.; Fukuda, A.; Yanagi, H.; Hoshino, K.; Nakamaru, Y.; Homma, A. Usefulness of the Video Head Impulse Test for the Evaluation of Vestibular Function in Patients with Otitis Media with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Otol. Neurotol. 2021, 42, e483–e488. [Google Scholar] [CrossRef]
- Imafuku, A.; Sawa, N.; Kawada, M.; Hiramatsu, R.; Hasegawa, E.; Yamanouchi, M.; Hoshino, J.; Ubara, Y.; Takaichi, K. Incidence and risk factors of new-onset hypertrophic pachymeningitis in patients with anti-neutrophil antibody-associated vasculitis: Using logistic regression and classification tree analysis. Clin. Rheumatol. 2019, 38, 1039–1046. [Google Scholar] [CrossRef]
- Yonekawa, T.; Murai, H.; Utsuki, S.; Matsushita, T.; Masaki, K.; Isobe, N.; Yamasaki, R.; Yoshida, M.; Kusunoki, S.; Sakata, K.; et al. A nationwide survey of hypertrophic pachymeningitis in Japan. J. Neurol. Neurosurg. Psychiatry. 2014, 85, 732–739. [Google Scholar] [CrossRef]
- Yoshida, N. Intractable otitis media—Pathogenesis and treatment of Eosinophilic otitis media (EOM) and otitis media with Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (OMAAV). Auris Nasus Larynx. 2023, 50, 171–179. [Google Scholar] [CrossRef]
- Tateyama, K.; Kodama, S.; Nomi, N.; Suzuki, M.; Kishibe, K.; Harabuchi, Y. Serological Study of Otitis Media with ANCA Associated Vasculitis (OMAAV). Nihon Jibiinkoka Gakkai Kaiho 2015, 118, 1133–1142. [Google Scholar] [CrossRef]
- Kato, K.; Sone, M.; Teranishi, M.; Yoshida, T.; Otake, H.; Nakashima, T.; Naganawa, S. Inner ear 3D-FLAIR magnetic resonance image evaluation of MPO-ANCA related angitis patients. Nihon Jibiinkoka Gakkai Kaiho. 2013, 116, 1192–1199. [Google Scholar] [CrossRef]
- Guan, W.J.; Ding, Y.X.; Liu, L.J.; Li, W.; Jing, L.J.; Zhang, X.; Zhang, L.J.; Li, H.; Cheng, S.H.; Liu, S.Y. Clinical analysis of 15 cases with myeloperoxidase antineutrophil cytoplasmic antibody associated hypertrophic pachymeningitis. Zhonghua Nei Ke Za Zhi. 2022, 61, 565–569. (In Chinese) [Google Scholar] [CrossRef]
- Peng, A.; Yang, X.; Wu, W.; Xiao, Z.; Xie, D.; Ge, S. Anti-neutrophil cytoplasmic antibody-associated hypertrophic cranial pachymeningitis and otitis media: A review of literature. Eur. Arch. Otorhinolaryngol. 2018, 275, 2915–2923. [Google Scholar] [CrossRef]
- Ishii, S.; Sugawara, S.; Yamakuni, R.; Sekino, H.; Ito, H. Hypertrophic Pachymeningitis Demonstrated by Whole-Body 67Ga Scintigraphy. Clin. Nucl. Med. 2022, 47, e149–e151. [Google Scholar] [CrossRef]
- Ono, N.; Niiro, H.; Ueda, A.; Sawabe, T.; Nishizaka, H.; Furugo, I.; Yoshizawa, S.; Yoshizawa, S.; Tsukamoto, H.; Kiyohara, C.; et al. Characteristics of MPO-ANCA-positive granulomatosis with polyangiitis: A retrospective multi-center study in Japan. Rheumatol. Int. 2015, 35, 555–559. [Google Scholar] [CrossRef]
- Gioffredi, A.; Maritati, F.; Oliva, E.; Buzio, C. Eosinophilic granulomatosis with polyangiitis: An overview. Front. Immunol. 2014, 5, 549. [Google Scholar] [CrossRef]
- Noh, J.Y.; Yasuda, S.; Sato, S.; Matsumoto, M.; Kunii, Y.; Noguchi, Y.; Mukasa, K.; Ito, K.; Ito, K.; Sugiyama, O.; et al. Clinical characteristics of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis caused by antithyroid drugs. J. Clin. Endocrinol. Metab. 2009, 94, 2806–2811. [Google Scholar] [CrossRef]
- Hiruma, M.; Sasano, Y.; Watanabe, N.; Yoshihara, A.; Ishii, S.; Yaguchi, Y.; Yoshimura Noh, J.; Sugino, K.; Ito, K. Propylthiouracil-induced otitis media with anti-neutrophil cytoplasmic antibody-associated vasculitis: A case report and review of the literature. Endocr. J. 2021, 68, 145–151. [Google Scholar] [CrossRef]
- Kimura, S.; Ikeda-Saito, M. Human myeloperoxidase and thyroid peroxidase, two enzymes with separate and distinct physiological functions, are evolutionarily related members of the same gene family. Proteins 1988, 3, 113–120. [Google Scholar] [CrossRef]
- Waldhauser, L.; Uetrecht, J. Oxidation of propylthiouracil to reactive metabolites by activated neutrophils. Implic. Agranulocytosis. Drug Metab. Dispos. 1991, 19, 354–359. [Google Scholar]
- Shirai, T.; Suzuki, J.; Kuniyoshi, S.; Tanno, Y.; Fujii, H. Granulomatosis with polyangiitis following Pfizer-BioNTech COVID-19 vaccination. Mod. Rheumatol. Case Rep. 2023, 7, 127–129. [Google Scholar] [CrossRef]
- Morita, S.; Nakamaru, Y.; Fukuda, A.; Fujiwara, K.; Suzuki, M.; Hoshino, K.; Honma, A.; Homma, A. The Quantification of Extracellular Trap Cell Death-Derived Products as Diagnostic Biomarkers for Otitis Media with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis and Eosinophilic Otitis Media. Otol. Neurotol. 2022, 43, e337–e343. [Google Scholar] [CrossRef]
- Fukuda, A.; Morita, S.; Nakamaru, Y.; Hoshino, K.; Fujiwara, K.; Homma, A. Differentiation Between Eosinophilic Otitis Media and Otitis Media Associated with Eosinophilic Granulomatosis with Polyangiitis. Otol. Neurotol. 2019, 40, e796–e802. [Google Scholar] [CrossRef]
- Iino, Y.; Tomioka-Matsutani, S.; Matsubara, A.; Nakagawa, T.; Nonaka, M. Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx. 2011, 38, 456–461. [Google Scholar] [CrossRef]
- Polianskis, M.; Ivaška, J.; Dadonienė, J.; Lengvenis, G.; Besusparis, J.; Rauba, D.; Morozas, A.; Ivaškienė, T.; Lesinskas, E. Immunoglobulin G4-Related Disease Presenting as Temporal Bone Lesion with Facial Nerve Palsy. ORL J. Otorhinolaryngol. Relat. Spec. 2022, 84, 473–479. [Google Scholar] [CrossRef]
- Ohno, K.; Matsuda, Y.; Arai, T.; Sugihara, T.; Iga, S.; Kimura, Y. Myeloperoxidase-Antineutrophil Cytoplasmic Antibody-Positive Otitis Media and Rhinosinusitis with Pathological Features of Immunoglobulin G4-Related Disease: A Case Report. Ann. Otol. Rhinol. Laryngol. 2016, 125, 516–521. [Google Scholar] [CrossRef]
- Okada, M.; Suemori, K.; Takagi, D.; Teraoka, M.; Yamada, H.; Hato, N. Comparison of Localized and Systemic Otitis Media With ANCA-Associated Vasculitis. Otol. Neurotol. 2017, 38, e506–e510. [Google Scholar] [CrossRef]
- Lubianca Neto, J.F.; Lubianca, J.P.N.; Lubianca, M.N. Viral Otitis Media and Acute Otitis Media and Recurrent Acute Otitis Media. An Evidence-Based Approach and Maria Beatriz Rotta Pereira, Manuel Ruttkay Pereira, Denise Rotta Ruttkay Pereira, Vlademir Cantarelli The Microbiology of Otitis Media, Biofilms and Its Implication in the Clinical Treatment. In Textbook of Otitis Media The Basics and Beyond, 1st ed.; Goycoolea, M.V., da Costa, S.S., De Souza, C., Paparella, M.M., Eds.; Springer: Cham, Switzerland, 2023; pp. 177–197. ISBN 978-3-031-40948-6. [Google Scholar]
- Chung, S.A.; Langford, C.A.; Maz, M.; Abril, A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2021, 73, 1366–1383. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, M.F.; Leach, A.J.; Brennan-Jones, C.G. Chronic suppurative otitis media. Lancet 2024, 403, 2339–2348. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Li, W.X.; Li, J. Screening for effective antibiotics in chronic suppurative otitis media. J. Clin. Otorhinolaryngol. Head Neck Surg. 2017, 31, 1243–1246. (In Chinese) [Google Scholar] [CrossRef]
- Brennan-Jones, C.G.; Head, K.; Chong, L.Y.; Burton, M.J.; Schilder, A.G.; Bhutta, M.F. Topical antibiotics for chronic suppurative otitis media. Cochrane Database Syst. Rev. 2020, 1, CD013051. [Google Scholar] [CrossRef]
- Hellmich, B.; Sanchez-Alamo, B.; Schirmer, J.H.; Berti, A.; Blockmans, D.; Cid, M.C.; Holle, J.U.; Hollinger, N.; Karadag, O.; Kronbichler, A.; et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann. Rheum. Dis. 2024, 83, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.R.W.; Merkel, P.A.; Schall, T.J.; Bekker, P.; ADVOCATE Study Group. Avacopan for the Treatment of ANCA-Associated Vasculitis [published correction appears in N. Engl. J. Med. 2024, 390, 388]. N. Engl. J. Med. 2021, 384, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, L.; Pagnoux, C.; Karras, A.; Khouatra, K.; Aumaître, O.; Cohen, P.; Maurier, F.; Decaux, O.; Ninet, J.; Gobert, P.; et al. Rituximab versus Azathioprine for Maintenance in ANCA-Associated Vasculitis. N. Engl. J. Med. 2014, 371, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- de Groot, K.; Harper, L.; Jayne, D.R.; Flores Suarez, L.F.; Gregorini, G.; Gross, W.L.; Luqmani, R.; Pusey, C.D.; Rasmussen, N.; Sinico, R.A.; et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized trial. Ann. Intern. Med. 2009, 150, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Sada, K.E.; Nagasaka, K.; Kaname, S.; Nango, E.; Kishibe, K.; Dobashi, H.; Hiromura, K.; Kawakami, T.; Bando, M.; Wada, T.; et al. Clinical practice guidelines of the Japan Research Committee of the Ministry of Health, Labour, and Welfare for Intractable Vasculitis for the management of microscopic polyangiitis and granulomatosis with polyangiitis: The 2023 update—Secondary publication. Mod Rheumatol. 2024, 34, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Bénard, V.; Farhat, C.; Zarandi-Nowroozi, M.; Durand, M.; Charles, P.; Puéchal, X.; Guillevin, L.; Pagnoux, C.; Makhzoum, J.P. Comparison of Two Rituximab Induction Regimens for Antineutrophil Cytoplasm Antibody-Associated Vasculitis: Systematic Review and Meta-Analysis. ACR Open Rheumatol. 2021, 3, 484–494. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.D.; Amy, K.; Langford Carol, A.; Merkel Peter, A.; Moosig, F.; Specks, U.; Cid Maria, C.; et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef]
- Qaisar, H.; Shenouda, M.; Shariff, M.; Cheema, A.; Tang, X.; Kaplan, A. Granulomatosis with Polyangiitis Manifesting as Refractory Otitis Media and Mastoiditis. Arch. Iran. Med. 2019, 22, 410–413. [Google Scholar]
- Dagum, P.; Roberson, J.B., Jr. Otologic Wegener’s granulomatosis with facial nerve palsy. Ann. Otol. Rhinol. Laryngol. 1998, 107, 555–559. [Google Scholar] [CrossRef]
- Yoshida, T.; Kobayashi, M.; Sugimoto, S.; Naganawa, S.; Sone, M. Labyrinthine calcification in ears with otitis media and antineutrophil cytoplasmic antibody-associated vasculitis (OMAAV): A report of two cases. Auris Nasus Larynx. 2023, 50, 299–304. [Google Scholar] [CrossRef]
- Bartov, N.; Dahan, T.; Halperin, D.; Katzenell, U. Cochlear Implantation in a Patient with Granulomatosis with Polyangiitis. Isr. Med. Assoc. J. 2022, 25, 834–835. [Google Scholar]
- Wang, Z.; Han, L.; Yu, L. Effects of surgery and topical medication on eosinophilic granulomatosis with polyangiitis with otitis media and sinusitis: A case report. J. Int. Med. Res. 2020, 48, 300060520920049. [Google Scholar] [CrossRef]
- Yoshida, N.; Hara, M.; Hasegawa, M.; Matsuzawa, S.; Shinnabe, A.; Kanazawa, H.; Iino, Y. Reversible cochlear function with ANCA-associated vasculitis initially diagnosed by otologic symptoms. Otol. Neurotol. 2014, 35, 114–120. [Google Scholar] [CrossRef]
- Costa, C.F.; Polanski, J.F. Wegener Granulomatosis: Otologic Manifestation as First Symptom. Int. Arch. Otorhinolaryngol. 2015, 19, 266–268. [Google Scholar] [CrossRef]
- Wackym, P.A.; Runge-Samuelson, C.L. Cochlear Implantation: Patient Evaluation and Device Selection. In Cummings Otolaryngology: Head and Neck Surgery, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 3, Chapter 160; pp. 2413–2424.e2. [Google Scholar]
- Nakamura, T.; Ganaha, A.; Tono, T.; Yamada, Y.; Okuda, T.; Shimoara, S.; Matsuda, Y. Combined Electric acoustic stimulation in a patient with otitis media with antineutrophil cytoplasmic antibody-associated vasculitis. Auris Nasus Larynx. 2022, 49, 1072–1077. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarczyk, M.S.; Jurkiewicz, D.; Niemczyk, S.; Rymarz, A. Aural Manifestations of Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis—Diagnosis, Symptoms, Treatment. J. Clin. Med. 2024, 13, 4298. https://doi.org/10.3390/jcm13154298
Kaczmarczyk MS, Jurkiewicz D, Niemczyk S, Rymarz A. Aural Manifestations of Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis—Diagnosis, Symptoms, Treatment. Journal of Clinical Medicine. 2024; 13(15):4298. https://doi.org/10.3390/jcm13154298
Chicago/Turabian StyleKaczmarczyk, Michał S., Dariusz Jurkiewicz, Stanisław Niemczyk, and Aleksandra Rymarz. 2024. "Aural Manifestations of Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis—Diagnosis, Symptoms, Treatment" Journal of Clinical Medicine 13, no. 15: 4298. https://doi.org/10.3390/jcm13154298






